Permeability Characteristics of a New Antifungal Topical Amphotericin B Formulation with γ-Cyclodextrins
Abstract
:1. Introduction
2. Results
2.1. Rheological Behavior
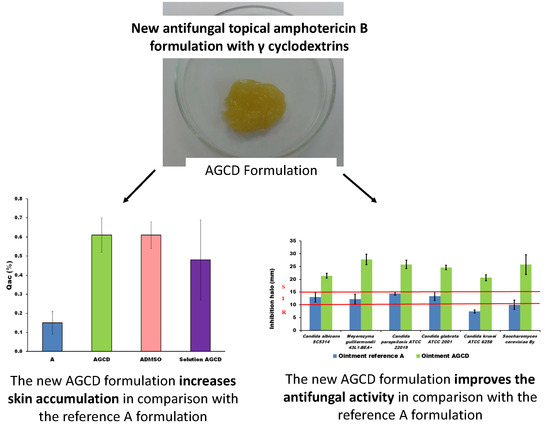
2.2. Permeability
2.3. Antifungal In Vitro Activity
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Formulations
4.3. Rheological Studies
4.4. Permeability
4.5. Antifungal In Vitro Activity
4.5.1. Disk Diffusion Test
4.5.2. Deep Diffusion Test
4.6. Statistic Data Treatment
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ben-Ami, R. Treatment of invasive candidiasis: A narrative review. J. Fungi 2018, 4, 97. [Google Scholar] [CrossRef] [PubMed]
- Torrado, J.J.; Espada, R.; Ballesteros, M.P.; Torrado-Santiago, S. Amphotericin B formulations and drug targeting. J. Pharm. Sci. 2008, 97, 2405–2425. [Google Scholar] [CrossRef] [PubMed]
- Mobasheri, M.; Attar, H.; Sorkhabadi, S.M.R.; Khamesipour, A.; Jaafari, M.R. Solubilization behavior of polyene antibiotics innanomicellar system: Insights from molecular dynamics simulation of the amphotericin B and nystatin interactions with polysorbate 80. Molecules 2016, 21, 6. [Google Scholar] [CrossRef]
- Luengo-Alonso, C.; Torrado, J.J.; Ballesteros, M.P.; Malfanti, A.; Bersani, S.; Salmaso, S.; Caliceti, P. A novel performing PEG-cholane nanoformulation for amphotericin B delivery. Int. J. Pharm. 2015, 495, 41–51. [Google Scholar] [CrossRef] [PubMed]
- Serrano, D.R.; Lalatsa, A.; Dea-Ayuela, M.A.; Bilbao-Ramos, P.E.; Garret, N.L.; Moger, J.; Guarro, J.; Capilla, J.; Ballesteros, M.P.; Schätzlein, A.G.; et al. Oral particle uptake and organ targeting drives the activity of amphotericin B nanoparticles. Mol. Pharm. 2015, 12, 420–431. [Google Scholar] [CrossRef] [PubMed]
- Grisin, T.; Bories, C.; Loiseau, P.M.; Bouchemal, K. Cyclodextrin-mediated self-associating chitosan micro-platelets act as a booster against Candida glabrata mucosal infections in immunocompetent mice. Int. J. Pharm. 2017, 519, 381–389. [Google Scholar] [CrossRef] [PubMed]
- Grisin, T.; Bories, C.; Bombardi, M.; Loiseau, P.M.; Rouffiac, V.; Solgadi, A.; Mallet, J.M.; Ponchel, G.; Bouchemal, K. Supramolecular chitosan micro-platelets synergistically enhance anti-candida albicans activity of amphotericin B using an immunocompetent murine model. Pharm. Res. 2017, 34, 1067–1082. [Google Scholar] [CrossRef] [PubMed]
- Vikmon, M.; Stadler-Szöke, A.; Szejtli, J. Solubilization of amphotericin B with γ-cyclodextrin. J. Antibiot. 1985, 38, 1822–1824. [Google Scholar] [CrossRef] [PubMed]
- Okada, Y.; Matsuda, K.; Hara, K.; Hamayasu, K.; Hashimoto, H.; Koizumi, K. Properties and the inclusion behavior of 6-O-alpha-d-galactosyl- and 6-O-alpha-d-mannosyl-cyclodextrins. Chem. Pharm. Bull. 1999, 47, 1564–1568. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.T.; Shin, B.K.; Garripelli, V.K.; Kim, J.K.; Davaa, E.; Jo, S.; Park, J.S. A thermosensitive vaginal gel formulation with HPγCD for the pH-dependent release and solubilization of amphotericin B. Eur. J. Pharm. Sci. 2010, 41, 399–406. [Google Scholar] [CrossRef] [PubMed]
- Kajtár, M.; Vikmon, M.; Morlin, E.; Szejtli, J. Aggregation of amphotericin B in the presence of gamma-cyclodextrin. Biopolymers 1989, 28, 1585–1596. [Google Scholar] [CrossRef] [PubMed]
- Legrand, P.; Chéron, M.; Leroy, L.; Bolard, J. Release of amphotericin B from delivery systems and its action against fungal and mammalian cells. J. Drug Target. 1997, 4, 311–319. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Brunete, J.A.; Dea, M.A.; Rama, S.; Bolás, F.; Alunda, J.M.; Torrado-Santiago, S.; Torrado, J.J. Amphotericin B molecular organization as an essential factor to improve activity/toxicity ratio in the treatment of visceral leishmaniasis. J. Drug Target. 2004, 12, 453–460. [Google Scholar] [CrossRef] [PubMed]
- Espada, R.; Valdespina, S.; Alfonso, C.; Rivas, G.; Ballesteros, M.P.; Torrado, J.J. Effect of aggregation state on the toxicity of different amphotericin B preparations. Int. J. Pharm. 2008, 361, 64–69. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Serrano, D.R.; Ruiz-Saldaña, H.K.; Molero, G.; Ballesteros, M.P.; Torrado, J.J. A novel formulation of solubilised amphotericin B designed for ophthalmic use. Int. J. Pharm. 2012, 437, 80–82. [Google Scholar] [CrossRef] [PubMed]
- Ruiz, H.K.; Serrano, D.R.; Dea-Ayuela, M.A.; Bilbao-Ramos, P.E.; Bolás-Fernández, F.; Torrado, J.J.; Molero, G. New amphotericin B-gamma cyclodextrin formulation for topical use with synergistic activity against diverse fungal species and Leishmania spp. Int. J. Pharm. 2014, 473, 148–157. [Google Scholar] [CrossRef] [PubMed]
- De Turris, V.; Teloni, R.; Chiani, P.; Bromuro, C.; Mariotti, S.; Pardini, M.; Nisini, R.; Torosantucci, A.; Gagliardi, M.C. Candida albicans targets a lipid raft/dectin-1 plattform to enter human monocytes and induce antigen specific T cells responses. PLoS ONE 2015, 10. [Google Scholar] [CrossRef] [PubMed]
- Viswanathan, G.; Jafurulla, M.; Kumar, G.A.; Raghunand, T.R.; Chattophardhyay, A. Dissecting the membrane cholesterol requirement for mycobacterial entry into host cells. Chem. Phys. Lipids 2015, 189, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Espinel-Ingroff, A.; Canton, E. Comparison of Neo-Sensitabs tablet diffusion assay with CLSI broth microdilution M38-A and disk diffusion methods for testing susceptibility of filamentous fungi with amphotericin B, caspofungin, itraconazole, posaconazole, and voriconazole. J. Clin. Microbiol. 2008, 46, 1793–1803. [Google Scholar] [CrossRef] [PubMed]
- Spanish Drug Agency Data Base. Available online: https://www.aemps.gob.es/cima/publico/home.html (accessed on 17 September 2018).
- Guinama Company Data Base. Available online: https://www.guinama.com/media/tecnico/93760_FT%20Orabase%20v.05.pdf (accessed on 17 September 2018).
- Stolz, R.; Hinnen, U.; Elsner, P. An evaluation of the relationship between ‘atopic skin’ and skin irritability in metalworker trainees. Contact Dermatitis 1997, 36, 281–284. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Bueno, T.; Rodríguez-Perdomo, Y.; Morales-Lacarrere, I.; Soler-Roger, D.M.; Martín-Viaña, N.P. Rheological behavior and extensibility of a semi-solid formulation of the aqueous extract of Rhizoma mangle L. Tecnol. Ciencia Ed. 2011, 26, 75–79. [Google Scholar]
- Espada, R.; Josa, J.M.; Valdespina, S.; Dea, M.A.; Ballesteros, M.P.; Alunda, J.M.; Torrado, J.J. HPLC assay for determination of amphotericin B in biological samples. Biomed. Chromatogr. 2008, 22, 402–407. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Not available. |





| Components | A | ADMSO | AGCD |
|---|---|---|---|
| AmB | 0.125 | 0.125 | 0.125 |
| Orabase® 1 | 99.875 | 87.375 | 87.375 |
| DMSO | - | 12.5 | - |
| γ-cyclodextrin | - | - | 12.5 |
| Components | Suspension A | Solution ADMSO | Solution AGCD |
|---|---|---|---|
| AmB | 0.125 | 0.125 | 0.125 |
| Purified water | 99.875 | - | 87.375 |
| DMSO | - | 99.875 | - |
| γ-cyclodextrin | - | - | 12.5 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
López-Castillo, C.; Rodríguez-Fernández, C.; Córdoba, M.; Torrado, J.J. Permeability Characteristics of a New Antifungal Topical Amphotericin B Formulation with γ-Cyclodextrins. Molecules 2018, 23, 3349. https://doi.org/10.3390/molecules23123349
López-Castillo C, Rodríguez-Fernández C, Córdoba M, Torrado JJ. Permeability Characteristics of a New Antifungal Topical Amphotericin B Formulation with γ-Cyclodextrins. Molecules. 2018; 23(12):3349. https://doi.org/10.3390/molecules23123349
Chicago/Turabian StyleLópez-Castillo, Carmen, Carmina Rodríguez-Fernández, Manuel Córdoba, and Juan J. Torrado. 2018. "Permeability Characteristics of a New Antifungal Topical Amphotericin B Formulation with γ-Cyclodextrins" Molecules 23, no. 12: 3349. https://doi.org/10.3390/molecules23123349