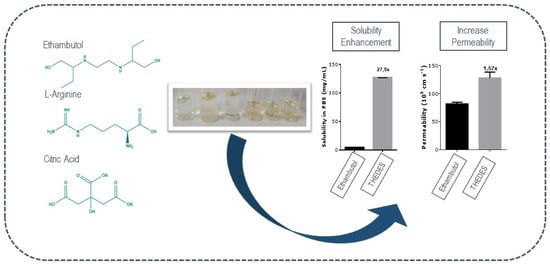
Properties of Therapeutic Deep Eutectic Solvents of l-Arginine and Ethambutol for Tuberculosis Treatment
Abstract
:1. Introduction
2. Results
2.1. Polarized Optical Microscopy
2.2. Differential Scanning Calorimetry
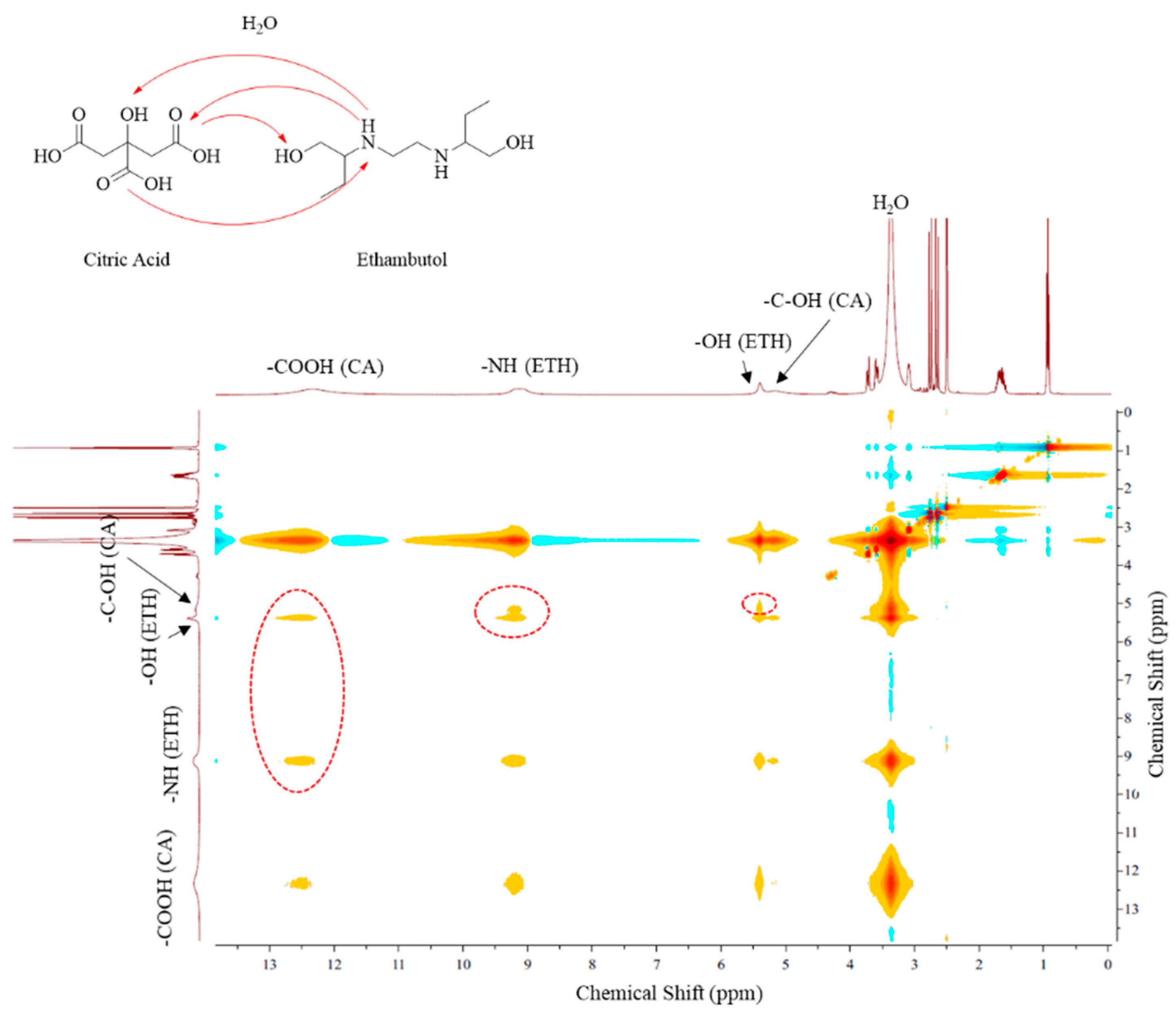
2.3. Nuclear Magnetic Resonance Studies
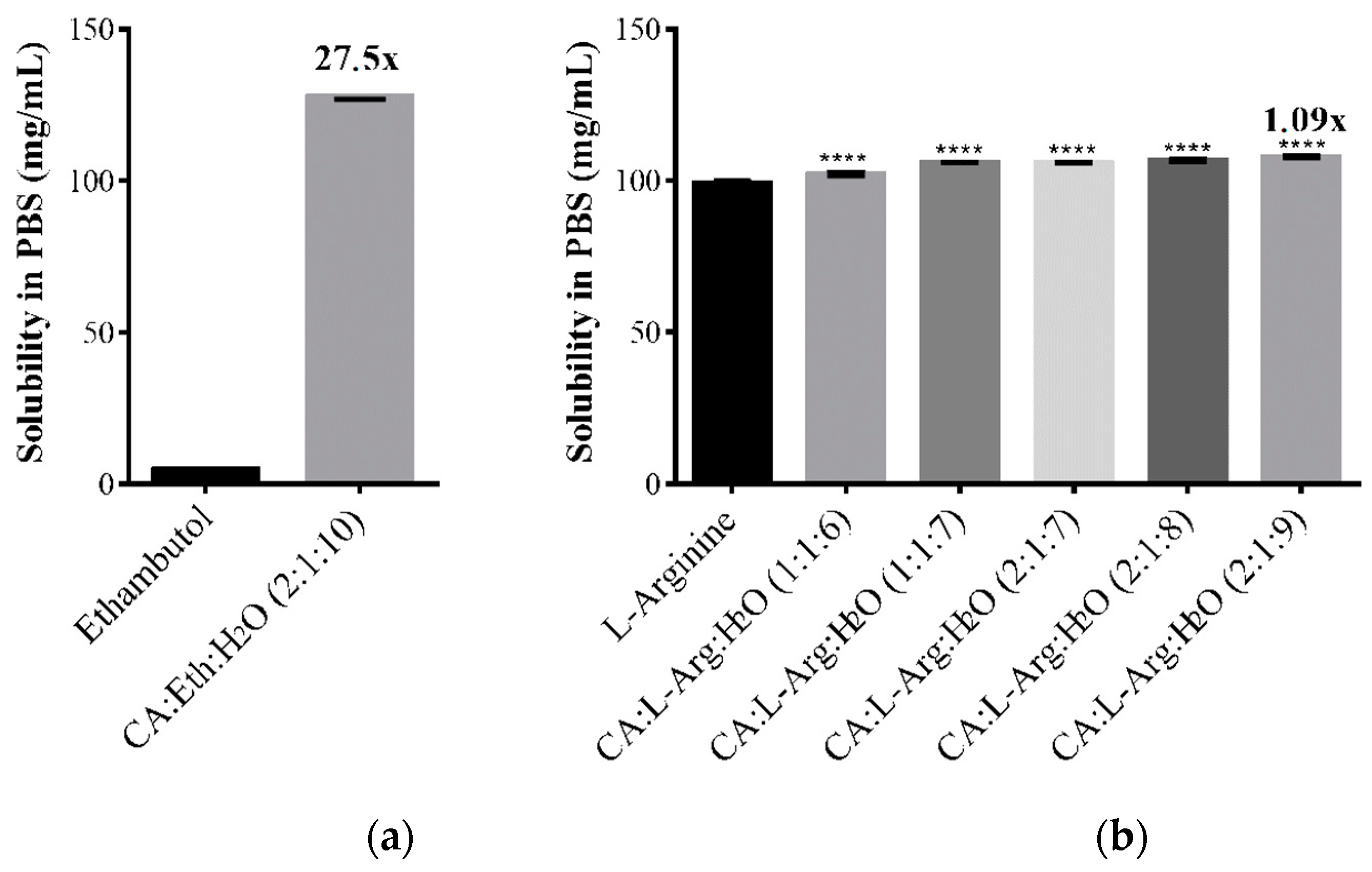
2.4. Solubility and Permeability Studies
2.5. Biological Performance
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Preparation of THEDES
4.3. Differential Scanning Calorimetry (DSC) Analysis—Thermal properties
4.4. Polarized Optical Microscopy (POM)
4.5. Nuclear Magnetic Resonance Studies (NMR)
4.6. Solubility and Permeability Studies
4.7. In Vitro Cytotoxicity and IC50 Evaluation
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Global Tuberculosis Report 2017; WHO: Geneva, Switzerland, 2017; ISBN 978-92-4-156551-6. [Google Scholar]
- Tiberi, S.; Buchanan, R.; Caminerom, J.A.; Centris, R.; Arbex, M.A.; Salazar, M.; Potter, J.; Migliori, G.B. The challenge of the new tuberculosis drugs. Presse Med. 2017, 46, e41–e51. [Google Scholar] [CrossRef] [PubMed]
- Pai, M.; Behr, M.A.; Dowdy, D.; Dheda, K.; Divangahi, M.; Boehme, C.C.; Ginsberg, A.; Swaminathan, S.; Spigelman, M.; Getahun, H.; et al. Tuberculosis. Nat. Rev. Dis. Primers 2016, 2, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Dheda, K.; Gumbo, T.; Gandhi, N.L.; Murray, M.; Theron, G.; Udwadia, Z.; Migliori, G.B.; Warren, R. Global control of tuberculosis: From extensively drug-resistant to untreatable tuberculosis. Lancet Respir. Med. 2014, 2, 321–338. [Google Scholar] [CrossRef]
- Duarte, A.R.C.; Ferreira, A.S.D.; Barreiros, S.; Cabrita, E.; Reis, R.L.; Paiva, A. A comparison between pure active pharmaceutical ingredients and therapeutic deep eutectic solvents: Solubility and permeability studies. Eur. J. Pharm. Biopharm. 2017, 114, 296–304. [Google Scholar] [CrossRef] [PubMed]
- Aroso, I.M.; Silva, J.C.; Mano, F.; Ferreira, A.S.D.; Dionísio, M.; Sá-Nogueira, I.; Barreiros, S.; Reis, R.L.; Paiva, A.; Duarte, A.R.C. Dissolution enhancement of active pharmaceutical ingredientes by therapeutic deep eutectic systems. Euro. J. Pharm. Biopharm. 2016, 98, 57–66. [Google Scholar] [CrossRef] [PubMed]
- Smith, E.L.; Abbott, A.P.; Ryder, K.S. Deep eutectic solvents (DESs) and their applications. Chem. Rev. 2014, 114, 11060–11082. [Google Scholar] [CrossRef] [PubMed]
- Abbott, A.P.; Ahmed, E.I.; Prasad, K.; Qader, I.B.; Ryder, K.S. Liquid pharmaceuticals formulation by eutectic formation. Fluid Phase Equilib. 2017, 448, 2–8. [Google Scholar] [CrossRef]
- Mbous, Y.P.; Hayyan, M.; Wong, W.F.; Looi, C.Y.; Hashim, M.A. Unraveling the cytotoxicity and metabolimic pathways of binary natural deep eutectic solvent systems. Sci. Rep. 2017, 7, 41257. [Google Scholar] [CrossRef]
- Verbeeck, R.K.; Günther, G.; Kibuule, D.; Hunter, C.; Rennie, T.W. Optimizing treatment outcome of first-line anti-tuberculosis drugs: The role of therapeutic drug monitoring. Eur. J. Clin. Pharmacol. 2016, 72, 905–916. [Google Scholar] [CrossRef]
- Rawat, J.; Jain, P.K.; Ravichandran, V.; Agrawal, R.K. Synthesis and evaluation of mutual prodrugs of isoniazid, p-amino salicylic acid and ethambutol. Arkivoc 2007, 1, 105–118. [Google Scholar]
- Farazi, A.; Shafaat, O.; Sofian, M.; Kahbazi, M. Arginine adjunctive therapy in active tuberculosis. Tuberc. Res. Treat. 2015, 2015, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Schön, T.; Idh, J.; Westman, A.; Elias, D.; Abate, E.; Diro, E.; Moges, F.; Kassu, A.; Ayele, B.; Forslund, T.; et al. Effects of a food supplement rich in arginine in patients with smear positive pulmonary tuberculosis—A randomized trial. Tuberculosis 2011, 91, 370–377. [Google Scholar] [CrossRef] [PubMed]
- Schön, T.; Elias, D.; Moges, F.; Melese, E.; Tessema, T.; Stendahl, O.; Britton, S.; Sundqvist, T. Arginine as an adjuvant to chemotherapy improves clinical outcome in active tuberculosis. Eur. Respir. J. 2003, 21, 483–488. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morrison, H.G.; Sun, C.C.; Neervannan, S. Characterization of thermal behavior of deep eutectic solvents and their potential as drug solubilization vehicles. Int. J. Pharm. 2009, 378, 136–139. [Google Scholar] [CrossRef] [PubMed]
- Craveiro, R.; Aroso, I.; Flammia, V.; Carvalho, T.; Viciosa, M.T.; Dionísio, M.; Barreiros, S.; Reis, R.L.; Duarte, A.R.C.; Paiva, A. Properties and termal behavior of natural deep eutectic solventes. J. Mol. Liq. 2016, 215, 534–550. [Google Scholar] [CrossRef]
- Lavor, E.P.; Freire, F.D.; Aragão, C.F.S.; Raffin, N.; Moura, T.F.A.L. Application of thermal analysis to the study of anti-tuberculosis drug compatibility. Part 1. J. Therm. Anal. Calorim. 2012, 108, 207–212. [Google Scholar] [CrossRef]
- Dai, Y.; Spronsen, J.V.; Witkamp, G.J.; Verpoorte, R.; Choi, Y.H. Natural deep eutectic solvents as new potential media for green technology. Anal. Chim. Acta 2013, 766, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Friesen, J.B.; McAlpine, J.B.; Lankin, D.C.; Chen, S.N.; Pauli, G.F. Natural deep eutectic solvents: Properties, applications, and perspectives. J. Nat. Prod. 2018, 81, 679–690. [Google Scholar] [CrossRef] [PubMed]
- Breemen, R.B.V.; Li, Y. Caco-2 cell permeability assays to measure drug absorption. Expert Opin. Drug Metab. Toxicol. 2005, 1, 175–185. [Google Scholar] [CrossRef] [PubMed]
- Dai, Y.; Witkamp, G.J.; Verpoorte, R.; Choi, Y.H. Tailoring properties of natural deep eutectic solvents with water to facilitate their applications. Food Chem. 2015, 187, 14–19. [Google Scholar] [CrossRef] [PubMed]
- Lindenberg, M.; Kopp, S.; Dressman, J.B. Classification of orally administered drugs on the World Health Organization Model list of essential medicines according to the biopharmaceutics classification system. Biopharmaceutics 2004, 58, 265–278. [Google Scholar] [CrossRef] [PubMed]
- Siongco, K.R.; Leron, R.B.; Caparanga, A.R.; Li, M.H. Molar heat capacities and electrical conductivities of two ammonium-based deep eutectic solvents and their aqueous solutions. Thermochim. Acta 2013, 556, 50–56. [Google Scholar] [CrossRef]
- Krstić, M.; Popović, M.; Dobričić, V.; Ibrić, S. Influence of solid drug delivery system formulation on poorly water-soluble drug dissolution and permeability. Molecules 2015, 20, 14684–14698. [Google Scholar] [CrossRef] [PubMed]
- Silva, J.M.; Duarte, A.R.C.; Caridade, S.G.; Picart, C.; Reis, R.L.; Mano, J.F. Tailored freestanding multilayered membranes based on chitosan and alginate. Biomacromolecules 2014, 15, 3817–3826. [Google Scholar] [CrossRef] [PubMed]
- Dienaite, L.; Pukalskiene, M.; Matias, A.A.; Pereira, C.V.; Pukalskas, A.; Venskutonis, P.R. Valorization of six Nepeta species by assessing the antioxidant potential, phytochemical composition and bioactivity of their extracts in the cell cultures. J. Funct. Foods 2018, 45, 512–522. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |



| THEDES | Molar Ratio | Solubility (mg/mL) |
|---|---|---|
| Ethambutol | 4.64 ± 0.07 | |
| Citric Acid:Ethambutol:H2O | 2:1:10 | 127.60 ± 0.69 |
| l-Arginine | 99.35 ± 0.54 | |
| Citric Acid:l-Arginine:H2O | 1:1:6 | 102.17 ± 0.56 |
| Citric Acid:l-Arginine:H2O | 1:1:7 | 106.05 ± 0.12 |
| Citric Acid:l-Arginine:H2O | 2:1:7 | 105.89 ± 0.15 |
| Citric Acid:l-Arginine:H2O | 2:1:8 | 106.77 ± 0.49 |
| Citric Acid:l-Arginine:H2O | 2:1:9 | 107.86 ± 0.33 |
| THEDES | Permeability (105 cm s−1) | Diffusion Coefficient (106 cm2 s−1) |
|---|---|---|
| thambutol | 81.9 ± 3.06 | 13.6 ± 1.01 |
| Citric Acid:Ethambutol:H2O (2:1:10) | 128.3 ± 10.4 | 17.4 ± 1.28 |
| l-Arginine | 36.6 ± 2.39 | 8.20 ± 0.39 |
| Citric Acid:l-Arginine:H2O (1:1:7) | 21.6 ± 0.21 | 5.59 ± 0.09 |
| Citric Acid:l-Arginine:H2O (2:1:9) | 23.2 ± 0.3 | 5.58 ± 0.1 |
| THEDES | Molar Ratio | IC50 (M) | pH |
|---|---|---|---|
| Ethambutol | 0.1007 | 6.21 | |
| Citric Acid:Ethambutol:H2O | 2:1:10 | 0.0647 | 5.61 |
| Citric Acid | 0.0033 | 7.08 | |
| l-Arginine | 0.1017 | 10.43 | |
| Citric Acid:l-Arginine:H2O | 1:1:6 | 0.0644 | 6.61 |
| Citric Acid:l-Arginine:H2O | 1:1:7 | 0.1085 | 5.88 |
| Citric Acid:l-Arginine:H2O | 2:1:7 | 0.0476 | 5.79 |
| Citric Acid:l-Arginine:H2O | 2:1:8 | 0.0662 | 5.48 |
| Citric Acid:l-Arginine:H2O | 2:1:9 | 0.0656 | 5.79 |
| Component A | Component B | Component C | Molar Ratio | Molar Mass * (g/mol) | Abbreviation | Visual Aspect |
|---|---|---|---|---|---|---|
| Citric Acid | Ethambutol | H2O | 2:1:10 | 61.02 | CA:ETH:H2O | Transparent liquid at room temperature |
| Citric Acid | l-Arginine | H2O | 1:1:6 | 61.56 | CA:L-Arg:H2O | |
| Citric Acid | l-Arginine | H2O | 1:1:7 | 56.72 | CA:L-Arg:H2O | |
| Citric Acid | l-Arginine | H2O | 2:1:7 | 77.02 | CA:L-Arg:H2O | |
| Citric Acid | l-Arginine | H2O | 2:1:8 | 67.15 | CA:L-Arg:H2O | |
| Citric Acid | l-Arginine | H2O | 2:1:9 | 63.06 | CA:L-Arg:H2O |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santos, F.; P.S. Leitão, M.I.; C. Duarte, A.R. Properties of Therapeutic Deep Eutectic Solvents of l-Arginine and Ethambutol for Tuberculosis Treatment. Molecules 2019, 24, 55. https://doi.org/10.3390/molecules24010055
Santos F, P.S. Leitão MI, C. Duarte AR. Properties of Therapeutic Deep Eutectic Solvents of l-Arginine and Ethambutol for Tuberculosis Treatment. Molecules. 2019; 24(1):55. https://doi.org/10.3390/molecules24010055
Chicago/Turabian StyleSantos, Filipa, Maria Inês P.S. Leitão, and Ana Rita C. Duarte. 2019. "Properties of Therapeutic Deep Eutectic Solvents of l-Arginine and Ethambutol for Tuberculosis Treatment" Molecules 24, no. 1: 55. https://doi.org/10.3390/molecules24010055