Modulation of ERK1/2 and Akt Pathways Involved in the Neurotrophic Action of Caffeic Acid Alkyl Esters
Abstract
:1. Introduction
2. Results
2.1. Chemistry
2.2. Effect of K252a and Caffeic Acid Ester Derivatives on the Viability of Serum-Deprived PC12 Cells
2.3. Assessment of TrkA Phosphorylation in PC12 Cells
2.4. Establishment of NIH/3T3-TrkA Cells
2.5. Assessment of TrkA Phosphorylation in NIH/3T3-TrkA Cells
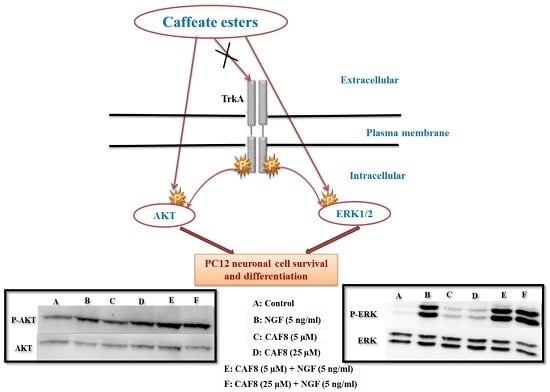
2.6. Assessment of ERK1/2 and Akt Phosphorylation in PC12 Cells
3. Discussion
4. Materials and Methods
4.1. Reagents
4.2. Chemistry
4.3. Cell Culture
4.4. Cell Viability Assay Using K252a
4.5. Immunoblotting
4.6. Establishment of Stable TrkA Overexpressing NIH/3T3 Cells
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Manach, C.; Scalbert, A.; Morand, C.; Rémésy, C.; Jiménez, L. Polyphenols: Food sources and bioavailability. Am. J. Clin. Nutr. 2004, 79, 727–747. [Google Scholar] [CrossRef] [PubMed]
- Firuzi, O.; Lacanna, A.; Petrucci, R.; Marrosu, G.; Saso, L. Evaluation of the antioxidant activity of flavonoids by “ferric reducing antioxidant power” assay and cyclic voltammetry. Biochim. Biophys. Acta 2005, 1721, 174–184. [Google Scholar] [CrossRef] [PubMed]
- Firuzi, O.; Mladěnka, P.; Petrucci, R.; Marrosu, G.; Saso, L. Hypochlorite scavenging activity of flavonoids. J. Pharm. Pharmacol. 2004, 56, 801–807. [Google Scholar] [CrossRef] [PubMed]
- Lemmens, K.J.; van de Wier, B.; Vaes, N.; Ghosh, M.; van Zandvoort, M.A.; van der Vijgh, W.J.; Bast, A.; Haenen, G.R. The flavonoid 7-mono-O-(β-hydroxyethyl)-rutoside is able to protect endothelial cells by a direct antioxidant effect. Toxicol. In Vitro 2014, 28, 538–543. [Google Scholar] [CrossRef] [PubMed]
- Martín, M.Á.; Fernández-Millán, E.; Ramos, S.; Bravo, L.; Goya, L. Cocoa flavonoid epicatechin protects pancreatic beta cell viability and function against oxidative stress. Mol. Nutr. Food Res. 2014, 58, 447–456. [Google Scholar] [CrossRef] [PubMed]
- Del Rio, D.; Rodriguez-Mateos, A.; Spencer, J.P.; Tognolini, M.; Borges, G.; Crozier, A. Dietary (poly) phenolics in human health: Structures, bioavailability, and evidence of protective effects against chronic diseases. Antioxid. Redox Signal. 2013, 18, 1818–1892. [Google Scholar] [CrossRef]
- Vauzour, D. Dietary polyphenols as modulators of brain functions: Biological actions and molecular mechanisms underpinning their beneficial effects. Oxid. Med. Cell. Longev. 2012, 914273. [Google Scholar] [CrossRef] [PubMed]
- Garrido, J.; Gaspar, A.; Garrido, E.M.; Miri, R.; Tavakkoli, M.; Poural, S.; Saso, L.; Borges, F.; Firuzi, O. Alkyl esters of hydroxycinnamic acids with improved antioxidant activity and lipophilicity protect PC12 cells against oxidative stress. Biochimie 2012, 94, 961–967. [Google Scholar] [CrossRef]
- Gaspar, A.; Martins, M.; Silva, P.; Garrido, E.M.; Garrido, J.; Firuzi, O.; Miri, R.; Saso, L.; Borges, F. Dietary phenolic acids and derivatives. Evaluation of the antioxidant activity of sinapic acid and its alkyl esters. J. Agric. Food Chem. 2010, 58, 11273–11280. [Google Scholar]
- Hwang, S.-L.; Shih, P.-H.; Yen, G.-C. Neuroprotective effects of citrus flavonoids. J. Agric. Food Chem. 2012, 60, 877–885. [Google Scholar] [CrossRef] [PubMed]
- Venkatesan, R.; Ji, E.; Kim, S.Y. Phytochemicals that regulate neurodegenerative disease by targeting neurotrophins: A comprehensive review. Biomed. Res. Int. 2015, 2015, 814068. [Google Scholar] [CrossRef] [PubMed]
- Mazaheri, M.; Moosavi-Movahedi, A.A.; Saboury, A.A.; Khodagholi, F.; Shaerzadeh, F.; Sheibani, N. Curcumin protects β-lactoglobulin Fibril Formation and fibril-induced neurotoxicity in PC12Cells. PLoS ONE 2015, 10, e0133206. [Google Scholar] [CrossRef] [PubMed]
- Moosavi, F.; Hosseini, R.; Saso, L.; Firuzi, O. Modulation of neurotrophic signaling pathways by polyphenols. Drug Des. Dev. Ther. 2016, 10, 23–42. [Google Scholar]
- Bhullar, K.S.; Rupasinghe, H. Polyphenols: Multipotent therapeutic agents in neurodegenerative diseases. Oxid. Med. Cell. Longev. 2013, 2013, 891748. [Google Scholar] [CrossRef] [PubMed]
- Ebrahimi, A.; Schluesener, H. Natural polyphenols against neurodegenerative disorders: Potentials and pitfalls. Ageing Res. Rev. 2012, 11, 329–345. [Google Scholar] [CrossRef] [PubMed]
- Clifford, M.N. Chlorogenic acids and other cinnamates–nature, occurrence, dietary burden, absorption and metabolism. J. Sci. Food Agric. 2000, 80, 1033–1043. [Google Scholar] [CrossRef]
- Jakobek, L.; Šeruga, M.; Jovanović, I.N.; Medvidović-Kosanović, M. Flavonols, phenolic acids and antioxidant activity of some red fruits. Dtsch. Lebensm. Rundsch. 2007, 103, 369–378. [Google Scholar]
- Papetti, A.; Daglia, M.; Aceti, C.; Sordelli, B.; Spini, V.; Carazzone, C.; Gazzani, G. Hydroxycinnamic acid derivatives occurring in Cichorium endivia vegetables. J. Pharm. Biomed. Anal. 2008, 48, 472–476. [Google Scholar] [CrossRef] [PubMed]
- Razzaghi-Asl, N.; Garrido, J.; Khazraei, H.; Borges, F.; Firuzi, O. Antioxidant properties of hydroxycinnamic acids: A review of structure-activity relationships. Curr. Med. Chem. 2013, 20, 4436–4450. [Google Scholar] [CrossRef] [PubMed]
- Firuzi, O.; Giansanti, L.; Vento, R.; Seibert, C.; Petrucci, R.; Marrosu, G.; Agostino, R.; Saso, L. Hypochlorite scavenging activity of hydroxycinnamic acids evaluated by a rapid microplate method based on the measurement of chloramines. J. Pharm. Pharmacol. 2003, 55, 1021–1027. [Google Scholar] [CrossRef] [PubMed]
- Ohara, K.; Uchida, A.; Nagasaka, R.; Ushio, H.; Ohshima, T. The effects of hydroxycinnamic acid derivatives on adiponectin secretion. Phytomedicine 2009, 16, 130–137. [Google Scholar] [CrossRef]
- Duchnowicz, P.; Broncel, M.; Podsędek, A.; Koter-Michalak, M. Hypolipidemic and antioxidant effects of hydroxycinnamic acids, quercetin, and cyanidin 3-glucoside in hypercholesterolemic erythrocytes (in vitro study). Eur. J. Nutr. 2012, 51, 435–443. [Google Scholar] [CrossRef] [PubMed]
- Heese, K.; Low, J.W.; Inoue, N. Nerve growth factor, neural stem cells and Alzheimer’s disease. Neurosignals 2006, 15, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Scarpi, D.; Cirelli, D.; Matrone, C.; Castronovo, G.; Rosini, P.; Occhiato, E.G.; Romano, F.; Bartali, L.; Clemente, A.M.; Bottegoni, G. Low molecular weight, non-peptidic agonists of TrkA receptor with NGF-mimetic activity. Cell Death Dis. 2012, 3, e339. [Google Scholar] [CrossRef] [PubMed]
- Longo, F.M.; Massa, S.M. Small-molecule modulation of neurotrophin receptors: A strategy for the treatment of neurological disease. Nat. Rev. Drug Dis. 2013, 12, 507–525. [Google Scholar] [CrossRef]
- Skaper, S.D. The neurotrophin family of neurotrophic factors: An overview. Methods Mol. Biol. 2012, 846, 1–12. [Google Scholar] [PubMed]
- Hosseini, R.; Moosavi, F.; Rajaian, H.; Silva, T.; Magalhães e Silva, D.; Soares, P.; Saso, L.; Edraki, N.; Miri, R.; Borges, F. Discovery of neurotrophic agents based on hydroxycinnamic acid scaffold. Chem. Biol. Drug Des. 2016, 88, 926–937. [Google Scholar] [CrossRef] [PubMed]
- Peng, C.-H.; Chiou, S.-H.; Chen, S.-J.; Chou, Y.-C.; Ku, H.-H.; Cheng, C.-K.; Yen, C.-J.; Tsai, T.-H.; Chang, Y.-L.; Kao, C.-L. Neuroprotection by Imipramine against lipopolysaccharide-induced apoptosis in hippocampus-derived neural stem cells mediated by activation of BDNF and the MAPK pathway. Eur. Neuropsychopharmacol. 2008, 18, 128–140. [Google Scholar] [CrossRef]
- Almeida, R.; Manadas, B.; Melo, C.; Gomes, J.; Mendes, C.; Graos, M.; Carvalho, R.; Carvalho, A.; Duarte, C. Neuroprotection by BDNF against glutamate-induced apoptotic cell death is mediated by ERK and PI3-kinase pathways. Cell Death Differ. 2005, 12, 1329–1343. [Google Scholar] [CrossRef] [Green Version]
- Angelucci, F.; Caltagirone, C.; Costa, A. Cognitive training in neurodegenerative diseases: A way to boost neuroprotective molecules? Neural Regener. Res. 2015, 10, 1754–1755. [Google Scholar] [CrossRef]
- Fukumoto, K.; Mizoguchi, H.; Takeuchi, H.; Horiuchi, H.; Kawanokuchi, J.; Jin, S.; Mizuno, T.; Suzumura, A. Fingolimod increases brain-derived neurotrophic factor levels and ameliorates amyloid β-induced memory impairment. Behav. Brain Res. 2014, 268, 88–93. [Google Scholar] [CrossRef] [PubMed]
- Sadan, O.; Shemesh, N.; Barzilay, R.; Dadon-Nahum, M.; Blumenfeld-Katzir, T.; Assaf, Y.; Yeshurun, M.; Djaldetti, R.; Cohen, Y.; Melamed, E. Mesenchymal stem cells induced to secrete neurotrophic factors attenuate quinolinic acid toxicity: A potential therapy for Huntington’s disease. Exp. Neurol. 2012, 234, 417–427. [Google Scholar] [CrossRef] [PubMed]
- Chao, M.V. Neurotrophins and their receptors: A convergence point for many signalling pathways. Nat. Rev. Neurosci. 2003, 4, 299–309. [Google Scholar] [CrossRef] [PubMed]
- Huang, E.J.; Reichardt, L.F. Trk receptors: Roles in neuronal signal transduction. Annu. Rev. Biochem. 2003, 72, 609–642. [Google Scholar] [CrossRef] [PubMed]
- Patapoutian, A.; Reichardt, L.F. Trk receptors: Mediators of neurotrophin action. Curr. Opin. Neurobiol. 2001, 11, 272–280. [Google Scholar] [CrossRef]
- Zampieri, N.; Chao, M. Mechanisms of neurotrophin receptor signalling. Biochem. Soc. Trans. 2006, 34, 607–611. [Google Scholar] [CrossRef]
- Zhao, J.; Cheng, Y.Y.; Fan, W.; Yang, C.B.; Ye, S.F.; Cui, W.; Wei, W.; Lao, L.X.; Cai, J.; Han, Y.F. Botanical drug puerarin coordinates with nerve growth factor in the regulation of neuronal survival and neuritogenesis via activating ERK1/2 and PI3K/Akt signaling pathways in the neurite extension process. CNS Neurosci. Ther. 2015, 21, 61–70. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Theus, M.H.; Wei, L. Role of ERK1/2 signaling in neuronal differentiation of cultured embryonic stem cells. Dev. Growth Differ. 2006, 48, 513–523. [Google Scholar] [CrossRef] [PubMed]
- Satoh, Y.; Kobayashi, Y.; Takeuchi, A.; Pagès, G.; Pouysségur, J.; Kazama, T. Deletion of ERK1 and ERK2 in the CNS causes cortical abnormalities and neonatal lethality: Erk1 deficiency enhances the impairment of neurogenesis in Erk2-deficient mice. J. Neurosci. 2011, 31, 1149–1155. [Google Scholar] [CrossRef]
- Li, Q.; Chen, M.; Liu, H.; Yang, L.; Yang, T.; He, G. The dual role of ERK signaling in the apoptosis of neurons. Front. Biosci. (Landmark Ed.) 2013, 19, 1411–1417. [Google Scholar] [CrossRef]
- Parmar, M.S.; Jaumotte, J.D.; Wyrostek, S.L.; Zigmond, M.J.; Cavanaugh, J.E. The role of ERK1, 2, and 5 in dopamine neuron survival during aging. Neurobiol. Aging 2014, 35, 669–679. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fang, X.-X.; Jiang, X.-L.; Han, X.-H.; Peng, Y.-P.; Qiu, Y.-H. Neuroprotection of interleukin-6 against NMDA-induced neurotoxicity is mediated by JAK/STAT3, MAPK/ERK, and PI3K/AKT signaling pathways. Cell. Mol. Neurobiol. 2013, 33, 241–251. [Google Scholar] [CrossRef] [PubMed]
- Luo, L.; Liu, X.-L.; Li, J.; Mu, R.-H.; Liu, Q.; Yi, L.-T.; Geng, D. Macranthol promotes hippocampal neuronal proliferation in mice via BDNF–TrkB–PI3K/Akt signaling pathway. Eur. J. Pharmacol. 2015, 762, 357–363. [Google Scholar] [CrossRef] [PubMed]
- Zou, Y.; Wang, B.; Fu, W.; Zhou, S.; Nie, Y.; Tian, S. Apelin-13 Protects PC12 Cells from Corticosterone-Induced Apoptosis Through PI3K and ERKs Activation. Neurochem. Res. 2016, 41, 1635–1644. [Google Scholar] [CrossRef] [PubMed]
- Omri, A.E.L.; Han, J.; Kawada, K.; Abdrabbah, M.B.; Isoda, H. Luteolin enhances cholinergic activities in PC12 cells through ERK1/2 and PI3K/Akt pathways. Brain Res. 2012, 1437, 16–25. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Yang, J.; Peng, L.; Zhao, J.; Mu, N.; Huang, J.; Lazarovici, P.; Chen, H.; Zheng, W. Gardenamide A attenuated cell apoptosis induced by serum deprivation insult via the ERK1/2 and PI3K/AKT signaling pathways. Neuroscience 2015, 286, 242–250. [Google Scholar] [CrossRef]
- Jang, S.-W.; Liu, X.; Yepes, M.; Shepherd, K.R.; Miller, G.W.; Liu, Y.; Wilson, W.D.; Xiao, G.; Blanchi, B.; Sun, Y.E. A selective TrkB agonist with potent neurotrophic activities by 7,8-dihydroxyflavone. Proc. Natl. Acad. Sci. USA 2010, 107, 2687–2692. [Google Scholar] [CrossRef] [Green Version]
- Ren, Q.; Zhang, J.-C.; Ma, M.; Fujita, Y.; Wu, J.; Hashimoto, K. 7,8-Dihydroxyflavone, a TrkB agonist, attenuates behavioral abnormalities and neurotoxicity in mice after administration of methamphetamine. Psychopharmacology 2014, 231, 159–166. [Google Scholar] [CrossRef]
- Tsai, T.; Klausmeyer, A.; Conrad, R.; Gottschling, C.; Leo, M.; Faissner, A.; Wiese, S. 7,8-Dihydroxyflavone leads to survival of cultured embryonic motoneurons by activating intracellular signaling pathways. Mol. Cell. Neurosci. 2013, 56, 18–28. [Google Scholar] [CrossRef]
- Moosavi, F.; Hosseini, R.; Rajaian, H.; Silva, T.; Magalhães, E.; Silva, D.; Saso, L.; Edraki, N.; Miri, R.; Borges, F.; Firuzi, O. Derivatives of caffeic acid, a natural antioxidant, as the basis for the discovery of novel nonpeptidic neurotrophic agents. Bioorg. Med. Chem. 2017, 25, 3235–3246. [Google Scholar] [CrossRef]
- Lin, C.-W.; Wu, M.-J.; Liu, I.C.; Su, J.-D.; Yen, J.-H. Neurotrophic and cytoprotective action of luteolin in PC12 cells through ERK-dependent induction of Nrf2-driven HO-1 expression. J. Agric. Food Chem. 2010, 58, 4477–4486. [Google Scholar] [CrossRef] [PubMed]
- El Omri, A.; Han, J.; Yamada, P.; Kawada, K.; Abdrabbah, M.B.; Isoda, H. Rosmarinus officinalis polyphenols activate cholinergic activities in PC12 cells through phosphorylation of ERK1/2. J. Ethnopharmacol. 2010, 131, 451–458. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Z.; Cai, L.; Zhou, X.; Su, C.; Xiao, F.; Gao, Q.; Luo, H. Methyl 3,4-dihydroxybenzoate promote rat cortical neurons survival and neurite outgrowth through the adenosine A2a receptor/PI3K/Akt signaling pathway. NeuroReport 2015, 26, 367–373. [Google Scholar] [CrossRef]
- Nguyen, C.N.; Kim, H.E.; Lee, S.G. Caffeoylserotonin Protects Human Keratinocyte HaCaT Cells against H2O2–Induced Oxidative Stress and Apoptosis through Upregulation of HO–1 Expression via Activation of the PI3K/Akt/Nrf2 Pathway. Phytother. Res. 2013, 27, 1810–1818. [Google Scholar] [CrossRef] [PubMed]
- Chai, L.; Guo, H.; Li, H.; Wang, S.; Wang, Y.-l.; Shi, F.; Hu, L.-M.; Liu, Y.; Adah, D. Scutellarin and caffeic acid ester fraction, active components of Dengzhanxixin injection, upregulate neurotrophins synthesis and release in hypoxia/reoxygenation rat astrocytes. J. Ethnopharmacol. 2013, 150, 100–107. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Jin, M.; Pi, R.; Zhang, J.; Chen, M.; Ouyang, Y.; Liu, A.; Chao, X.; Liu, P.; Liu, J. Protective effects of caffeic acid and caffeic acid phenethyl ester against acrolein-induced neurotoxicity in HT22 mouse hippocampal cells. Neurosci. Lett. 2013, 535, 146–151. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.; Baarine, M.; Singh, I. Therapeutic potential of caffeic acid phenethyl ester in neurodegenerative diseases. In Caffeic Acid: Biological Properties. Structure and Health Effects; Nova Science Publishers: New York, NY, USA, 2015. [Google Scholar]
- Shen, J.N.; Xu, L.X.; Shan, L.; Zhang, W.D.; Li, H.L.; Wang, R. Neuroprotection of (+)-2-(1-hydroxyl-4-oxocyclohexyl) ethyl caffeate against hydrogen peroxide and lipopolysaccharide induced injury via modulating arachidonic acid network and p38-MAPK signaling. Curr. Alzheimer Res. 2015, 9, 892–902. [Google Scholar] [CrossRef]
- Silva, R.B.; Santos, N.A.; Martins, N.M.; Ferreira, D.A.; Barbosa, F., Jr.; Souza, V.O.; Kinoshita, A.; Baffa, O.; Del-Bel, E.; Santos, A.C. Caffeic acid phenethyl ester protects against the dopaminergic neuronal loss induced by 6-hydroxydopamine in rats. Neuroscience 2013, 233, 86–94. [Google Scholar] [CrossRef] [PubMed]
- Chien, M.Y.; Chuang, C.H.; Chern, C.M.; Liou, K.T.; Liu, D.Z.; Hou, Y.C.; Shen, Y.C. Salvianolic acid A alleviates ischemic brain injury through the inhibition of inflammation and apoptosis and the promotion of neurogenesis in mice. Free Radic. Biol. Med. 2016, 99, 508–519. [Google Scholar] [CrossRef] [PubMed]
- Teng, K.K.; Angelastro, J.M.; Cunningham, M.E.; Greene, L.A. Cultured PC12 cells: A model for neuronal function, differentiation, and survival. Cell Biol. (Third Ed.) 2006, 1, 171–176. [Google Scholar]
- Goc, A.; Sabbineni, H.; Abdalla, M.; Somanath, P.R. p70 S6-kinase mediates the cooperation between Akt1 and Mek1 pathways in fibroblast-mediated extracellular matrix remodeling. Biochim. Biophys. Acta Mol. Cell Res. 2015, 7, 1626–1635. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.C.; Chen, S.J.; Chien, C.L. Erythropoietin produced by genetic–modified NIH/3T3 fibroblasts enhances the survival of degenerating neurons. Brain Behav. 2015, 8, e00356. [Google Scholar] [CrossRef] [PubMed]
- Gu, G.J.; Wu, D.; Lund, H.; Sunnemark, D.; Kvist, A.J.; Milner, R.; Eckersley, S.; Nilsson, L.N.; Agerman, K.; Landegren, U.; et al. Elevated MARK2-dependent phosphorylation of Tau in Alzheimer’s disease. J. Alzheimers Dis. 2013, 3, 699–713. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are not available from the authors. |







| Compound | Structure |
|---|---|
| CAF1: C1 |  |
| CAF2: C2 |  |
| CAF3: C3 |  |
| CAF4: C4 |  |
| CAF5: C6 |  |
| CAF6: C8 |  |
| CAF7: C10 |  |
| CAF8: C12 |  |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hosseini, R.; Moosavi, F.; Silva, T.; Rajaian, H.; Hosseini, S.Y.; Bina, S.; Saso, L.; Miri, R.; Borges, F.; Firuzi, O. Modulation of ERK1/2 and Akt Pathways Involved in the Neurotrophic Action of Caffeic Acid Alkyl Esters. Molecules 2018, 23, 3340. https://doi.org/10.3390/molecules23123340
Hosseini R, Moosavi F, Silva T, Rajaian H, Hosseini SY, Bina S, Saso L, Miri R, Borges F, Firuzi O. Modulation of ERK1/2 and Akt Pathways Involved in the Neurotrophic Action of Caffeic Acid Alkyl Esters. Molecules. 2018; 23(12):3340. https://doi.org/10.3390/molecules23123340
Chicago/Turabian StyleHosseini, Razieh, Fatemeh Moosavi, Tiago Silva, Hamid Rajaian, Seyed Younes Hosseini, Samaneh Bina, Luciano Saso, Ramin Miri, Fernanda Borges, and Omidreza Firuzi. 2018. "Modulation of ERK1/2 and Akt Pathways Involved in the Neurotrophic Action of Caffeic Acid Alkyl Esters" Molecules 23, no. 12: 3340. https://doi.org/10.3390/molecules23123340