In Vitro Neuroprotection of Rat Hippocampal Neurons by Manninotriose and Astragaloside IV Against Corticosterone-Induced Toxicity
Abstract
:1. Introduction
2. Results
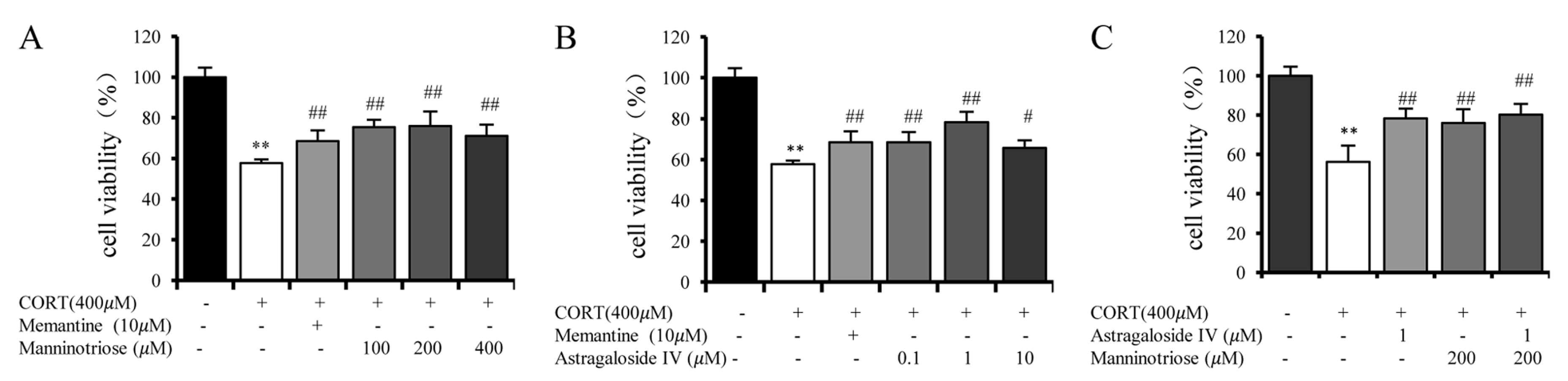
2.1. Effect of Manninotriose and AS-IV on the Viability of CORT Injured Hippocampal Neurons
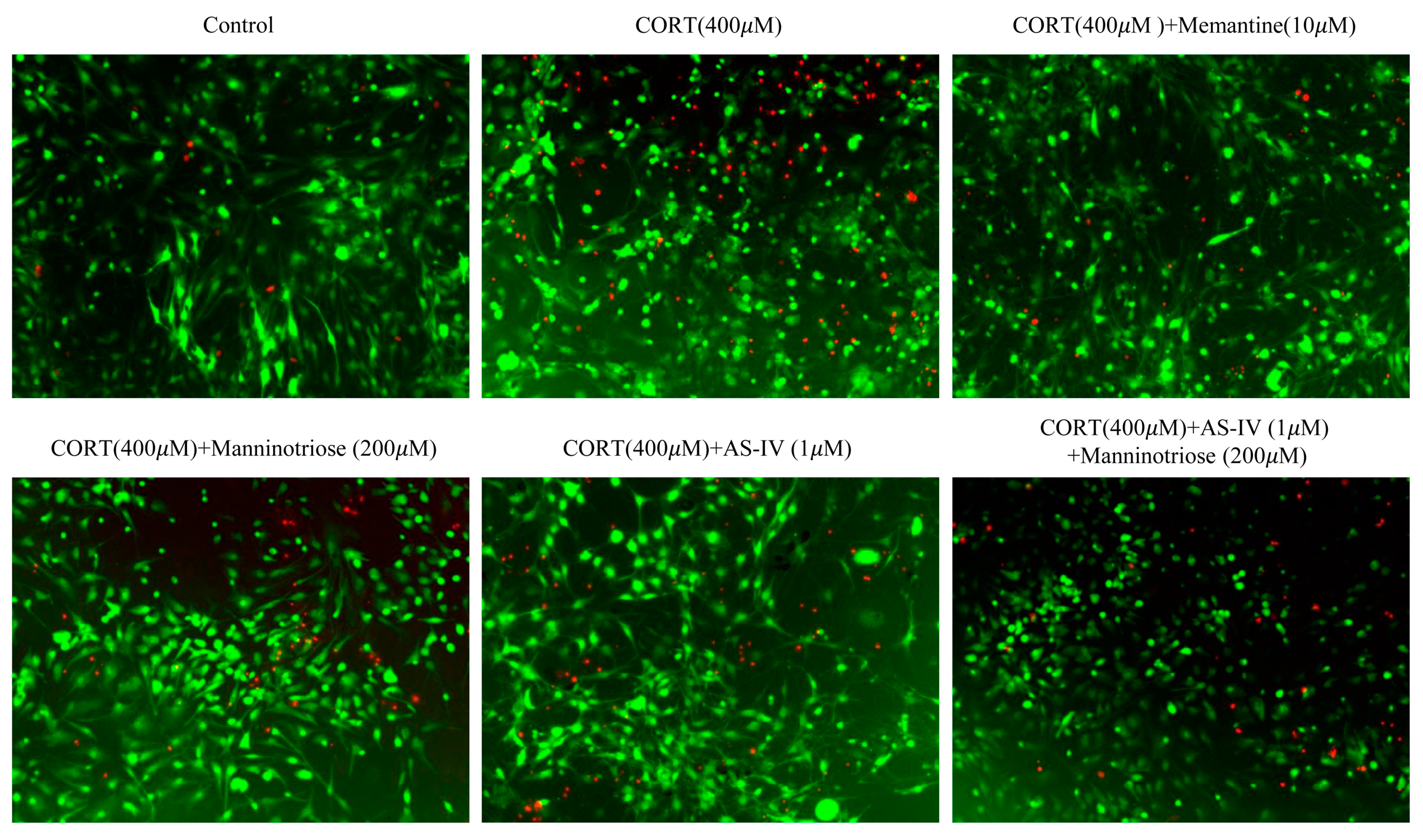
2.2. Effect of Manninotriose and AS-IV on the Survival of the Neurons Detected by FDA/PI Double Staining
2.3. Effects of Manninotriose and AS-IV on the GR Level in CORT Injured Hippocampal Neurons
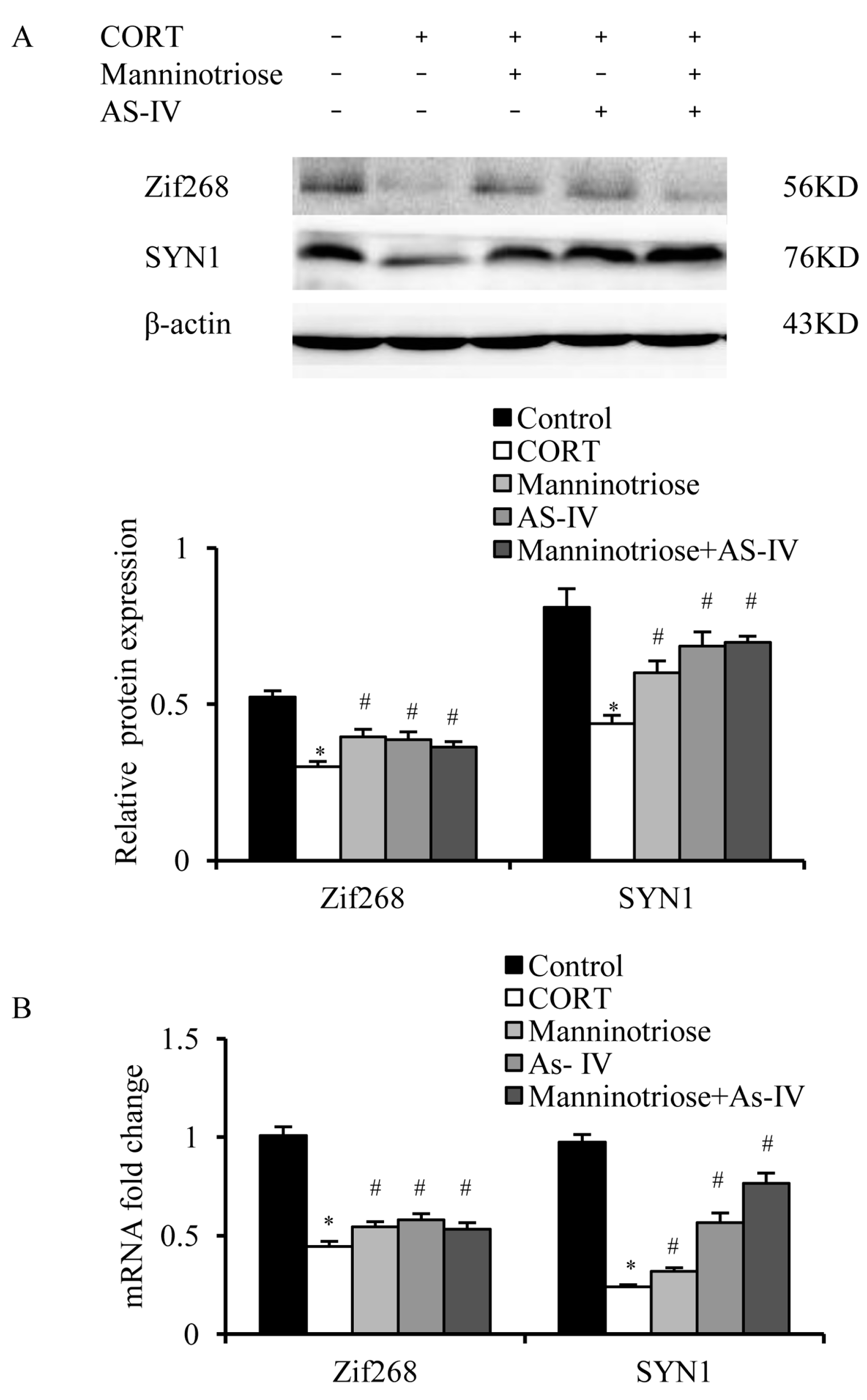
2.4. Effects of Manninotriose and AS-IV on CORT-Induced Changes in Zif268 and SYN1 Gene Expression in the Hippocampal Neurons
2.5. Effects of Manninotriose and AS-IV on CpG Island Methylation in the SYN1 Gene Promoter of CORT Injured Hippocampal Neurons
3. Discussion
4. Materials and Methods
4.1. Chemical Compounds
4.2. Primary Culture of Hippocampal Neurons
4.3. Determination of Cell Viability
4.4. FDA/PI Staining
4.5. Western Blotting
4.6. RNA Extraction and qRT-PCR
4.7. DNA Isolation, Bisulfite Conversion and Methylation Analysis
4.8. Statistical Analysis
Author Contributions
Funding
Conflicts of Interest
References
- Rosenzweig, E.S.; Barnes, C.A. Impact of aging on hippocampal function: Plasticity, network dynamics, and cognition. Prog. Neurobiol. 2003, 69, 143–179. [Google Scholar] [CrossRef]
- Roth, E.D.; Roth, T.L.; Money, K.M.; SenGupta, S.; Eason, D.E.; Sweatt, J.D. DNA methylation regulates neurophysiological spatial representation in memory formation. Neuroepigenetics 2015, 2, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Jones, M.W.; Errington, M.L.; French, P.J.; Fine, A.; Bliss, T.V.; Garel, S.; Charnay, P.; Bozon, B.; Laroche, S.; Davis, S. A requirement for the immediate early gene Zif268 in the expression of late LTP and long-term memories. Nat. Neurosci. 2001, 4, 289–296. [Google Scholar] [CrossRef]
- Blalock, E.M.; Chen, K.C.; Sharrow, K.; Herman, J.P.; Porter, N.M.; Foster, T.C.; Landfield, P.W. Gene microarrays in hippocampal aging: Statistical profiling identifies novel processes correlated with cognitive impairment. J. Neurosci. 2003, 23, 3807–3819. [Google Scholar] [CrossRef]
- Rowe, W.B.; Blalock, E.M.; Chen, K.C.; Kadish, I.; Wang, D.; Barrett, J.E.; Thibault, O.; Porter, N.M.; Rose, G.M.; Landfield, P.W. Hippocampal expression analyses reveal selective association of immediate-early, neuroenergetic, and myelinogenic pathways with cognitive impairment in aged rats. J. Neurosci. 2007, 27, 3098–3110. [Google Scholar] [CrossRef]
- Wang, Q.; Van Heerikhuize, J.; Aronica, E.; Kawata, M.; Seress, L.; Joels, M.; Swaab, D.F.; Lucassen, P.J. Glucocorticoid receptor protein expression in human hippocampus; stability with age. Neurobiol. Aging 2013, 34, 1662–1673. [Google Scholar] [CrossRef] [Green Version]
- Lupien, S.J.; de Leon, M.; de Santi, S.; Convit, A.; Tarshish, C.; Nair, N.P.V.; Thakur, M.; McEwen, B.S.; Hauger, R.L.; Meaney, M.J. Cortisol levels during human aging predict hippocampal atrophy and memory deficits. Nat. Neurosci. 1998, 1, 69. [Google Scholar] [CrossRef]
- Yu, N.K.; Baek, S.H.; Kaang, B.K. DNA methylation-mediated control of learning and memory. Mol. Brain 2011, 4, 5. [Google Scholar] [CrossRef] [Green Version]
- Zovkic, I.B.; Guzman-Karlsson, M.C.; Sweatt, J.D. Epigenetic regulation of memory formation and maintenance. Learn. Mem. 2013, 20, 61–74. [Google Scholar] [CrossRef] [Green Version]
- Sanchez-Mut, J.V.; Graff, J. Epigenetic Alterations in Alzheimer’s Disease. Front. Behav. Neurosci. 2015, 9, 347. [Google Scholar] [CrossRef]
- Hernandez, D.G.; Nalls, M.A.; Gibbs, J.R.; Arepalli, S.; van der Brug, M.; Chong, S.; Moore, M.; Longo, D.L.; Cookson, M.R.; Traynor, B.J.; et al. Distinct DNA methylation changes highly correlated with chronological age in the human brain. Hum. Mol. Genet. 2011, 20, 1164–1172. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Dai, W.; Zhang, X.; Gong, Z.; Jin, G. Mannotriose regulates learning and memory signal transduction in the hippocampus. Neural Regen. Res. 2013, 8, 3020–3026. [Google Scholar]
- Tohda, C.; Tamura, T.; Matsuyama, S.; Komatsu, K. Promotion of axonal maturation and prevention of memory loss in mice by extracts of Astragalus mongholicus. Br. J. Pharmacol. 2006, 149, 532–541. [Google Scholar] [CrossRef] [Green Version]
- Sun, Q.; Jia, N.; Wang, W.; Jin, H.; Xu, J.; Hu, H. Protective effects of astragaloside IV against amyloid beta1-42 neurotoxicity by inhibiting the mitochondrial permeability transition pore opening. PLoS ONE 2014, 9, e98866. [Google Scholar] [CrossRef]
- Chan, W.S.; Durairajan, S.S.; Lu, J.H.; Wang, Y.; Xie, L.X.; Kum, W.F.; Koo, I.; Yung, K.K.; Li, M. Neuroprotective effects of Astragaloside IV in 6-hydroxydopamine-treated primary nigral cell culture. Neurochem. Int. 2009, 55, 414–422. [Google Scholar] [CrossRef]
- Bizon, J.L.; Helm, K.A.; Han, J.S.; Chun, H.J.; Pucilowska, J.; Lund, P.K.; Gallagher, M. Hypothalamic-pituitary-adrenal axis function and corticosterone receptor expression in behaviourally characterized young and aged Long-Evans rats. Eur. J. Neurosci. 2001, 14, 1739–1751. [Google Scholar] [CrossRef]
- Day, J.J.; Sweatt, J.D. DNA methylation and memory formation. Nat. Neurosci. 2010, 13, 1319–1323. [Google Scholar] [CrossRef] [Green Version]
- Sultan, F.A.; Day, J.J. Epigenetic mechanisms in memory and synaptic function. Epigenomics 2011, 3, 157–181. [Google Scholar] [CrossRef] [Green Version]
- Zannas, A.S.; Arloth, J.; Carrillo-Roa, T.; Iurato, S.; Roh, S.; Ressler, K.J.; Nemeroff, C.B.; Smith, A.K.; Bradley, B.; Heim, C.; et al. Lifetime stress accelerates epigenetic aging in an urban, African American cohort: Relevance of glucocorticoid signaling. Genome Biol. 2015, 16, 266. [Google Scholar] [CrossRef]
- Paonessa, F.; Latifi, S.; Scarongella, H.; Cesca, F.; Benfenati, F. Specificity protein 1 (Sp1)-dependent activation of the synapsin I gene (SYN1) is modulated by RE1-silencing transcription factor (REST) and 5′-cytosine-phosphoguanine (CpG) methylation. J. Biol. Chem. 2013, 288, 3227–3239. [Google Scholar] [CrossRef]
- Haberman, R.P.; Quigley, C.K.; Gallagher, M. Characterization of CpG island DNA methylation of impairment-related genes in a rat model of cognitive aging. Epigenetics 2012, 7, 1008–1019. [Google Scholar] [CrossRef] [Green Version]
- Mpofana, T.; Daniels, W.M.; Mabandla, M.V. Exposure to Early Life Stress Results in Epigenetic Changes in Neurotrophic Factor Gene Expression in a Parkinsonian Rat Model. Parkinsons Dis. 2016, 2016, 6438783. [Google Scholar] [CrossRef]
- Lee, R.S.; Tamashiro, K.L.; Yang, X.; Purcell, R.H.; Harvey, A.; Willour, V.L.; Huo, Y.; Rongione, M.; Wand, G.S.; Potash, J.B. Chronic corticosterone exposure increases expression and decreases deoxyribonucleic acid methylation of Fkbp5 in mice. Endocrinology 2010, 151, 4332–4343. [Google Scholar] [CrossRef]
- Swirnoff, A.H.; Milbrandt, J. DNA-binding specificity of NGFI-A and related zinc finger transcription factors. Mol. Cell Biol. 1995, 15, 2275–2287. [Google Scholar] [CrossRef]
- Koldamova, R.; Schug, J.; Lefterova, M.; Cronican, A.A.; Fitz, N.F.; Davenport, F.A.; Carter, A.; Castranio, E.L.; Lefterov, I. Genome-wide approaches reveal EGR1-controlled regulatory networks associated with neurodegeneration. Neurobiol. Dis. 2014, 63, 107–114. [Google Scholar] [CrossRef] [Green Version]
- Penner, M.R.; Parrish, R.R.; Hoang, L.T.; Roth, T.L.; Lubin, F.D.; Barnes, C.A. Age-related changes in Egr1 transcription and DNA methylation within the hippocampus. Hippocampus 2016, 26, 1008–1020. [Google Scholar] [CrossRef]
- Sapolsky, R.M. The possibility of neurotoxicity in the hippocampus in major depression: A primer on neuron death. Biol. Psychiatry 2000, 48, 755–765. [Google Scholar] [CrossRef]
- Kim, S.; Kang, I.-H.; Nam, J.-B.; Cho, Y.; Chung, D.-Y.; Kim, S.-H.; Kim, J.-S.; Cho, Y.-D.; Hong, E.-K.; Sohn, N.-W.; et al. Ameliorating the Effect of Astragaloside IV on Learning and Memory Deficit after Chronic Cerebral Hypoperfusion in Rats. Molecules 2015, 20, 1904–1921. [Google Scholar] [CrossRef] [Green Version]
- Xu, X.L.; Chen, X.J.; Ji, H.; Li, P.; Bian, Y.Y.; Yang, D.; Xu, J.D.; Bian, Z.P.; Zhang, J.N. Astragaloside IV improved intracellular calcium handling in hypoxia-reoxygenated cardiomyocytes via the sarcoplasmic reticulum Ca-ATPase. Pharmacology 2008, 81, 325–332. [Google Scholar] [CrossRef]
- Banker, G.A.; Cowan, W.M. Rat hippocampal neurons in dispersed cell culture. Brain Res. 1977, 126, 397–425. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |


| Gene | Primers Sequence |
|---|---|
| Zif268(NM_012551.2) | Forward GCGAACAACCCTACGAGCAC Reverse GCGGCCAGTATAGGTGATGG |
| SYN1(NM_001110782.2) | Forward GACAACCAACATGACTTCCAGG Reverse TTCCAGTTCCCTGACACTGATG |
| β-actin(NM_031144.3) | Forward CCCATCTATGAGGGTTACGC Reverse TTTAATGTCACGCACGATTTC |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, J.; Yin, F.; Jin, G.; Zhang, X.; Zhang, L.; Gong, Z.; Kang, X.; Hu, H. In Vitro Neuroprotection of Rat Hippocampal Neurons by Manninotriose and Astragaloside IV Against Corticosterone-Induced Toxicity. Molecules 2018, 23, 3339. https://doi.org/10.3390/molecules23123339
Zheng J, Yin F, Jin G, Zhang X, Zhang L, Gong Z, Kang X, Hu H. In Vitro Neuroprotection of Rat Hippocampal Neurons by Manninotriose and Astragaloside IV Against Corticosterone-Induced Toxicity. Molecules. 2018; 23(12):3339. https://doi.org/10.3390/molecules23123339
Chicago/Turabian StyleZheng, Jing, Fang Yin, Guoqin Jin, Xueli Zhang, Lina Zhang, Zhangbin Gong, Xiangping Kang, and Haiyan Hu. 2018. "In Vitro Neuroprotection of Rat Hippocampal Neurons by Manninotriose and Astragaloside IV Against Corticosterone-Induced Toxicity" Molecules 23, no. 12: 3339. https://doi.org/10.3390/molecules23123339




