The Impact of O-Glycosylation on Cyanidin Interaction with POPC Membranes: Structure-Activity Relationship
Abstract
:1. Introduction
2. Results and Discussion
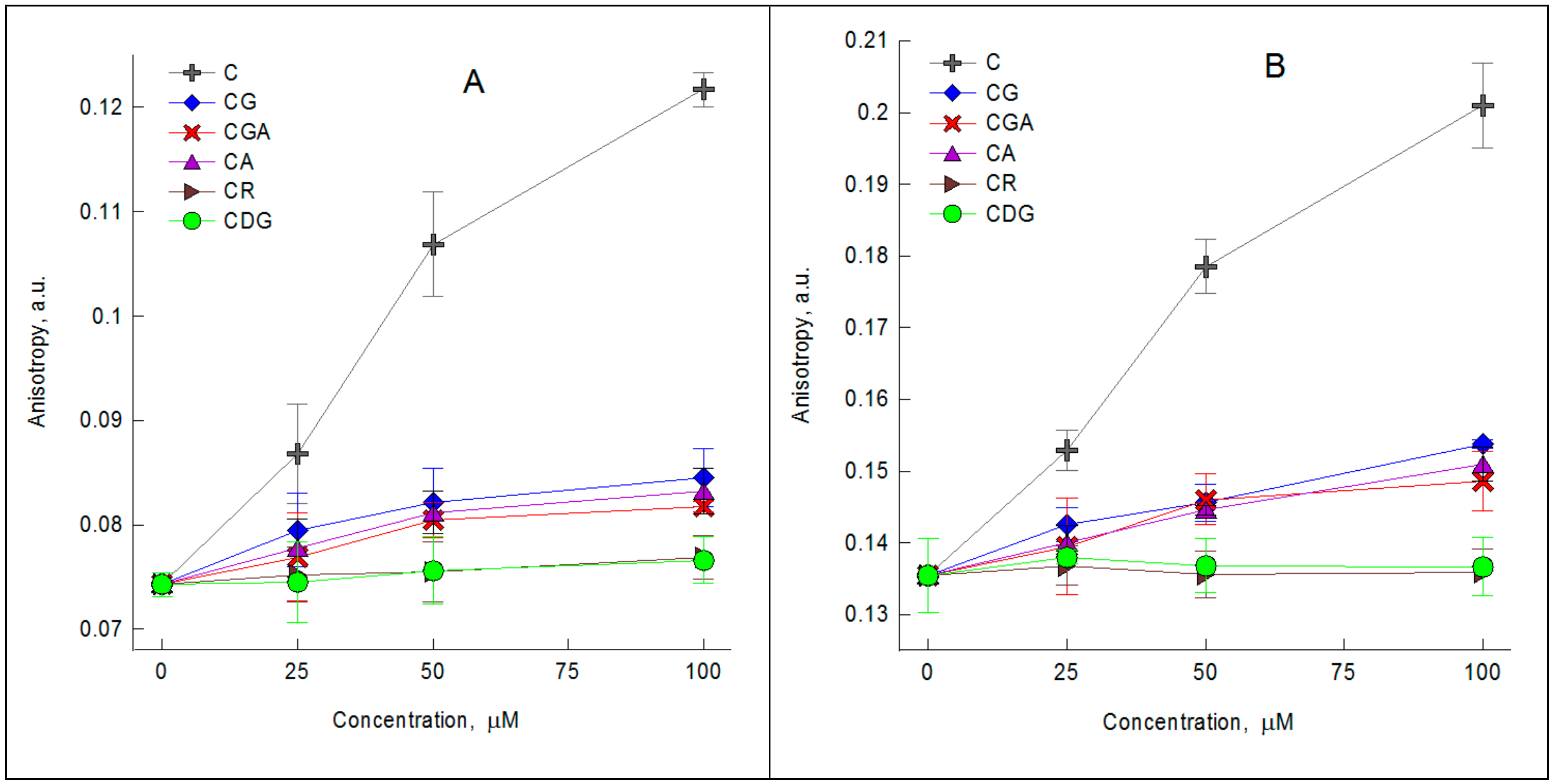
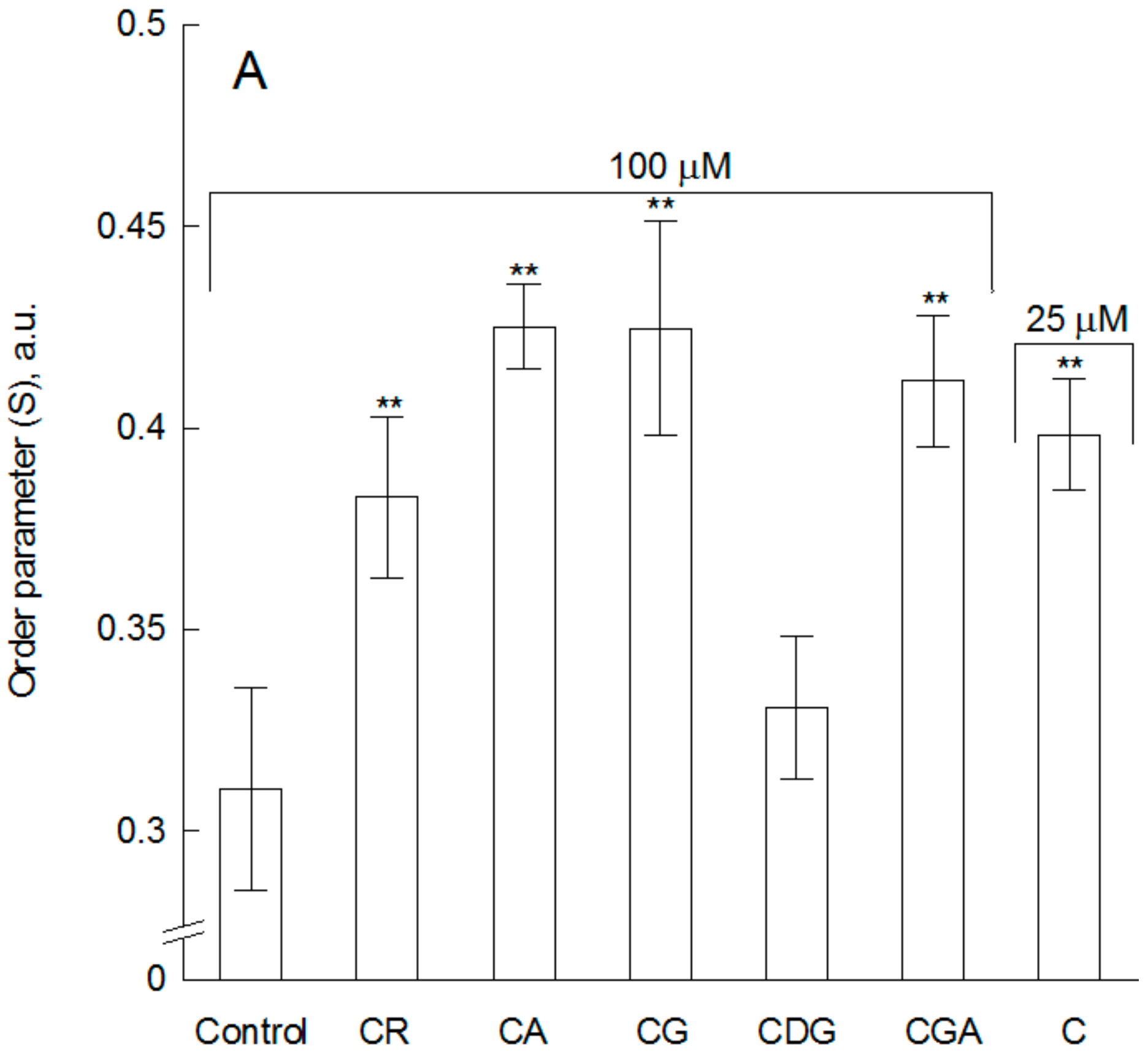
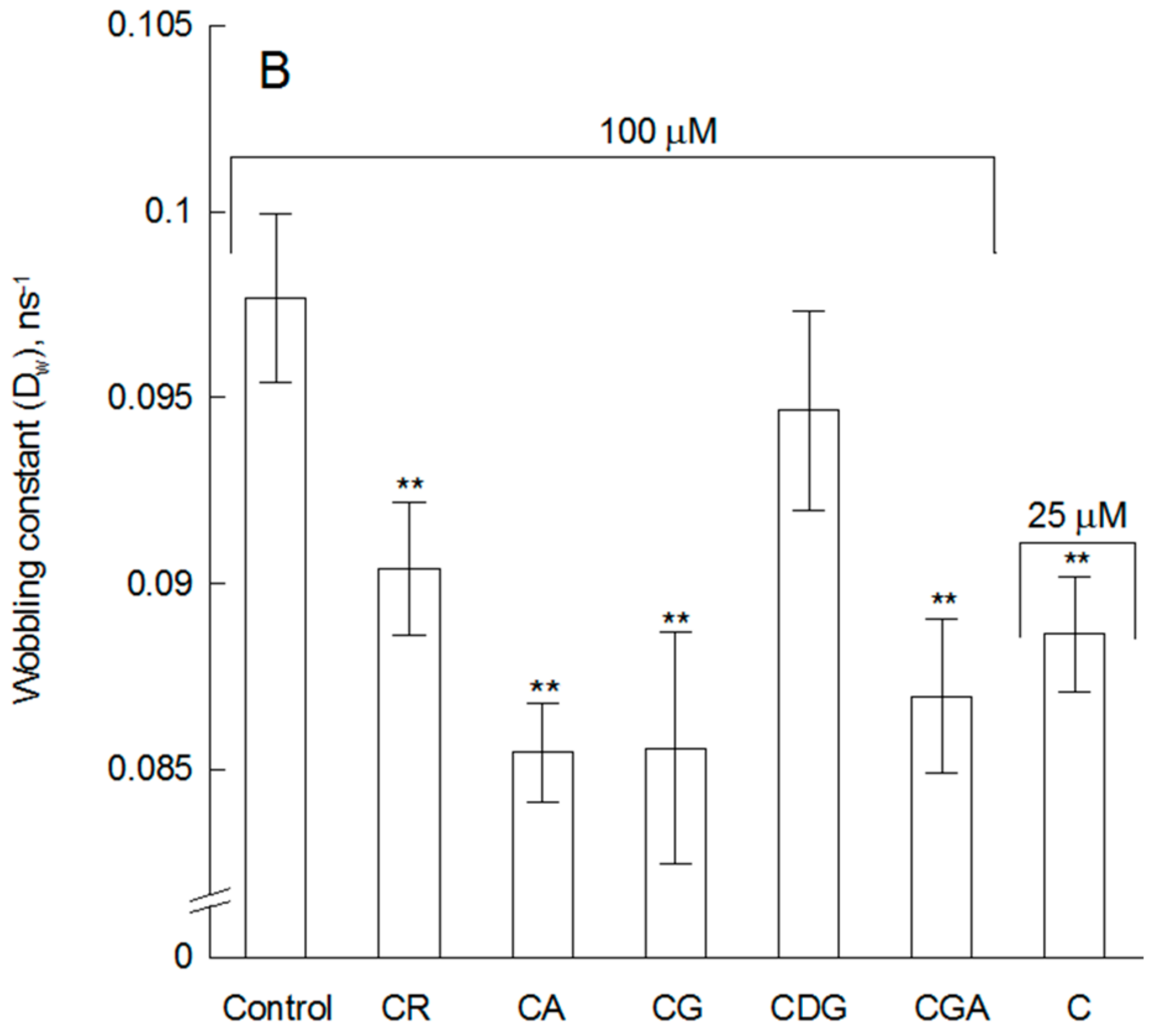
2.1. The Impact of the Compounds on the Physical Properties of Lipid Membrane—Fluorescence Spectroscopy Studies
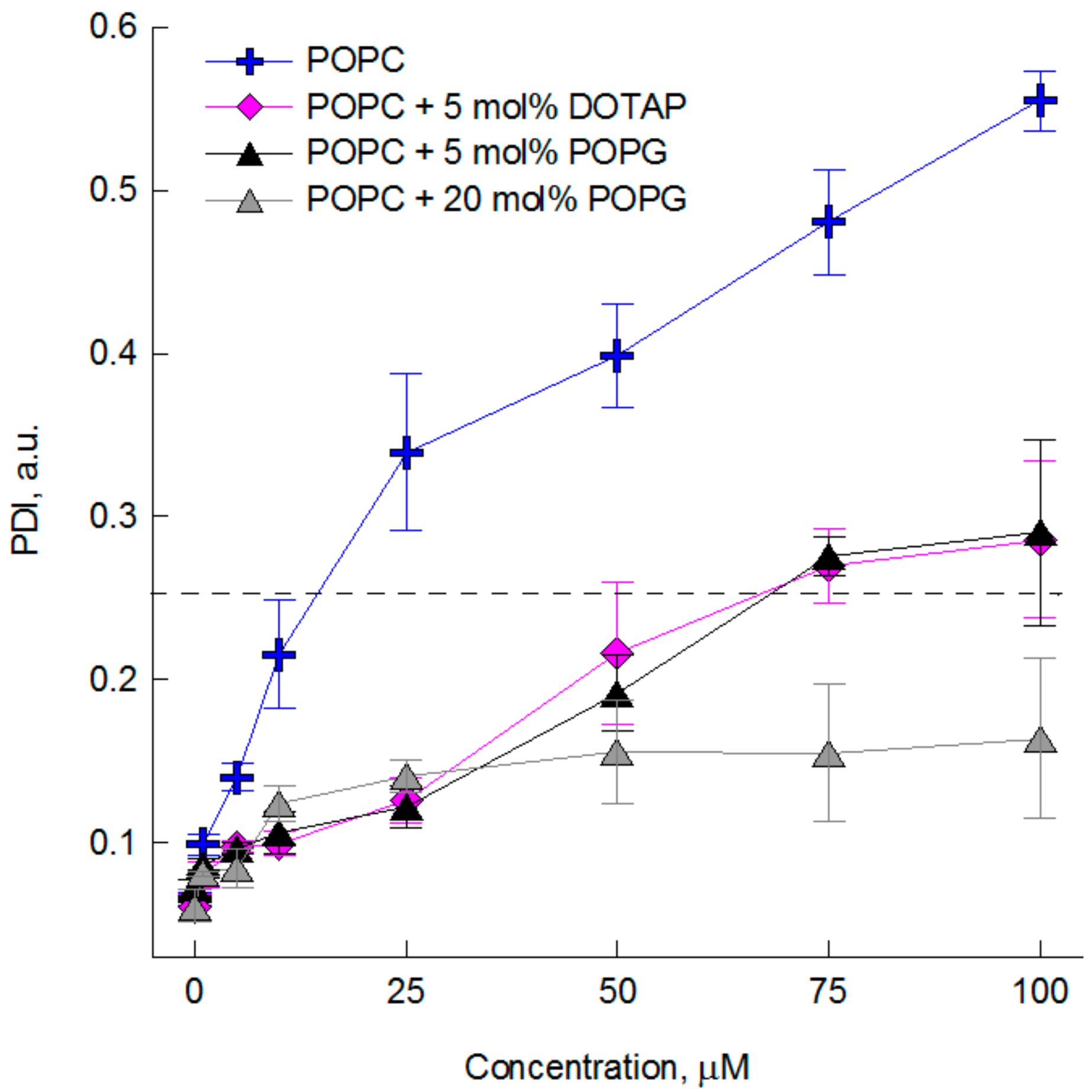
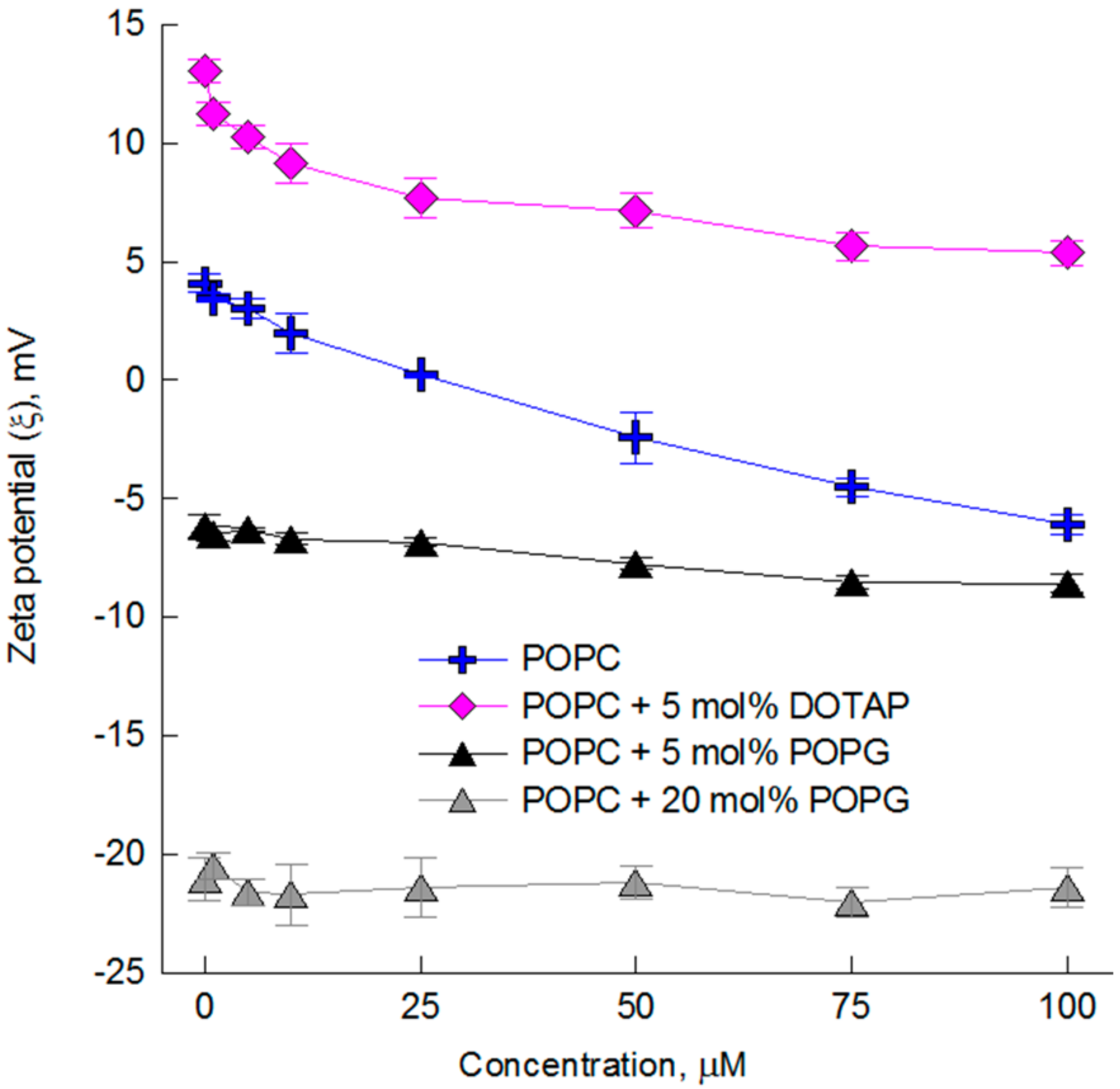
2.2. The Impact of the Compounds on the Physical Properties of Lipid Membrane—Dynamic and Electrophoretic Light Scattering Studies
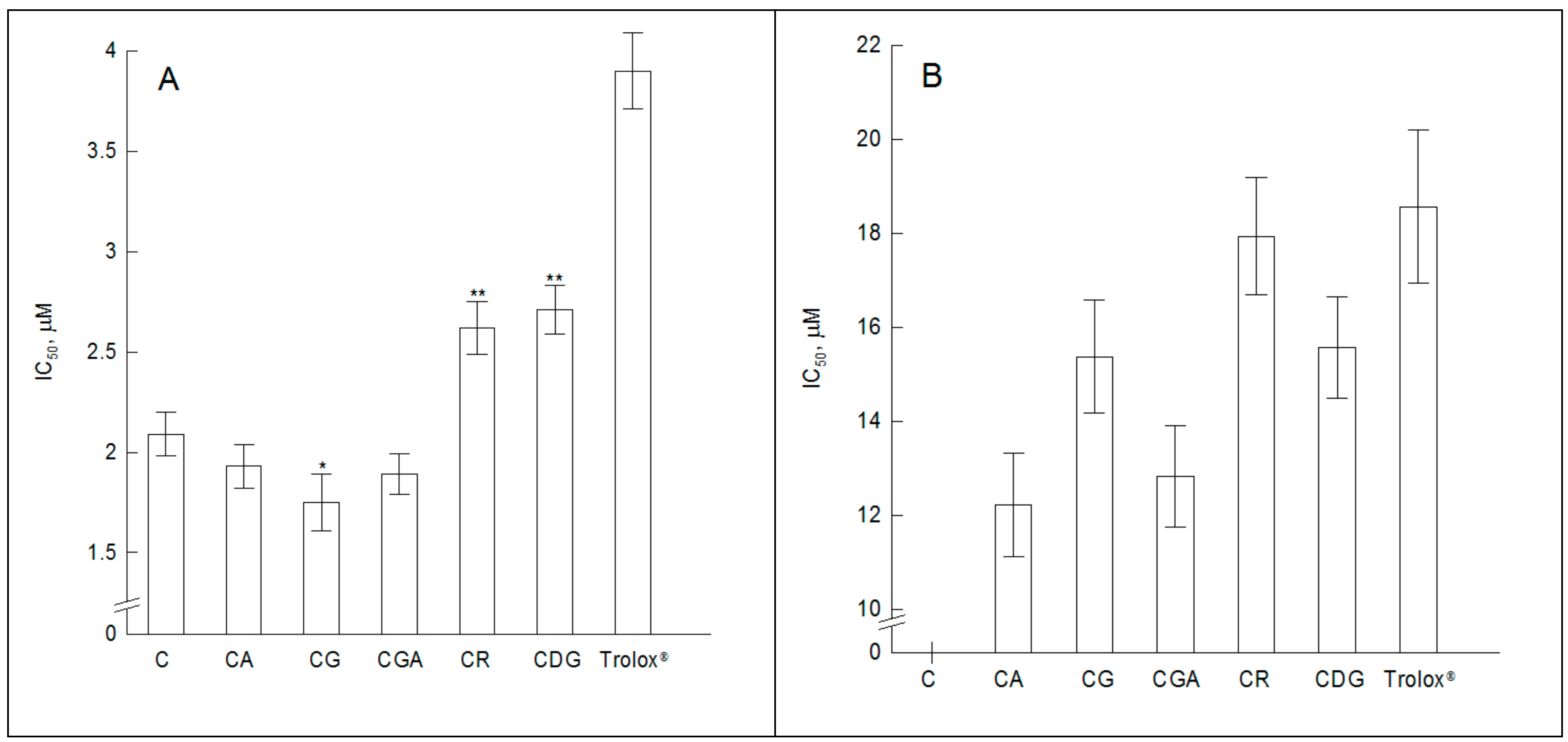
2.3. The Antioxidant Activity of the Compounds—Fluorescence Spectroscopy Studies
3. Materials and Methods
3.1. Anthocyanin and Anthocyanidins
3.2. Lipids, Liposomes and Reagents
3.3. LUVs
3.4. Cyanidin-Membrane Interaction Studies
3.4.1. Steady-State Fluorescence Spectroscopy
3.4.2. Time-Resolved Fluorescence Spectroscopy
3.4.3. Dynamic Light Scattering
3.4.4. Electrophoretic Light Scattering
3.4.5. Fluorimetric Studies of the Antioxidant Activity of the Compounds
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Kong, J.M.; Chia, L.S.; Goh, N.K.; Chia, T.F.; Brouillard, R. Analysis and biological activities of anthocyanins. Phytochemistry 2003, 64, 923–933. [Google Scholar] [CrossRef]
- Takeoka, G.; Dao, L.T. Anthocyanins. In Methods of Analysis for Functional Foods and Nutraceuticals; Hurst, W.J., Ed.; CRC Taylor & Francis Group: Boca Raton, FL, USA, 2002; pp. 219–241. [Google Scholar]
- Prior, R.L.; Wu, X. Anthocyanins: Structural characteristics that result in unique metabolic patterns and biological activities. Free Radic. Res. 2006, 40, 1014–1028. [Google Scholar] [CrossRef] [PubMed]
- Andersen, Ø.M.; Jordheim, M. The anthocyanins. In Flavonoids: Chemistry, Biochemistry and Applications; Andersen, Ø.M., Markham, K.R., Eds.; CRC Taylor & Francis Group: Boca Raton, FL, USA, 2006; pp. 471–551. [Google Scholar]
- Křen, V. Chemical biology and biomedicine of glycosylated natural compounds. In Glycoscience: Chemistry and Chemical Biology I–III; Fraser-Reid, B.O., Tatsuta, K., Thiem, J., Eds.; Springer: Berlin, Germany, 2001; pp. 2471–2529. [Google Scholar]
- Heim, K.E.; Tagliaferro, A.R.; Bobilya, D.J. Flavonoid antioxidants: Chemistry, metabolism and structure-activity relationships. J. Nutr. Biochem. 2002, 13, 572–584. [Google Scholar] [CrossRef]
- Kähkönen, M.P.; Heinonen, M. Antioxidant activity of anthocyanins and their aglycons. J. Agric. Food Chem. 2003, 51, 628–633. [Google Scholar] [CrossRef] [PubMed]
- Castañeda-Ovando, A.; Pacheco-Hernández, M.; Páez-Hernández, M.; Rodríguez, J.A.; Galán-Vidal, C.A. Chemical studies of anthocyanins: A review. Food Chem. 2009, 113, 859–871. [Google Scholar] [CrossRef]
- Iacobucci, G.A.; Sweeny, J.G. The chemistry of anthocyanins, anthocyanidins and related flavylium salts. Tetrahedron 1983, 39, 3005–3038. [Google Scholar] [CrossRef]
- Borkowski, T.; Szymusiak, H.; Gliszczynska-Swigło, A.; Tyrakowska, B. The effect of 3-O-β-glucosylation on structural transformations of anthocyanins. Food Res. Int. 2005, 38, 1031–1037. [Google Scholar] [CrossRef]
- Nayak, B.; Berrios, J.; Powers, J.R.; Tang, J. Thermal degradation of anthocyanins from purple potato (cv. Purple Majesty) and impact on antioxidant capacity. J. Agric. Food Chem. 2011, 59, 11040–11049. [Google Scholar] [CrossRef] [PubMed]
- Landi, M.; Tattini, M.; Gould, K.S. Multiple functional roles of anthocyanins in plant-environment interactions. Environ. Exp. Bot. 2015, 119, 4–17. [Google Scholar] [CrossRef]
- Miguel, M.G. Anthocyanins: Antioxidant and/or anti-inflammatory activities. J. Appl. Pharm. Sci. 2011, 1, 7–15. [Google Scholar]
- Galvano, F.; La Fauci, L.; Vitaglione, P.; Fogliano, V.; Vanella, L.; Felgines, C. Bioavailability, antioxidant and biological properties of the natural free-radical scavengers cyanidin and related glycosides. Ann. Ist. Super. Sanità 2007, 43, 382–393. [Google Scholar] [PubMed]
- Pojer, E.; Mattivi, F.; Johnson, D.; Stockley, C.S. The case for anthocyanin consumption to promote human health: A Review. Compr. Rev. Food Sci. Food Saf. 2013, 12, 483–508. [Google Scholar] [CrossRef]
- Xiao, J. Dietary flavonoid aglycones and their glycosides: Which show better biological significance? Crit. Rev. Food Sci. Nutr. 2017, 57, 1874–1905. [Google Scholar] [CrossRef] [PubMed]
- Van der Paal, J.; Neyts, E.C.; Verlackt, C.C.W.; Bogaerts, A. Effect of lipid peroxidation on membrane permeability of cancer and normal cells subjected to oxidative stress. Chem. Sci. 2016, 7, 489–498. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scheidt, H.A.; Pampel, A.; Nissler, L.; Gebhardt, R.; Huster, D. Investigation of the membrane localization and distribution of flavonoids by high-resolution magic angle spinning NMR spectroscopy. Biochim. Biophys. Acta 2004, 1663, 97–107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rokic, V.; Ota, A.; Sokolovic, D.; Ulrih, N.P. Interaction of cyanidin and cyanidin 3-0-β-glucospyranoside with model lipid membrane. J. Therm. Anal. Calorim. 2017, 127, 1467–1477. [Google Scholar] [CrossRef]
- Strugala, P.; Dudra, A.; Gabrielska, J. Interaction between mimic lipid membrane and acytalted and nonacytalted cyanidin and its bioactivity. J. Agric. Food Chem. 2016, 64, 7414–7422. [Google Scholar] [CrossRef] [PubMed]
- Parasassi, T.; Krasnowska, E.K.; Bagatolli, L.; Gratton, E. Laurdan and Prodan as polarity-sensitive fluorescent membrane probes. J. Fluoresc. 1998, 8, 365–373. [Google Scholar] [CrossRef]
- Sanchez, S.A.; Tricerri, M.A.; Gunther, G.; Gratton, E. Laurdan Generalized Polarization: From cuvette to microscope. In Modern Research and Educational Topics in Microscopy; Méndez-Vilas, A., Díaz, J., Eds.; Formatex: Badajoz, Spain, 2007; pp. 1007–1014. [Google Scholar]
- Repáková, J.; Čapková, P.; Holopainen, M.J.; Vattulainen, I. Distribution, orientation, and dynamics of DPH probes in DPPC bilayer. J. Phys. Chem. B 2004, 108, 13438–13448. [Google Scholar] [CrossRef]
- Lentz, B.R. Use of fluorescent probes to monitor molecular order and motions within liposome bilayers. Chem. Phys. Lipids 1993, 64, 99–116. [Google Scholar] [CrossRef]
- Heyn, M.P. Determination of lipid order parameters and rotational correlation times from fluorescence depolarization experiments. FEBS let. 1979, 108, 359–364. [Google Scholar] [CrossRef] [Green Version]
- Tsuchiya, H. Effects of red wine flavonoid components on biomembranes and cell proliferation. Int. J. Wine Res. 2011, 3, 9–17. [Google Scholar] [CrossRef]
- Tsuchiya, H. Structure-dependent membrane interaction of flavonoids associated with their bioactivity. Food Chem. 2010, 120, 1089–1096. [Google Scholar] [CrossRef]
- Giusti, M.M.; Wrolstad, R.E. Anthocyanins. Characterization and measurement of anthocyanins by UV–Visible spectroscopy. In Current Protocols in Food Analytical Chemistry; Wrolstad, R.E., Ed.; John Wiley & Sons: New York, NY, USA, 2001; pp. F1.2.1–F1.2.13. [Google Scholar]
- Ahmadiani, N.; Robbins, R.J.; Collins, T.M.; Giusti, M.M. Molar absorptivity (ε) and spectral characteristics of cyanidin-based anthocyanins from red cabbage. Food Chem. 2015, 197, 900–906. [Google Scholar] [CrossRef] [PubMed]
- Bonarska-Kujawa, D.; Pruchnik, H.; Kleszczynska, H. Interaction of selected anthocyanins with erythrocytes and liposome membranes. Cell. Mol. Biol. Lett. 2012, 17, 289–308. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lehtonen, J.Y.A.; Adlercreutz, H.; Kinnunen, P.K.J. Binging of daidzein to the liposomes. Biochim. Biophys. Acta 1996, 1285, 91–100. [Google Scholar] [CrossRef]
- Hendrich, A.B.; Malon, R.; Pola, A.; Shirataki, Y.; Motohashi, N.; Michalak, K. Differential interaction of Sophora isoflavonoids with lipid bilayers. Eur. J. Pharm. Sci. 2002, 16, 201–208. [Google Scholar] [CrossRef]
- Huh, N.W.; Porter, N.A.; McIntosh, T.J.; Simon, S.A. The interaction of polyphenols with bilayers: Conditions for increasing bilayer adhesion. Biophys. J. 1996, 71, 3261–3277. [Google Scholar] [CrossRef]
- Brouillard, R.; Chassaing, S.; Isorez, G.; Kueny-Stotz, M.; Figueiredo, P. The visible flavonoids or anthocyanins: From research to applications. In Recent Advances in Polyphenol Research; Santos-Buelga, C., Escribano-Bailon, M.T., Lattanzio, V., Eds.; Blackwell Publishing Ltd.: Oxford, UK, 2010; Volume 2, pp. 1–22. [Google Scholar]
- Bockmann, R.A.; Hac, A.; Heimburg, T.; Grubmuller, H. Effect of sodium chloride on a lipid bilayer. Biophys. J. 2003, 85, 1647–1655. [Google Scholar] [CrossRef]
- Makino, K.; Yamada, T.; Kimura, M.; Oka, T.; Ohshima, H.; Kondo, T. Temperature- and ionic strength-induced conformational changes in the lipid head group region of liposomes as suggested by zeta potential data. Biophys. Chem. 1991, 41, 175–183. [Google Scholar] [CrossRef]
- Chibowski, E.; Szcześ, A. Zeta potential and surface charge of DPPC and DOPC liposomes in the presence of PLC enzyme. Adsorption 2016, 22, 755–765. [Google Scholar] [CrossRef] [Green Version]
- Janosi, L.; Gorfe, A. Simulating POPC and POPC/POPG bilayers: Conserved packing and altered surface reactivity. J. Chem. Theory Comput. 2010, 6, 3267–3273. [Google Scholar] [CrossRef] [PubMed]
- Cyboran, S.; Oszmiański, J.; Kleszczyńska, H. Modification of the properties of biological membrane and its protection against oxidation by Actinidia arguta leaf extract. Chem. Biol. Interact. 2014, 222, 50–59. [Google Scholar] [CrossRef] [PubMed]
- Parasassi, T.; De Stasio, G.; Ravagnan, G.; Rusch, R.M.; Gratton, E. Quantitation of lipid phases in phospholipid vesicles by the generalized polarization of Laurdan fluorescence. Biophys. J. 1991, 60, 179–189. [Google Scholar] [CrossRef] [Green Version]
- Lakowicz, J.R. Fluorescence Polarization in Principles of Fluorescence Spectroscopy; Plenum Press: New York, NY, USA, 2006; pp. 353–382. [Google Scholar]
- Kinosita, K.; Ikegami, A.; Kawato, S. On the wobbling-in-cone analysis of fluorescence anisotropy decay. Biophys. J. 1982, 37, 461–464. [Google Scholar] [CrossRef] [Green Version]
- Jahnig, F. Structural order of lipids and proteins in membranes: evaluation of fluorescence anisotropy data. Proc. Natl. Acad. Sci. USA 1979, 76, 6361–6365. [Google Scholar] [CrossRef] [PubMed]
- Kawato, S.; Kinosita, K.; Ikegami, A. Dynamic structure of lipid bilayers studied by nanosecond fluorescence techniques. Biochemistry 1977, 16, 2319–2324. [Google Scholar] [CrossRef] [PubMed]
- Lipari, G.; Szabo, A. Effect of librational motion on fluorescence depolarization and nuclear magnetic resonance relaxation in macromolecules and membranes. Biophys. J. 1980, 30, 489–506. [Google Scholar] [CrossRef] [Green Version]
- Koppel, D.E. Analysis of macromolecular polydispersity in intensity correlation spectroscopy: The method of Cumulants. J. Chem. Phys. 1972, 57, 4814–4820. [Google Scholar] [CrossRef]
- Hunter, R.J. Zeta Potential in Colloid Science; Academic Press: London, UK, 1981; p. 71. [Google Scholar]
- Smoluchowski, M.V. Bulletin International de l’Académie des Sciences de Cracovie. Classe des Sciences Mathématiques et Naturelles. 1916, p. 182. Available online: https://www.biodiversitylibrary.org/bibliography/13192#/summary (accessed on 25 September 2018).
| Abbr. | Name | Trade Name | Substitution Pattern | Structure * | |
|---|---|---|---|---|---|
| R1 | R2 | ||||
| C | Cyanidin | - | H | H | 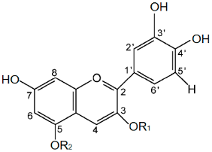 |
| CG | Cyanidin-3-O-glucoside | Chrysanthemin | glucose | H | |
| CR | Cyanidin-3-O-rutinoside | Keracyanin | rutinose | H | |
| CGA | Cyanidin-3-O-galactoside | Ideain | galactose | H | |
| CDG | Cyanidin-3-5-O-di-glucoside | Cyanin | glucose | glucose | |
| CA | Cyanidin-3-O-arabinoside | - | arabinose | H | |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cyboran-Mikołajczyk, S.; Jurkiewicz, P.; Hof, M.; Kleszczyńska, H. The Impact of O-Glycosylation on Cyanidin Interaction with POPC Membranes: Structure-Activity Relationship. Molecules 2018, 23, 2771. https://doi.org/10.3390/molecules23112771
Cyboran-Mikołajczyk S, Jurkiewicz P, Hof M, Kleszczyńska H. The Impact of O-Glycosylation on Cyanidin Interaction with POPC Membranes: Structure-Activity Relationship. Molecules. 2018; 23(11):2771. https://doi.org/10.3390/molecules23112771
Chicago/Turabian StyleCyboran-Mikołajczyk, Sylwia, Piotr Jurkiewicz, Martin Hof, and Halina Kleszczyńska. 2018. "The Impact of O-Glycosylation on Cyanidin Interaction with POPC Membranes: Structure-Activity Relationship" Molecules 23, no. 11: 2771. https://doi.org/10.3390/molecules23112771




