Semisynthesis and Biological Evaluation of Oleanolic Acid 3-O-β-d-Glucuronopyranoside Derivatives for Protecting H9c2 Cardiomyoblasts against H2O2-Induced Injury
Abstract
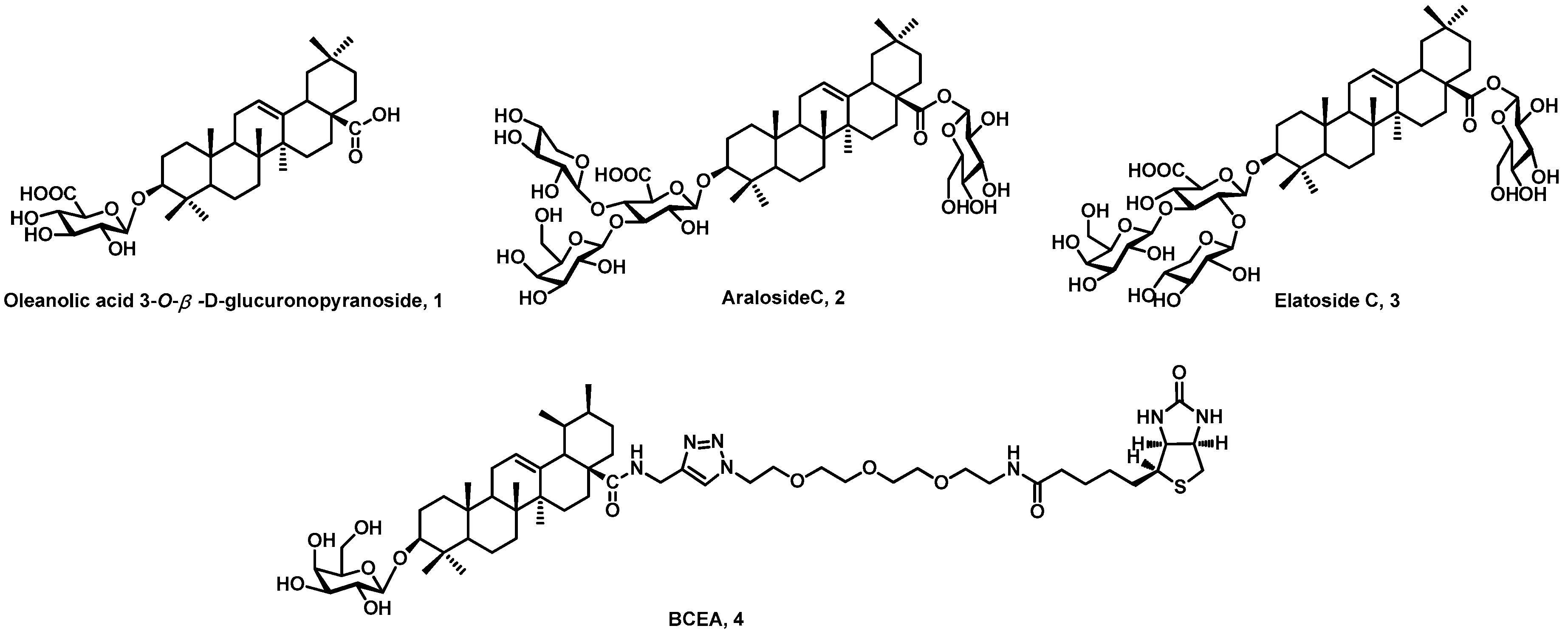
:1. Introduction
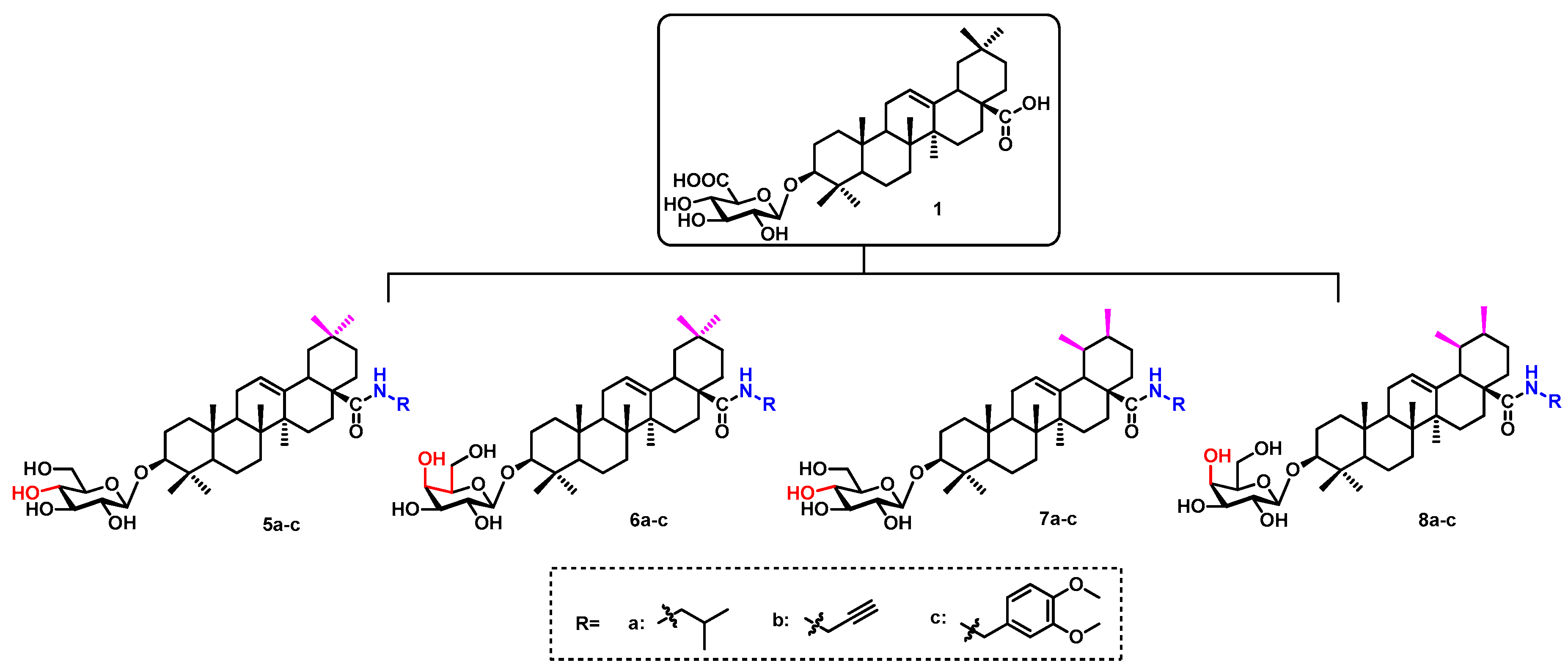
2. Results and Discussion
2.1. Chemistry
2.2. Biological Results and Discussion
3. Experimental Section
3.1. General Information
3.2. Chemistry
3.2.1. General Procedure for the Synthesis of Compounds 10a–10b
3.2.2. General Procedure for the Synthesis of Compounds 11a–11d
3.2.3. General Procedure for the Synthesis of Compounds 12a–12d
3.2.4. General Procedure for the Synthesis of Compounds 13a–c, 14a–c, 15a–c, 16a–c and compounds 5a–c, 6a–c, 7a–c, 8a–c
3.3. Evaluation of the Biological Activity
3.3.1. Cell Culture and Treatment
3.3.2. Determination of Cell Viability
3.3.3. Statistic Analysis
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Chaudhari, K.; Hamad, B.; Syed, B.A. Antithrombotic drugs market. Nat. Rev. Drug Discov. 2014, 13, 571–572. [Google Scholar] [CrossRef] [PubMed]
- Li, C.L.; Dong, M.H.; Ren, Y.J.; Li, L.H. Design, synthesis, biological evaluation and molecular docking of novel dabigatran derivatives as potential thrombin inhibitors. RSC Adv. 2015, 5, 23737–23748. [Google Scholar] [CrossRef]
- Megson, L.L.; Whitfield, P.D.; Zabetakis, L. Lipids and cardiovascular disease: Where does dietary intervention sit alongside statin therapy? Food Funct. 2016, 7, 2603–2614. [Google Scholar] [CrossRef] [PubMed]
- Brown, A.; Reynolds, L.R.; Bruemmer, D. Intensive glycemic control and cardiovascular disease: An update. Nat. Rev. Cardiol. 2010, 7, 369–375. [Google Scholar] [CrossRef] [PubMed]
- Morrish, N.J.; Wang, S.L.; Stevens, L.K.; Fuller, J.H.; Keen, H. Mortality and causes of death in the WHO multinational study of vascular disease in diabetes. Diabetologia 2001, 44, S14–S21. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.F.; Fan, S.R.; Mao, Y.G.; Ji, Y.; Jin, L.Q.; Lu, J.X.; Chen, X.M. Cardiovascular protective effect of polysaccharide from Ophiopogon japonicus in diabetic rats. Int. J. Biol. Macromol. 2016, 82, 505–513. [Google Scholar] [CrossRef] [PubMed]
- Baranov, A.I. Medicinal uses of ginseng and related plants in the Soviet Union: Recent trends in the Soviet literature. J. Ethnopharmacol. 1982, 6, 339–353. [Google Scholar] [CrossRef]
- Jia, Y.L.; Dong, X.Y.; Zhou, P.F.; Liu, X.H.; Pan, L.L.; Xin, H.; Zhu, Y.Z.; Wang, Y. The synthesis and biological evaluation of novel Danshensu-cysteine analog conjugates as cardiovascular-protective agents. Eur. J. Med. Chem. 2012, 55, 176–187. [Google Scholar] [CrossRef] [PubMed]
- Nhiem, N.X.; Lim, H.Y.; Kiem, P.V.; Minh, C.V.; Thu, V.K.; Tai, B.H.; Quang, T.H.; Song, S.B.; Kim, Y.H. Oleanane-type triterpene saponins from the bark of Aralia elata and their NF-kappa B inhibition and PPAR activation signal pathway. Bioorg. Med. Chem. Lett. 2011, 21, 6143–6147. [Google Scholar] [CrossRef] [PubMed]
- Sun, G.B.; Xu, H.B.; Wen, F.C.; Zhang, W.; Ding, T.; Sun, X.B. Protective effects of aralosides on cultured myocardial cells subjected to anoxia/reoxygenation injury. Chin. Pharmacol. Bull. 2006, 22, 1092–1095. [Google Scholar]
- Wen, F.C.; Xu, H.B.; Zhang, W.; Ding, T.; Sun, X.B. Studies on antimyocardiac ischemia of Aralia elata saponin. World Sci. Technol. Mod. Tradit. Chin. Med. Mater. Med. 2005, 7, 5–8. [Google Scholar]
- Deng, H.W.; Li, Y.J.; Shen, N.; Chen, X.; Zhou, Z.C. Protective effect of aralosides of Aralia elataon experimental myocardial ischemia of rats. Chin. J. Pharmacol. 1988, 2, 20–23. [Google Scholar]
- Wang, M.; Xu, X.D.; Xu, H.B.; Wen, F.C.; Zhang, X.P.; Sun, H.; Yao, F.; Sun, G.B.; Sun, X.B. Effect of the total saponins of Aralia elata (Miq) Seem on cardiac contractile function and intracellular calcium cycling regulation. J. Ethnopharmacol. 2014, 155, 240–247. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Ha, Y.W.; Jeong, C.S.; Kim, Y.S.; Park, Y. Isolation and tandem mass fragmentations of an anti-inflammatory compound from Aralia elata. Arch. Pharm. Res. 2009, 32, 831–840. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Song, S.; Lu, H.; Chen, G.; Xu, S.; Sagara, Y.; Kitaoka, N.; Manabe, M.; Kodama, H. Effect of three triterpenoid compounds isolated from root bark of Aralia elataon stimulus-induced superoxide generation and tyrosyl phosphorylation and translocation of p47(phox) and p67(phox) to cell membrane in human neutrophil. Clin. Chim. Acta 2003, 336, 65–72. [Google Scholar] [CrossRef]
- Wang, M.; Meng, X.B.; Yu, Y.L.; Sun, G.B.; Xu, X.D.; Zhang, X.P.; Dong, X.; Ye, J.X.; Xu, H.B.; Sun, Y.F. Elatoside C protects against hypoxia/reoxygenation-induced apoptosis in H9c2 cardiomyocytes through the reduction of endoplasmic reticulum stress partially depending on STAT3 activation. Apoptosis 2014, 19, 1727–1735. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Sun, G.B.; Zhang, J.Y.; Luo, Y.; Yu, Y.L.; Xu, X.D.; Meng, X.B.; Zhang, M.D.; Lin, W.B.; Sun, X.B. Elatoside C protects the heart from ischaemia/reperfusion injury through the modulation of oxidative stress and intracellular Ca2+ homeostasis. Int. J. Cardiol. 2015, 185, 167–176. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Tian, Y.; Du, Y.Y.; Sun, G.B.; Xu, X.D.; Jiang, H.; Xu, H.B.; Meng, X.B.; Zhang, J.Y.; Ding, S.L. Protective effects of Araloside C against myocardial ischaemia/reperfusion injury: Potential involvement of heat shock protein 90. J. Cell. Mol. Med. 2017, 21, 1870–1880. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Wang, S.; Shang, H.; Wang, M.; Sun, G.B.; Xu, X.D.; Sun, X. The proteomic profiling of calenduloside E targets in HUVEC: Design, synthesis and application of biotinylated probe BCEA. RSC Adv. 2017, 7, 6259–6265. [Google Scholar] [CrossRef]
- Sun, G.B.; Sun, X.; Wang, M.; Ye, J.X.; Si, J.Y.; Xu, H.B.; Meng, X.B.; Qin, M.; Sun, J.; Wang, H.W.; et al. Oxidative stress suppression by luteolin-induced heme oxygenase-1 expression. Toxicol. Appl. Pharmacol. 2012, 265, 229–240. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds 5–8a–c are available from the authors. |


© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tian, Y.; Sun, Z.; Wang, W.; Shang, H.; Wang, B.; Deng, D.; Ma, G.; Wu, H.; Zhu, N.; Xu, X.; et al. Semisynthesis and Biological Evaluation of Oleanolic Acid 3-O-β-d-Glucuronopyranoside Derivatives for Protecting H9c2 Cardiomyoblasts against H2O2-Induced Injury. Molecules 2018, 23, 44. https://doi.org/10.3390/molecules23010044
Tian Y, Sun Z, Wang W, Shang H, Wang B, Deng D, Ma G, Wu H, Zhu N, Xu X, et al. Semisynthesis and Biological Evaluation of Oleanolic Acid 3-O-β-d-Glucuronopyranoside Derivatives for Protecting H9c2 Cardiomyoblasts against H2O2-Induced Injury. Molecules. 2018; 23(1):44. https://doi.org/10.3390/molecules23010044
Chicago/Turabian StyleTian, Yu, Zhonghao Sun, Wenqian Wang, Hai Shang, Baoqi Wang, Di Deng, Guoxu Ma, Haifeng Wu, Nailiang Zhu, Xudong Xu, and et al. 2018. "Semisynthesis and Biological Evaluation of Oleanolic Acid 3-O-β-d-Glucuronopyranoside Derivatives for Protecting H9c2 Cardiomyoblasts against H2O2-Induced Injury" Molecules 23, no. 1: 44. https://doi.org/10.3390/molecules23010044




