Synthesis of Novel Reactive Disperse Silicon-Containing Dyes and Their Coloring Properties on Silicone Rubbers
Abstract
:1. Introduction
2. Experimental
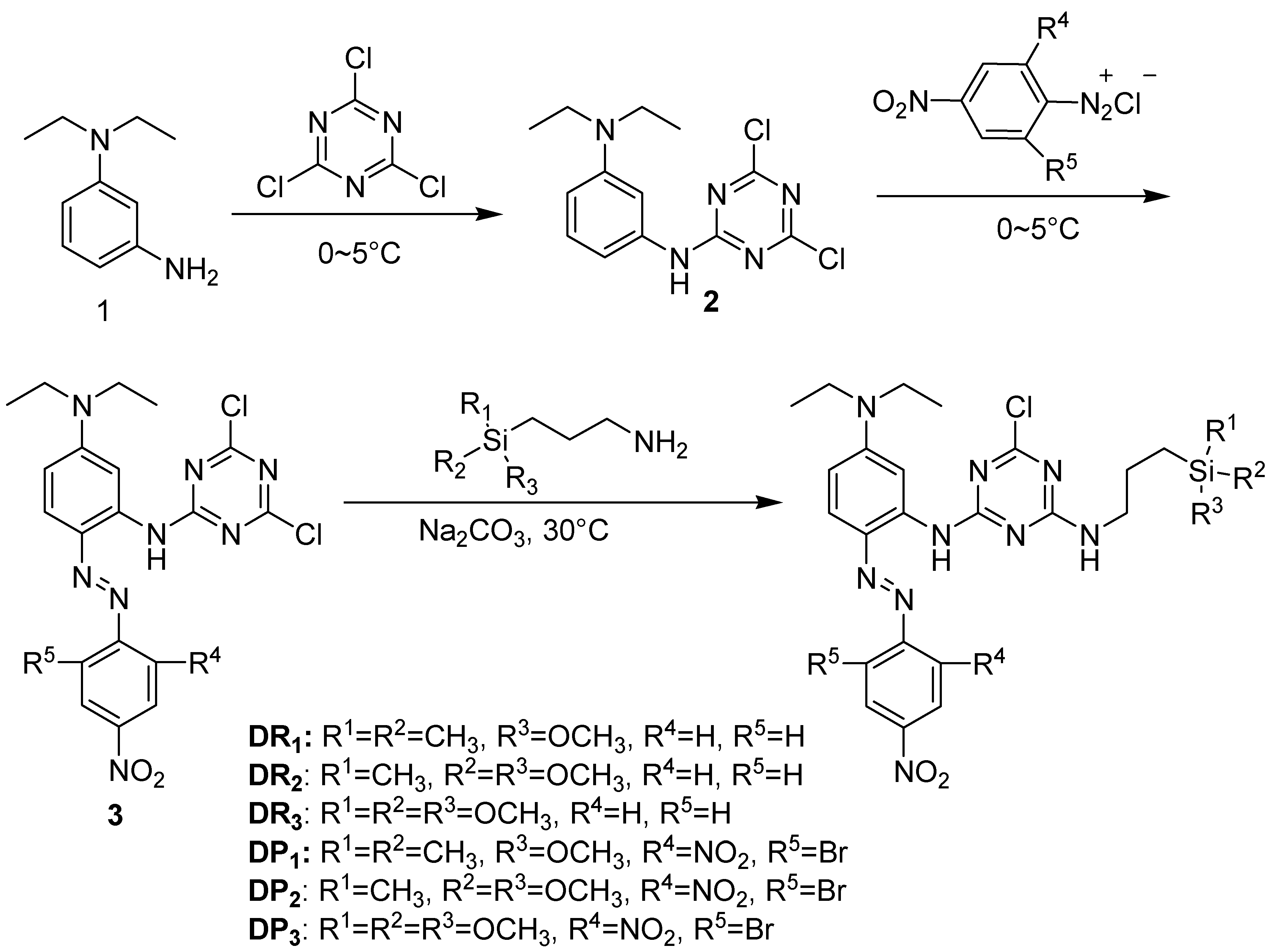
2.1. Synthesis of Reactive Disperse Silicon-Containing Dyes
2.1.1. Materials
2.1.2. Synthesis of Red Dye DR1
2.1.3. Synthesis of Purple Dye DP1
2.1.4. Characterization
2.1.5. Structure Characterization Results of Reactive Disperse Silicon-Containing Dyes (Please see in Supplementary Materials)
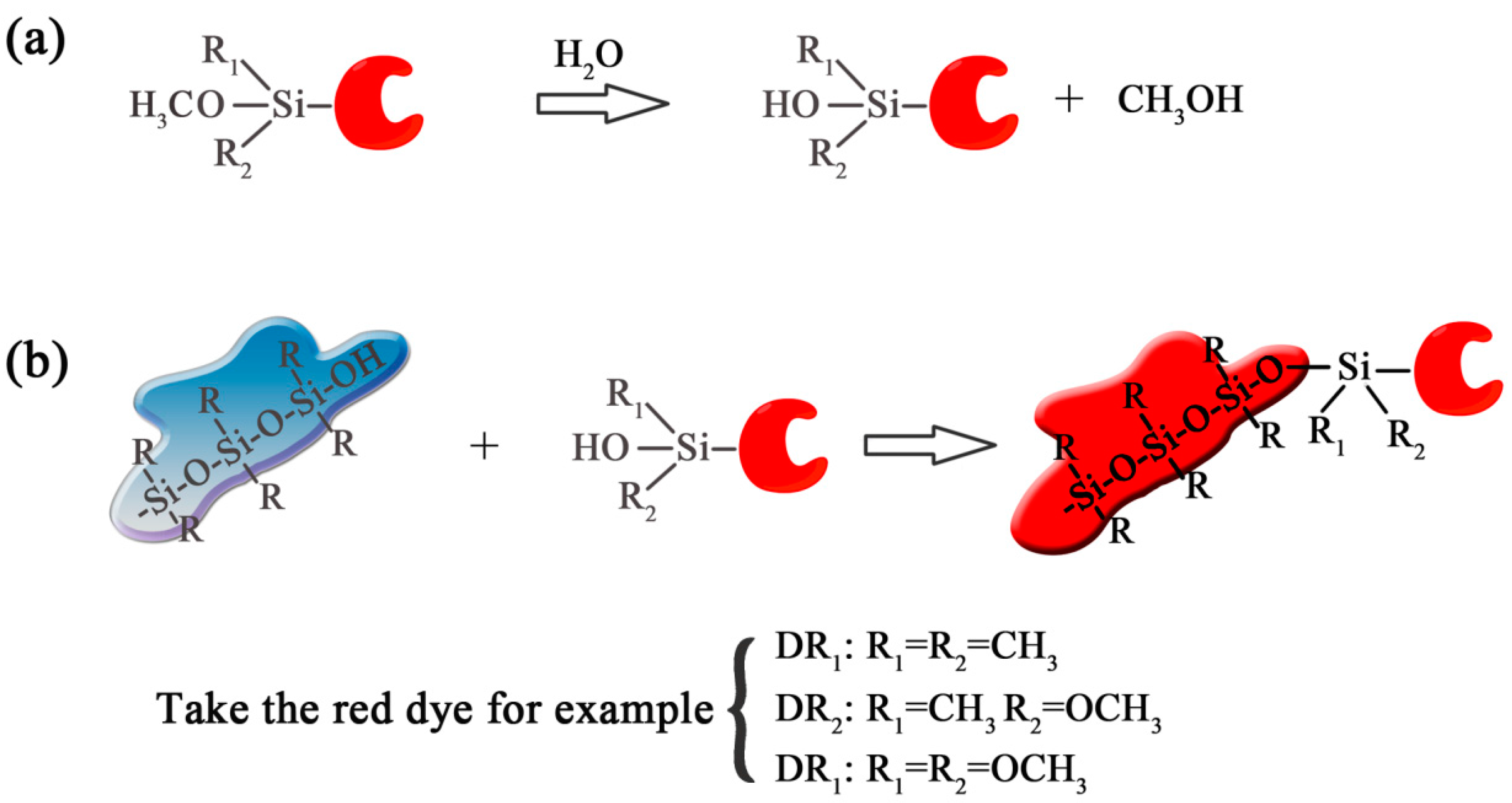
2.2. Coloring the Silicone Rubbers
2.2.1. Coloring Method
2.2.2. Color Fastness Test
2.2.3. Color Strength and Other Colorimetric Data Measurement
2.2.4. Mechanical Property Test
3. Results and Discussion
3.1. Dyes Synthesis and Structure Characterization
3.2. Color Fastness of the Colored Rubbers
3.3. Color Strength and Other Colorimetric Data of the Colored Rubbers
3.4. Mechanical Properties of the Colored Rubber
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Forbes, C.J.; Mccoy, C.F.; Murphy, D.J. Modified silicone elastomer vaginal gels for sustained release of antiretroviral HIV microbicides. J. Pharm. Sci. 2014, 103, 1422–1432. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McCoy, C.F.; Murphy, D.J.; Boyd, P. Packing polymorphism of dapivirine and its impact on the performance of a dapivirine-releasing silicone elastomer vaginal ring. J. Pharm. Sci. 2017, 106, 2015–2025. [Google Scholar] [CrossRef] [PubMed]
- Mokkaphan, J.; Banlunara, W.; Palaga, T. Silicone surface with drug nanodepots for medical devices. ACS Appl. Mater. Interface 2014, 6, 20188–20196. [Google Scholar] [CrossRef] [PubMed]
- Aliyar, H.; Schalau, G., II. Recent developments in silicones for topical and transdermal drug delivery. Ther. Deliv. 2015, 6, 827–839. [Google Scholar] [CrossRef] [PubMed]
- Roggero, A.; Dantras, E.; Paulmier, T. Inorganic fillers influence on the radiation-induced ageing of a space-used silicone elastomer. Polym. Degrad. Stab. 2016, 128, 126–133. [Google Scholar] [CrossRef] [Green Version]
- Roggero, A.; Dantras, E.; Paulmier, T. Electrical behavior of a silicone elastomer under simulated space environment. J. Phys. D Appl. Phys. 2015, 48, 135302. [Google Scholar] [CrossRef]
- Roggero, A.; Dantras, E.; Paulmier, T. Electrical conductivity of a silicone network upon electron irradiation: Influence of formulation. J. Phys. D Appl. Phys. 2016, 49, 505303. [Google Scholar] [CrossRef]
- Song, S.W.; Li, Z.; Zhao, H. Electric field control in DC cable test termination by nano silicone rubber composite. AIP Adv. 2017, 7. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhou, Y.; Chen, M. Electrical tree initiation in silicone rubber under DC and polarity reversal voltages. J. Electrost. 2017, 88, 207–213. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhou, Y.; Liu, R. Three-dimensional morphology and spherical growth mechanisms of electrical trees in silicone rubber. J. Electrost. 2015, 76, 83–88. [Google Scholar] [CrossRef]
- Li, X.; Zhang, D.; Chen, Y. Silicone rubber/hollow silica spheres composites with enhanced mechanical and electrical insulating performances. Mater. Lett. 2017, 205, 240–244. [Google Scholar] [CrossRef]
- Madsen, F.B.; Yu, L.; Daugaard, A.E. A new soft dielectric silicone elastomer matrix with high mechanical integrity and low losses. RSC Adv. 2015, 5, 10254–10259. [Google Scholar] [CrossRef] [Green Version]
- Quinsaat, J.E.Q.; Alexandru, M.; Nüesch, F.A. Highly stretchable dielectric elastomer composites containing high volume fractions of silver nanoparticles. J. Mater. Chem. A 2015, 3, 14675–14685. [Google Scholar] [CrossRef]
- Xiang, H.P.; Rong, M.Z.; Zhang, M.Q. A facile method for imparting sunlight driven catalyst-free self-healability and recyclability to commercial silicone elastomer. Polymer 2017, 108, 339–347. [Google Scholar] [CrossRef]
- Guo, L.M.; Lv, Y.A.; Deng, Z.F. Effect of Strain Rate on Tension Response of Filled Silicone Rubber. Appl. Mech. Mater. 2017, 863, 112–116. [Google Scholar] [CrossRef]
- Gan, L.; Shang, S.; Yuen, C.W.M. Facile preparation of graphenenanoribbon filled silicone rubber nanocomposite with improved thermal and mechanical properties. Compos. Part B 2015, 69, 237–242. [Google Scholar] [CrossRef]
- Gan, L.; Shang, S.; Jiang, S. Impact of vinyl concentration of a silicone rubber on the properties of the graphene oxide filled silicone rubber composites. Compos. Part B 2016, 84, 294–300. [Google Scholar] [CrossRef]
- Wang, J.; Ji, C.; Yan, Y. Mechanical and ceramifiable properties of silicone rubber filled with different inorganic fillers. Polym. Degrad. Stab. 2015, 121, 149–156. [Google Scholar] [CrossRef]
- Chatterjee, T.; Wiessner, S.; Naskar, K. Novel thermoplastic vulcanizates (TPVs) based on silicone rubber and polyamide exploring peroxide cross-link. Express Polym. Lett. 2014, 8, 220–231. [Google Scholar] [CrossRef]
- Jiang, L.; Betts, A.; Kennedy, D. Improving the electromechanical performance of dielectric elastomers using silicone rubber and dopamine coated barium titanate. Mater. Des. 2015, 85, 733–742. [Google Scholar] [CrossRef]
- Guiotti, A.M.; Goiato, M.C.; Dos Santos, D.M. Comparison of conventional and plant-extract disinfectant solutions on the hardness and color stability of a maxillofacial elastomer after artificial aging. J. Prosthet. Dent. 2016, 115, 501–508. [Google Scholar] [CrossRef] [PubMed]
- Eltayyar, N.H.; Alshimy, A.M.; Abushelib, M.N. Evaluation of intrinsic color stability of facial silicone elastomer reinforced with different nanoparticles. Alex. Dent. J. 2016, 41, 50–54. [Google Scholar]
- Mancuso, D.N.; Goiato, M.C.; Santos, D.M. Color stability after accelerated aging of two silicones, pigmented or not, for use in facial prostheses. Braz. Oral Res. 2009, 23, 144–148. [Google Scholar] [CrossRef] [PubMed]
- Al-Harbi, F.A.; Ayad, N.M.; Saber, M.A. Mechanical behavior and color change of facial prosthetic elastomers after outdoor weathering in a hot and humid climate. J. Prosthet. Dent. 2015, 113, 146–151. [Google Scholar] [CrossRef] [PubMed]
- Dan, X.; Zhu, L.; Yu-Xin, P.; Jiao, G.; Hui-Fen, Q.; Wei, H. Post-modification of 2-formylthiophene based heterocyclic azo dyes. Dyes Pigment. 2016, 133, 143–152. [Google Scholar]
- Xiao-Lei, Z.; Jiao, G.; Hui-Fen, Q.; Wei, H. pH-induced azo-keto and azo-enol tautomerism for 6-(3-methoxypropylamino)pyridin-2-one based thiophene azo dyes. Dyes Pigment. 2017, 147, 318–326. [Google Scholar]
- Kew-Yu, C.; Che-Wei, C.; Hsing-Yang, T. 1,6- and 1,7-Regioisomers of highly soluble amino-substituted perylene tetracarboxylic dianhydrides: Synthesis, optical and electrochemical properties. Materials 2015, 8, 4943–4960. [Google Scholar]
Sample Availability: Samples of the compounds DR1–DR3 and DP1–DP3 are available from the authors. |
| Dyes | Washing | Rubbing | Sublimation | |||||
|---|---|---|---|---|---|---|---|---|
| Color Change | Polyester | Cotton | Dry | Wet | Color Change | Polyester | Cotton | |
| DR1 | 4–5 | 4–5 | 4–5 | 4–5 | 4–5 | 4–5 | 4–5 | 5 |
| DR2 | 4–5 | 4–5 | 4–5 | 4–5 | 4–5 | 4 | 4–5 | 4–5 |
| DR3 | 4–5 | 4–5 | 4–5 | 4–5 | 4–5 | 4 | 4–5 | 4–5 |
| DP1 | 4–5 | 4–5 | 4–5 | 4–5 | 4–5 | 4 | 4–5 | 5 |
| DP2 | 4–5 | 4–5 | 4–5 | 4–5 | 4–5 | 4–5 | 4–5 | 4–5 |
| DP3 | 4–5 | 4–5 | 4–5 | 4–5 | 4–5 | 4–5 | 4–5 | 4–5 |
| DR1 | DR2 | DR3 | DP1 | DP2 | DP3 | |
|---|---|---|---|---|---|---|
| K/S | 10.72 | 9.89 | 11.09 | 10.67 | 9.27 | 10.52 |
| L* | 37.6 | 42.87 | 37.15 | 30 | 32.25 | 30.6 |
| C* | 39.57 | 45.96 | 39.54 | 27.31 | 28.55 | 29.37 |
| h* | 12.93 | 14.45 | 11.05 | 314.14 | 314.43 | 315.77 |
| a* | 38.56 | 44.51 | 38.81 | 19.02 | 19.99 | 21.04 |
| b* | 8.85 | 11.47 | 7.58 | −19.6 | −20.39 | −20.49 |
| X | 14.86 | 20.12 | 14.59 | 7.78 | 8.98 | 8.29 |
| Y | 9.87 | 13.07 | 9.62 | 6.24 | 7.19 | 6.48 |
| Z | 7.83 | 9.79 | 7.97 | 12.98 | 14.9 | 13.75 |
| x | 0.4565 | 0.4682 | 0.4533 | 0.2882 | 0.289 | 0.2906 |
| y | 0.303 | 0.3041 | 0.299 | 0.231 | 0.2315 | 0.2273 |
| YI E313 | 104.82 | 114.26 | 102.06 | −76.93 | −75.67 | −77.5 |
| WI E313 | −56.78 | −64.7 | −47.6 | 196.73 | 196.19 | 201.35 |
| Tensile Strength (MPa) | Elongation at Break (%) | 100% Tensile Stress at a Given Elongation (MPa) | Tearing Strength (kN/m) | Hardness (Shore Hardness A) | Resilience (%) | |
|---|---|---|---|---|---|---|
| Blank | 6.09 | 381.50 | 1.68 | 20.42 | 51.3 | 50.0 |
| DR1 | 5.60 | 348.67 | 1.74 | 30.37 | 52.0 | 53.3 |
| DR2 | 6.03 | 396.78 | 1.65 | 28.15 | 51.9 | 51.2 |
| DR3 | 6.12 | 372.15 | 1.67 | 31.23 | 52.2 | 50.9 |
| Tensile Strength (MPa) | Elongation at Break (%) | 100% Tensile Stress at a Given Elongation (MPa) | Tearing Strength (kN/m) | Hardness (Shore Hardness A) | Resilience (%) | |
|---|---|---|---|---|---|---|
| Blank | 6.09 | 381.50 | 1.68 | 20.42 | 51.3 | 50.0 |
| DP1 | 6.01 | 392.00 | 1.62 | 28.57 | 51.7 | 50.0 |
| DP2 | 5.89 | 386.23 | 1.63 | 23.65 | 51.0 | 51.3 |
| DP3 | 6.20 | 379.23 | 1.71 | 26.77 | 50.4 | 49.2 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, N.; Zhang, S.; Tang, B.; Ma, W.; Qiu, J. Synthesis of Novel Reactive Disperse Silicon-Containing Dyes and Their Coloring Properties on Silicone Rubbers. Molecules 2018, 23, 127. https://doi.org/10.3390/molecules23010127
Yu N, Zhang S, Tang B, Ma W, Qiu J. Synthesis of Novel Reactive Disperse Silicon-Containing Dyes and Their Coloring Properties on Silicone Rubbers. Molecules. 2018; 23(1):127. https://doi.org/10.3390/molecules23010127
Chicago/Turabian StyleYu, Ning, Shufen Zhang, Bingtao Tang, Wei Ma, and Jinjing Qiu. 2018. "Synthesis of Novel Reactive Disperse Silicon-Containing Dyes and Their Coloring Properties on Silicone Rubbers" Molecules 23, no. 1: 127. https://doi.org/10.3390/molecules23010127





