Effects of Sleep Quality on Melatonin Levels and Inflammatory Response after Major Abdominal Surgery in an Intensive Care Unit
Abstract
:1. Introduction
2. Results
3. Discussion
4. Materials and Methods
Statistics
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Reiter, R.J. Melatonin: Clinical relevance. Best Pract. Res. Clin. Endocrinol. Metab. 2003, 17, 273–285. [Google Scholar] [CrossRef]
- Rajaratnam, S.; Cohen, D.; Rogers, N. Melatonin and melatonin analogues. Sleep Med. Clin. 2009, 4, 179–193. [Google Scholar] [CrossRef]
- Claustrat, B.; Brun, J.; Chazot, G. The basic physiology and pathophysiology of melatonin. Sleep Med. Rev. 2005, 9, 11–24. [Google Scholar] [CrossRef] [PubMed]
- Shilo, L.; Dagan, Y.; Smorjik, Y.; Weinberg, U.; Dolev, S.; Komptel, B.; Shenkman, L. Effect of melatonin on sleep quality of COPD intensive care patients: A pilot study. Chronobiol. Int. 2000, 17, 71–76. [Google Scholar] [CrossRef] [PubMed]
- Shilo, L.; Dagan, Y.; Smorjik, Y.; Weinberg, U.; Dolev, S.; Komptel, B.; Balaum, H.; Shenkman, L. Patients in the intensive care unit suffer from severe lack of sleep associated with loss of normal melatonin secretion pattern. Am. J. Med. Sci. 1999, 317, 278. [Google Scholar] [CrossRef]
- Carrillo-Vico, A.; Lardone, P.J.; Naji, L.; Fernández-Santos, J.M.; Martín-Lacave, I.; Guerrero, J.M.; Calvo, J.R. Beneficial pleiotropic actions of melatonin in an experimental model of septic shock in mice: Regulation of pro-/anti-inflammatory cytokine network, protection against oxidative damage and anti-apoptotic effects. J. Pineal Res. 2005, 39, 400–408. [Google Scholar] [CrossRef] [PubMed]
- Veneroso, C.; Tunon, M.J.; Gonzalez-Gallego, J.; Collado, P.S. Melatonin reduces cardiac inflammatory injury induced by acute exercise. J. Pineal Res. 2009, 47, 184–191. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.J.; Mei, G.P.; Liu, J.; Li, Y.L.; Zuo, D.; Liu, S.J.; Zhao, T.B.; Lin, M.T. Therapeutic effects of melatonin on heatstroke-induced multiple organ dysfunction syndrome in rats. J. Pineal Res. 2011, 50, 436–444. [Google Scholar] [CrossRef] [PubMed]
- Mishra, A.; Paul, S.; Swarnakar, S. Downregulation of matrix metalloproteinase-9 by melatonin during prevention of alcohol-induced liver injury in mice. Biochimie 2011, 93, 854–866. [Google Scholar] [CrossRef] [PubMed]
- Tocharus, J.; Khonthun, C.; Chongthammakun, S.; Govitrapong, P. Melatonin attenuates methamphetamine-induced overexpression of pro-inflammatory cytokines in microglial cell lines. J. Pineal Res. 2010, 48, 347–352. [Google Scholar] [CrossRef] [PubMed]
- Bekyarova, G.; Tancheva, S.; Hristova, M. Protective effect of melatonin against oxidative hepatic injury after experimental thermal trauma. Methods Find Exp. Clin. Pharmacol. 2009, 31, 11–14. [Google Scholar] [CrossRef] [PubMed]
- Riutta, A.; Ylitalo, P.; Kaukinen, S. Diurnal variation of melatonin and cortisol is maintained in non-septic intensive care patients. Intensive Care Med. 2009, 35, 1720–1727. [Google Scholar] [CrossRef] [PubMed]
- Hu, R.F.; Jiang, X.Y.; Zeng, Y.M.; Chen, X.Y.; Zhang, Y.H. Effects of earplugs and eye masks on nocturnal sleep, melatonin and cortisol in a simulated intensive care unit environment. Crit. Care 2010, 14, R66. [Google Scholar] [CrossRef] [PubMed]
- Kvarnström, A.L.; Sarbinowski, R.T.; Bengtson, J.P.; Jacobsson, L.M.; Bengtsson, A.L. Complement activation and interleukin response in major abdominal surgery. Scand. J. Immunol. 2012, 75, 510–516. [Google Scholar] [CrossRef] [PubMed]
- Esrefoglu, M.; Cetin, A.; Taslidere, E.; Elbe, H.; Ates, B.; Tok, O.E.; Aydin, M.S. Therapeutic effects of melatonin and quercetin in improvement of hepatic steatosis in rats through supression of oxidative damage. Bratisl. Lek. Listy 2017, 118, 347–354. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.S.; Li, Y.Y.; Cui, W.; Li, L.B.; Zhang, Z.C.; Tian, B.P.; Zhang, G.S. Melatonin attenuates pain hypersensitivity and decreases astrocyte-mediated spinal neuroinflammation in a rat model of oxaliplatin-induced pain. Inflammation 2017. [Google Scholar] [CrossRef] [PubMed]
- Shaki, F.; Ashari, S.; Ahangar, N. Melatonin can attenuate ciprofloxacin induced nephrotoxicity: Involvement of nitric oxide and TNF-α. Biomed. Pharmacother. 2016, 11, e0166010. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zhang, J.; Zhao, Q.; Chen, Q.; Sun, Y.; Jin, Y.; Wu, J. Melatonin induces anti-inflammatory effects to play a protective role via endoplasmic reticulum stress in acute pancreatitis. Cell. Physiol. Biochem. 2016, 40, 1094–1104. [Google Scholar] [CrossRef] [PubMed]
- Jiang, T.; Chang, Q.; Cai, J.; Fan, J.; Zhang, X.; Xu, G. Protective effects of melatonin on retinal inflammation and oxidative stress in experimental diabetic retinopathy. Oxid. Med. Cell. Longev. 2016, 2016, 3528274. [Google Scholar] [CrossRef] [PubMed]
- Richards, K.C.; O’Sullivan, P.S.; Phillips, R.L. Measurement of sleep in critically ill patients. J. Nurs. Meas. 2000, 8, 131–144. [Google Scholar] [PubMed]
- Geyik, S.; Yiğiter, R.; Akçalı, A.; Deniz, H.; Geyik, A.M.; Elçi, M.A.; Hafız, E. The Effect of Circadian Melatonin Levels on Inflammation and Neurocognitive Functions Following Coronary Bypass Surgery. Ann. Thorac. Cardiovasc. Surg. 2015, 21, 466–473. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are available from the authors. |
| Control Group Mean ± S.Error | Experimental Group Mean ± S.Error | p | |
| Age | 55.50 (±1.74) | 53.35 (±1.91) | 0.410 |
| Gender (number of males) | 16 | 15 | |
| Richards–Campbell sleep quality scores POD1 | 41.65 (±1.60) | 49.95 (±1.56) | 0.001 |
| Median (25–75%) | Median (25–75%) | ||
| Operation duration | 3.50 (2.50–4.75) | 3.00 (2.50–3.88) | 0.602 |
| Richards–Campbell sleep quality scores POD3 | 46.50 (43.00–52.50) | 60.50 (52.75–62.75) | <0.001 |
| Control Group Mean ± S.Error/ Median (25–75%) | Experimental Group Mean ± S.Error/ Median (25–75%) | p | |
|---|---|---|---|
| Preoperative | |||
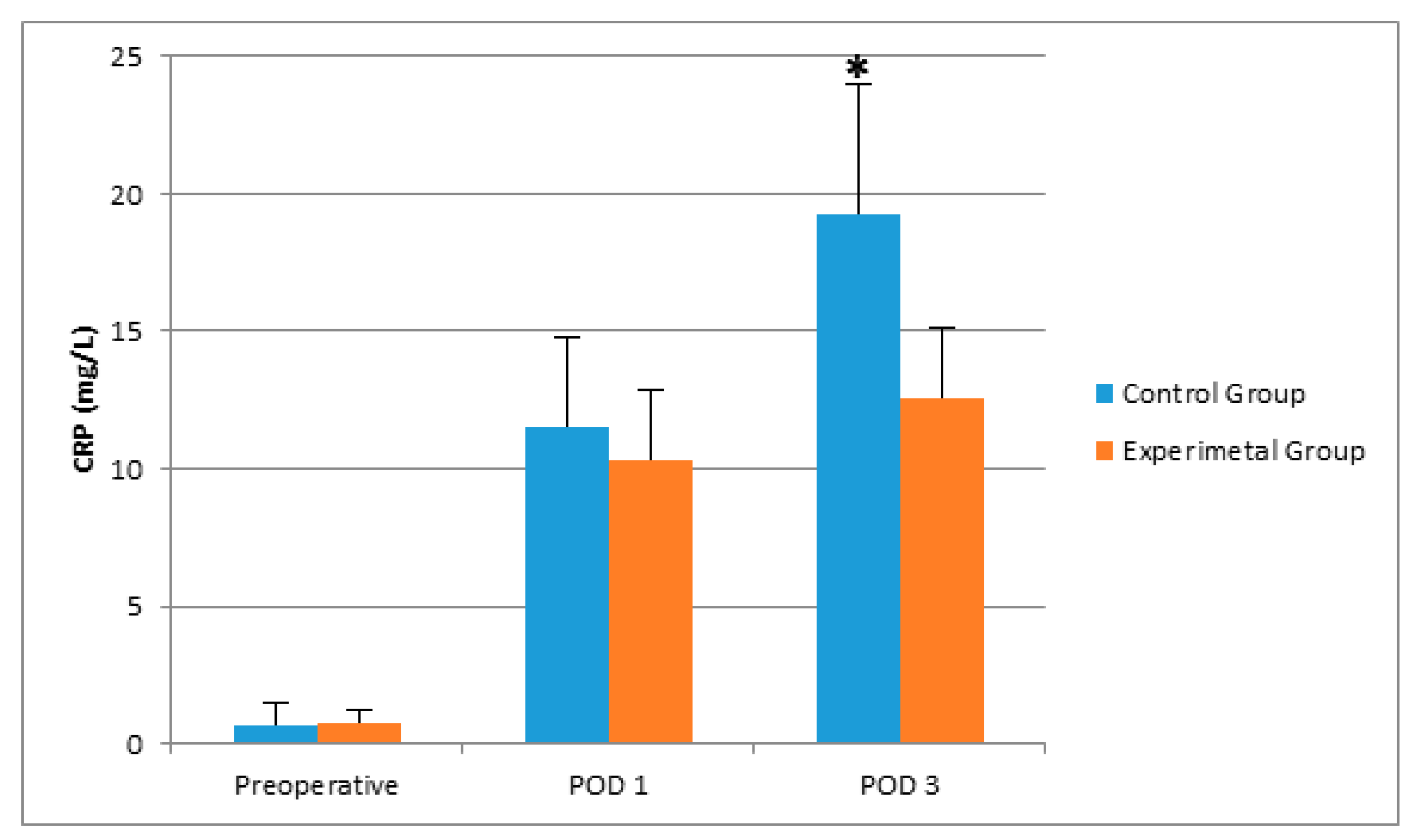
| CRP (mg/L) | 0.66 (0.34–1.84) | 0.78 (0.34–1.35) | 0.820 |
| IL-1 (pg/mL) | 8.54 (2.79–33.75) | 14.79 (6.23–30.56) | 0.355 |
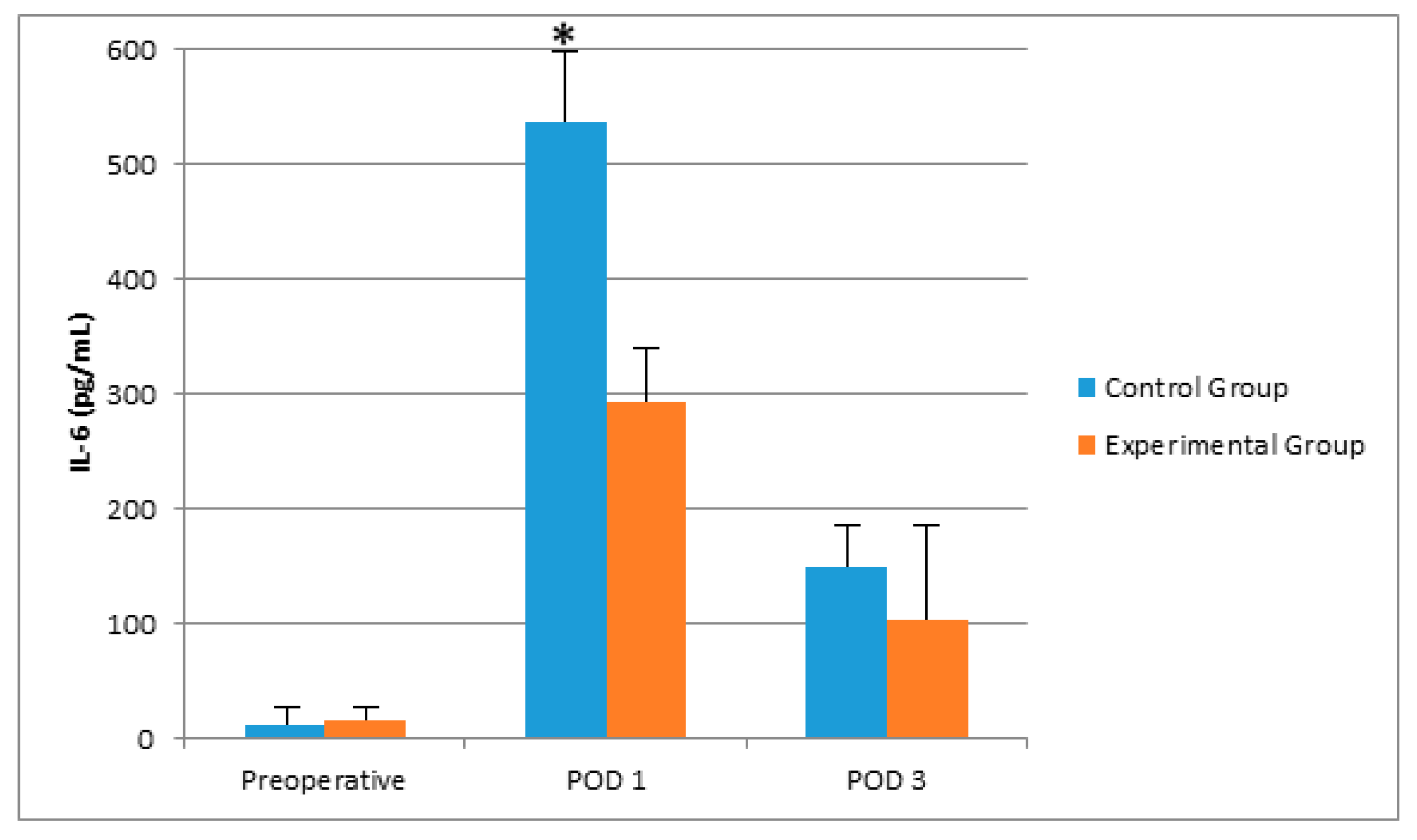
| IL-6 (pg/mL) | 12.47 (8.33–26.95) | 16.29 (8.86–31.03) | 0.529 |
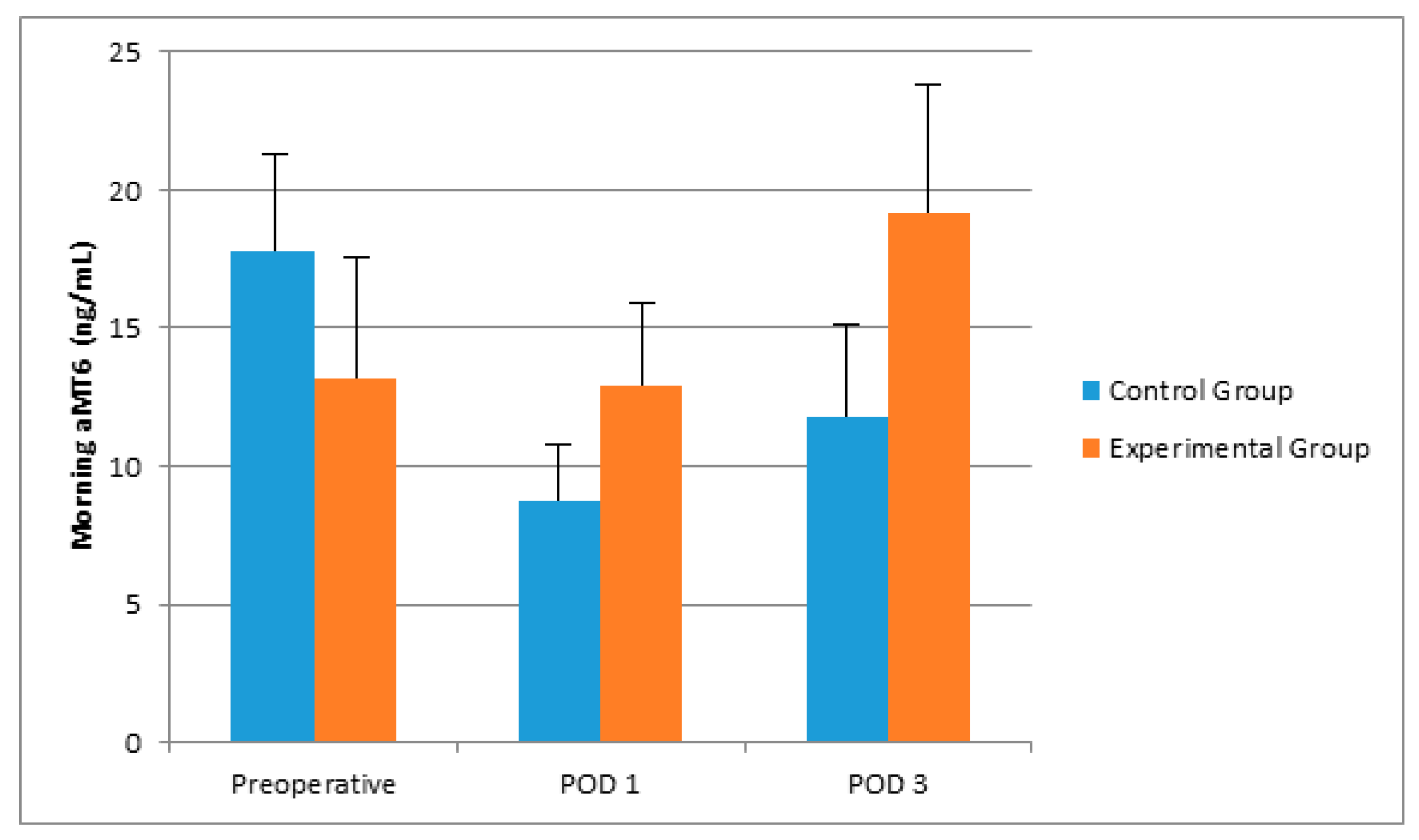
| Morning aMT6 (ng/mL) | 17.76 (4.40–25.18) | 13.17 (7.60–30.56) | 0.989 |
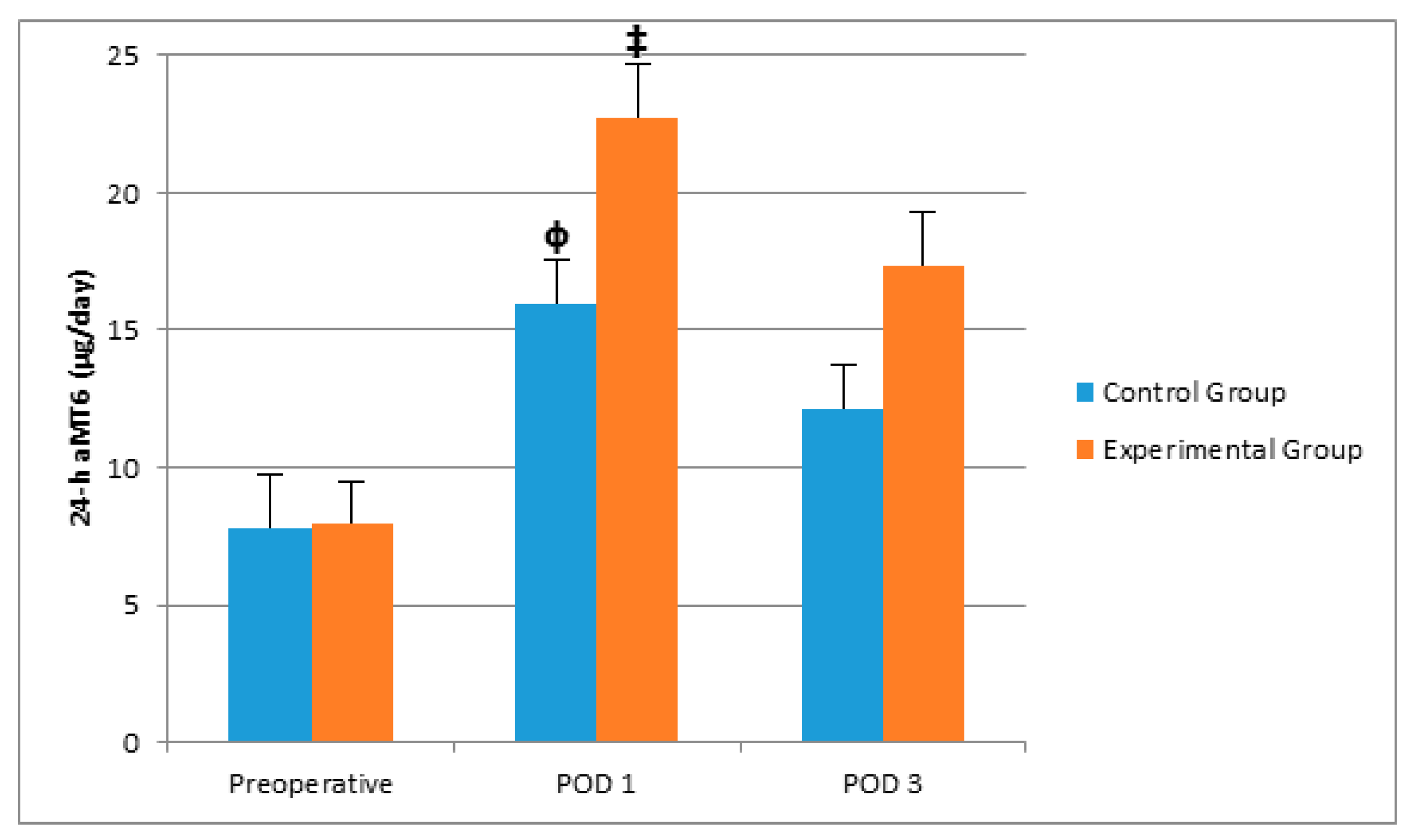
| 24-h aMT6 (µg/day) | 7.82 (3.97–16.56) | 7.99 (4.60–11.76) | 0.947 |
| POD 1 | |||
| CRP (mg/L) | 11.51 (±0.99) | 10.28 (±0.74) | 0.330 |
| IL-1 (pg/mL) | 25.70 (±3.53) | 19.10 (±2.62) | 0.141 |
| IL-6 (pg/mL) | 537.31 (282.26–678.15) | 293.24 (195.28–454.44) | 0.013 |
| Morning aMT6 (ng/mL) | 8.70 (4.76–16.64) | 12.91 (7.81–26.68) | 0.211 |
| 24-h aMT6 (µg/day) | 15.92 (±2.22) | 22.73 (±3.52) | 0.112 |
| POD 3 | |||
| CRP (mg/L) | 19.28 (±1.35) | 12.56 (±1.02) | <0.001 |
| IL-1 (pg/mL) | 23.55 (4.68–39.77) | 17.15 (5.40–37.26) | 0.883 |
| IL-6 (pg/mL) | 148.93 (87.35–175.10) | 103.97 (83.70–143.66) | 0.183 |
| Morning aMT6 (ng/mL) | 11.78 (5.91–34.78) | 19.14 (10.13–29.38) | 0.383 |
| 24-h aMT6 (µg/day) | 12.10 (7.89–21.38) | 17.34 (7.58–34.70) | 0.327 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yaşar, N.F.; Badak, B.; Canik, A.; Baş, S.Ş.; Uslu, S.; Öner, S.; Ateş, E. Effects of Sleep Quality on Melatonin Levels and Inflammatory Response after Major Abdominal Surgery in an Intensive Care Unit. Molecules 2017, 22, 1537. https://doi.org/10.3390/molecules22091537
Yaşar NF, Badak B, Canik A, Baş SŞ, Uslu S, Öner S, Ateş E. Effects of Sleep Quality on Melatonin Levels and Inflammatory Response after Major Abdominal Surgery in an Intensive Care Unit. Molecules. 2017; 22(9):1537. https://doi.org/10.3390/molecules22091537
Chicago/Turabian StyleYaşar, Necdet Fatih, Bartu Badak, Ağgül Canik, Sema Şanal Baş, Sema Uslu, Setenay Öner, and Ersin Ateş. 2017. "Effects of Sleep Quality on Melatonin Levels and Inflammatory Response after Major Abdominal Surgery in an Intensive Care Unit" Molecules 22, no. 9: 1537. https://doi.org/10.3390/molecules22091537





