Cloning and Characterization of Two Iridoid Synthase Homologs from Swertia Mussotii
Abstract
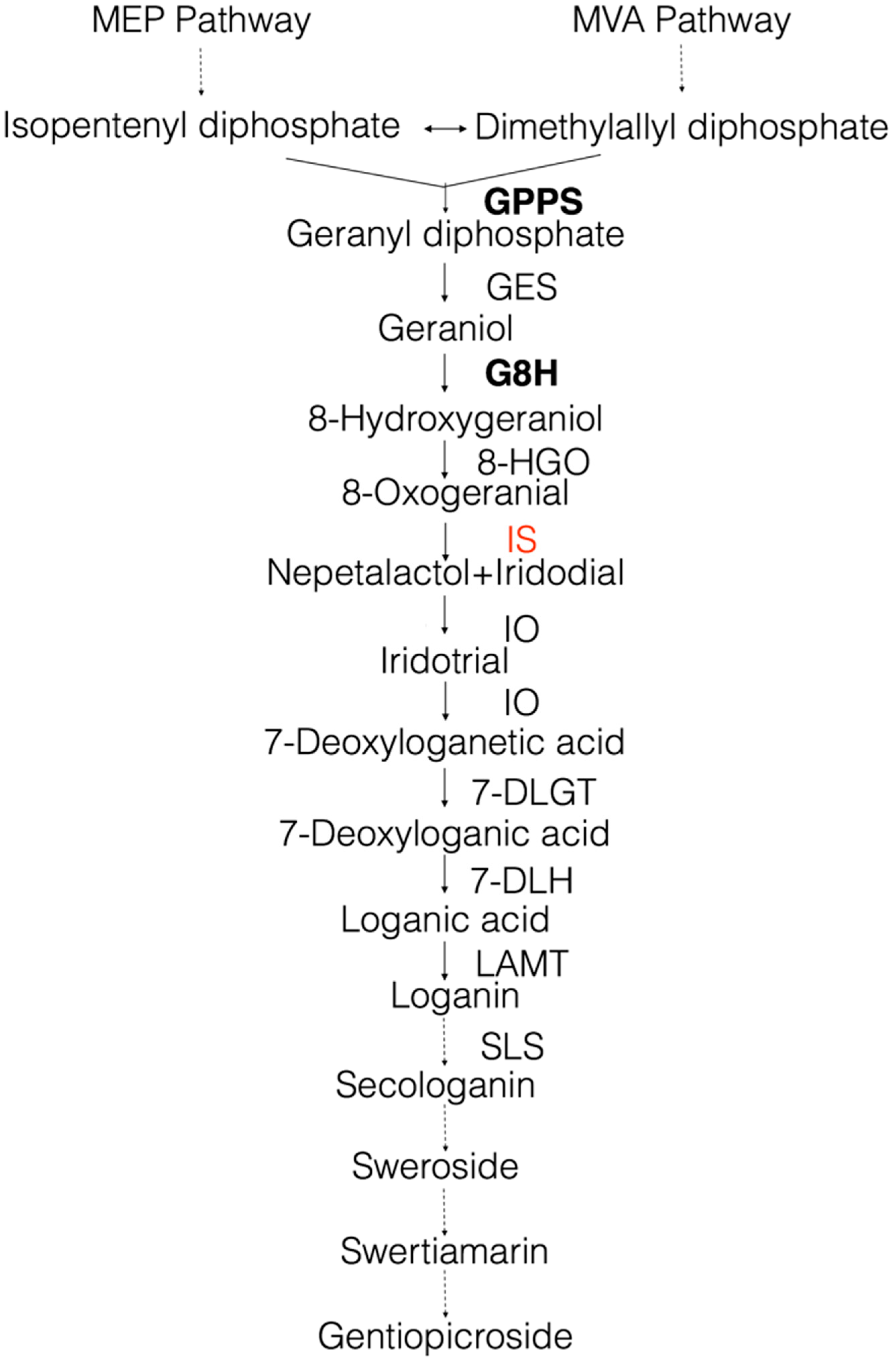
1. Introduction
2. Results
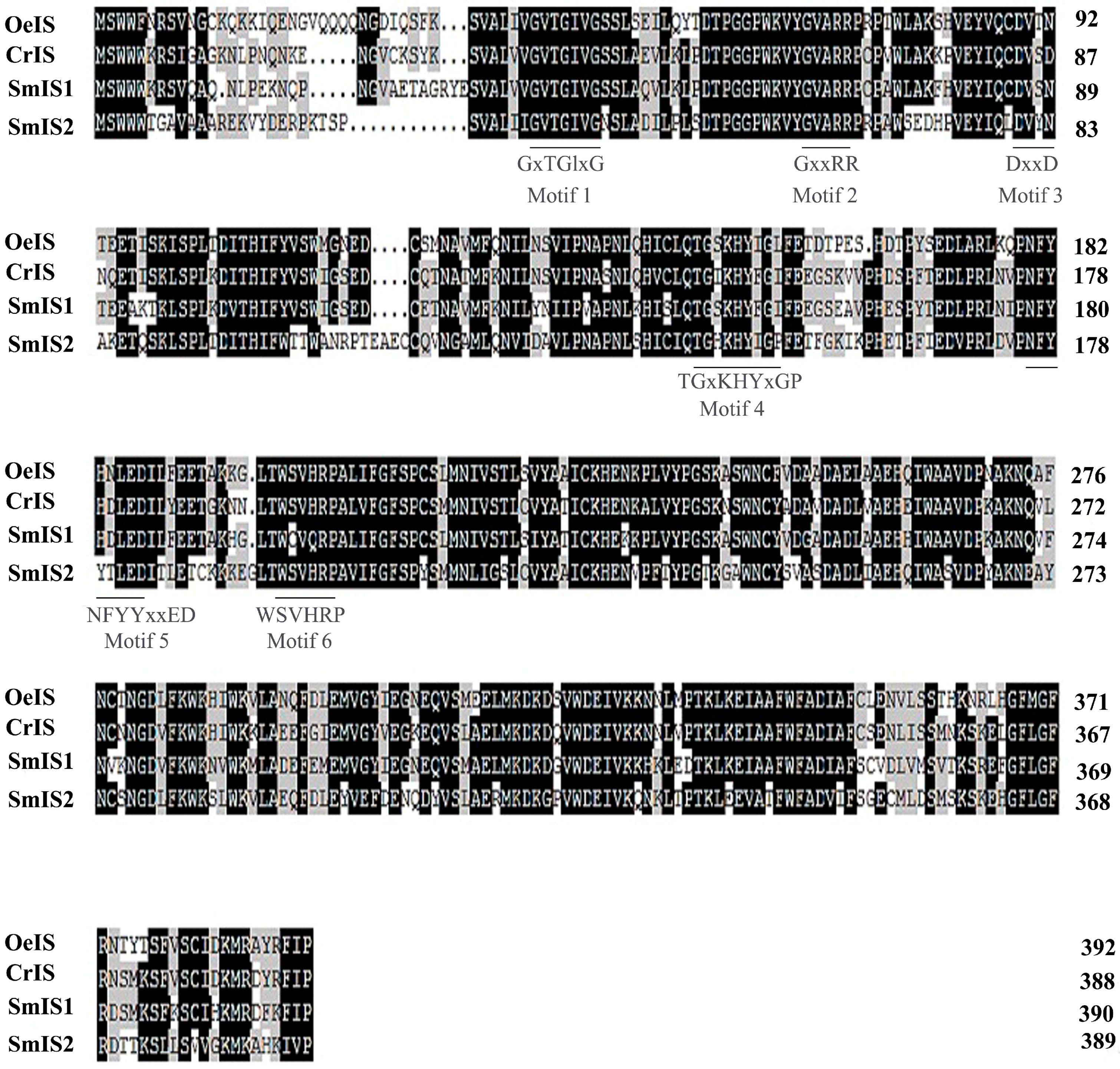
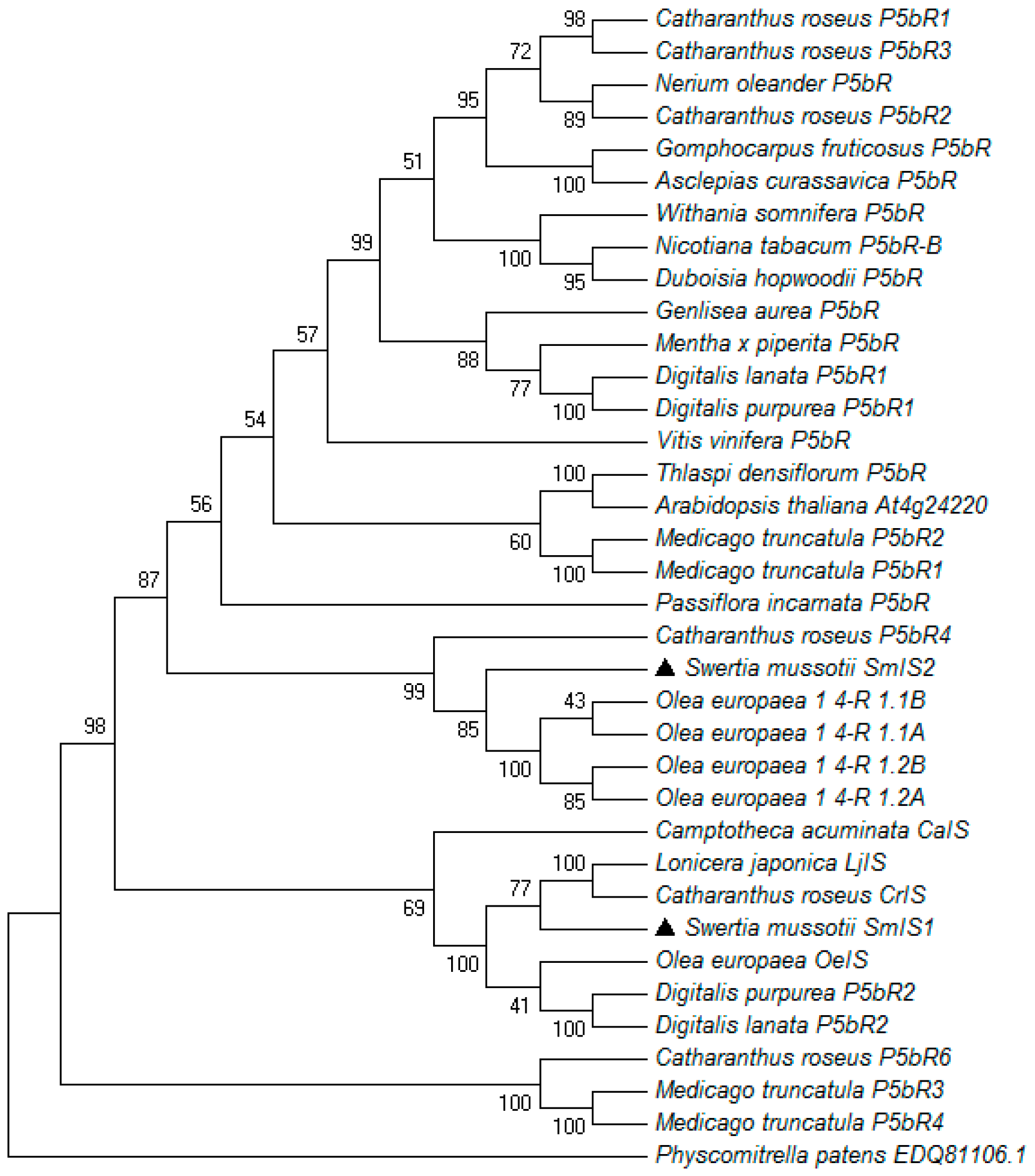
2.1. Cloning, Sequencing and Phylogenetic Analysis of SmIS1 and SmIS2 cDNAs
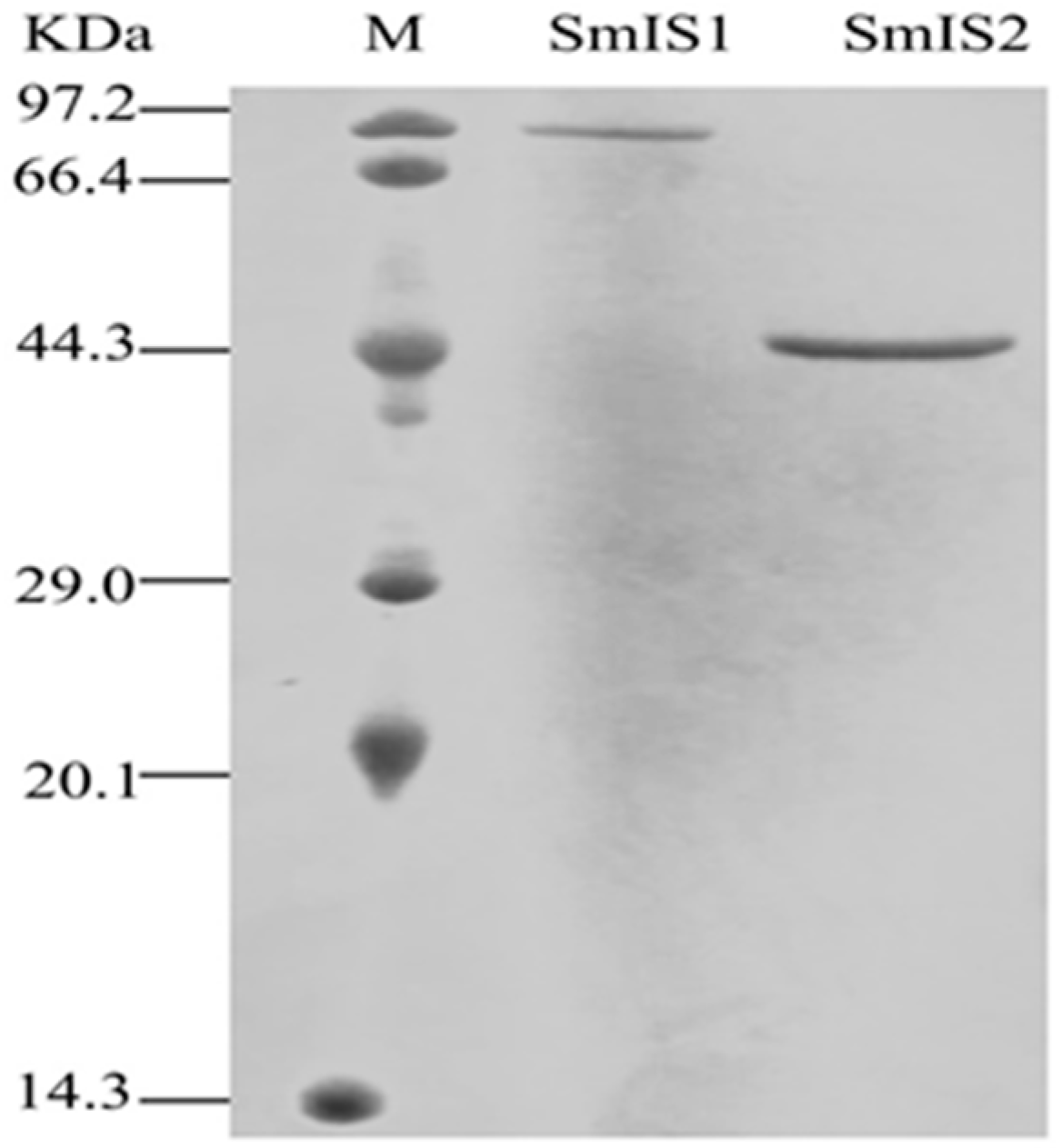
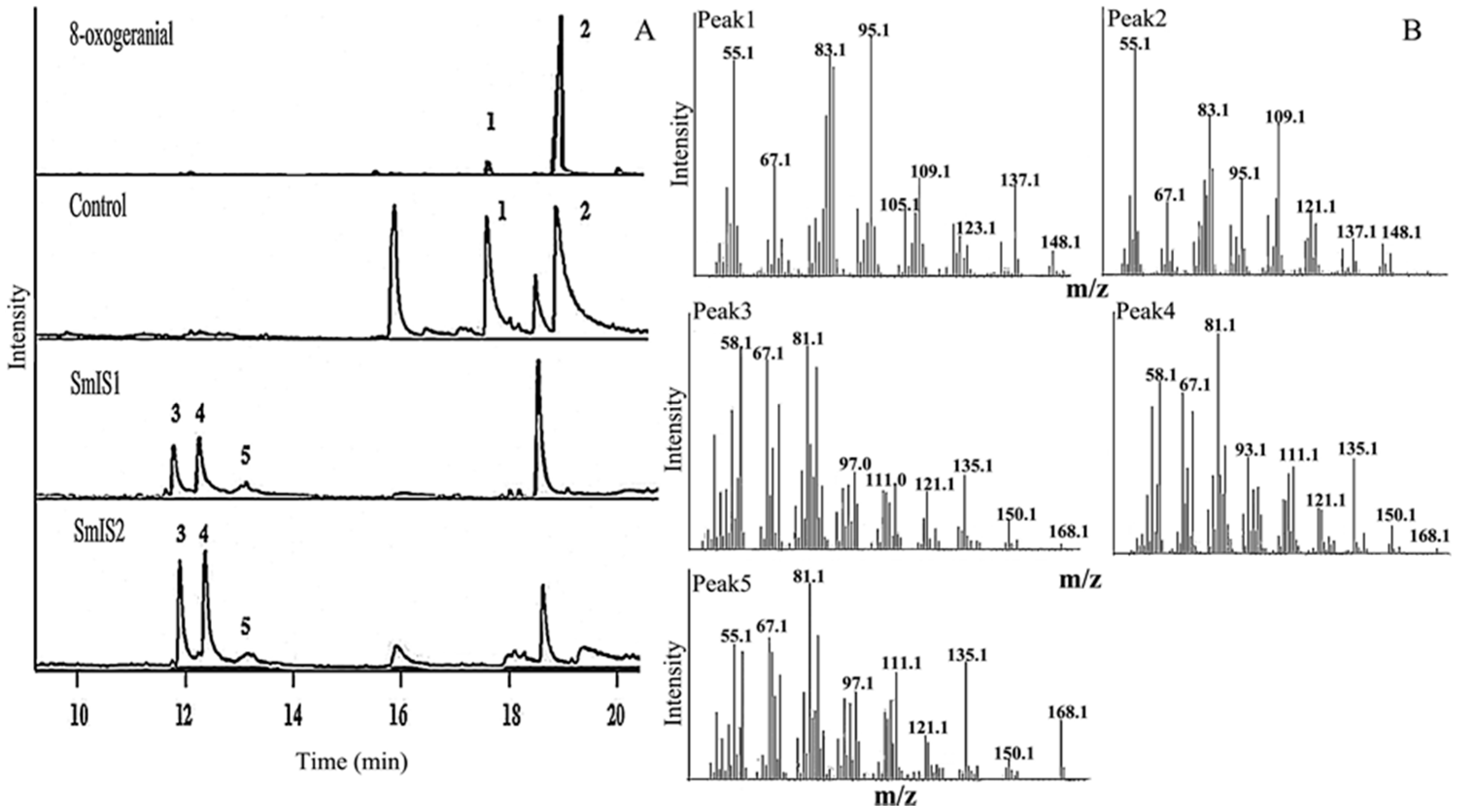
2.2. Functional Characterization and Substrate Preferences of Recombinant SmIS1 and SmIS2
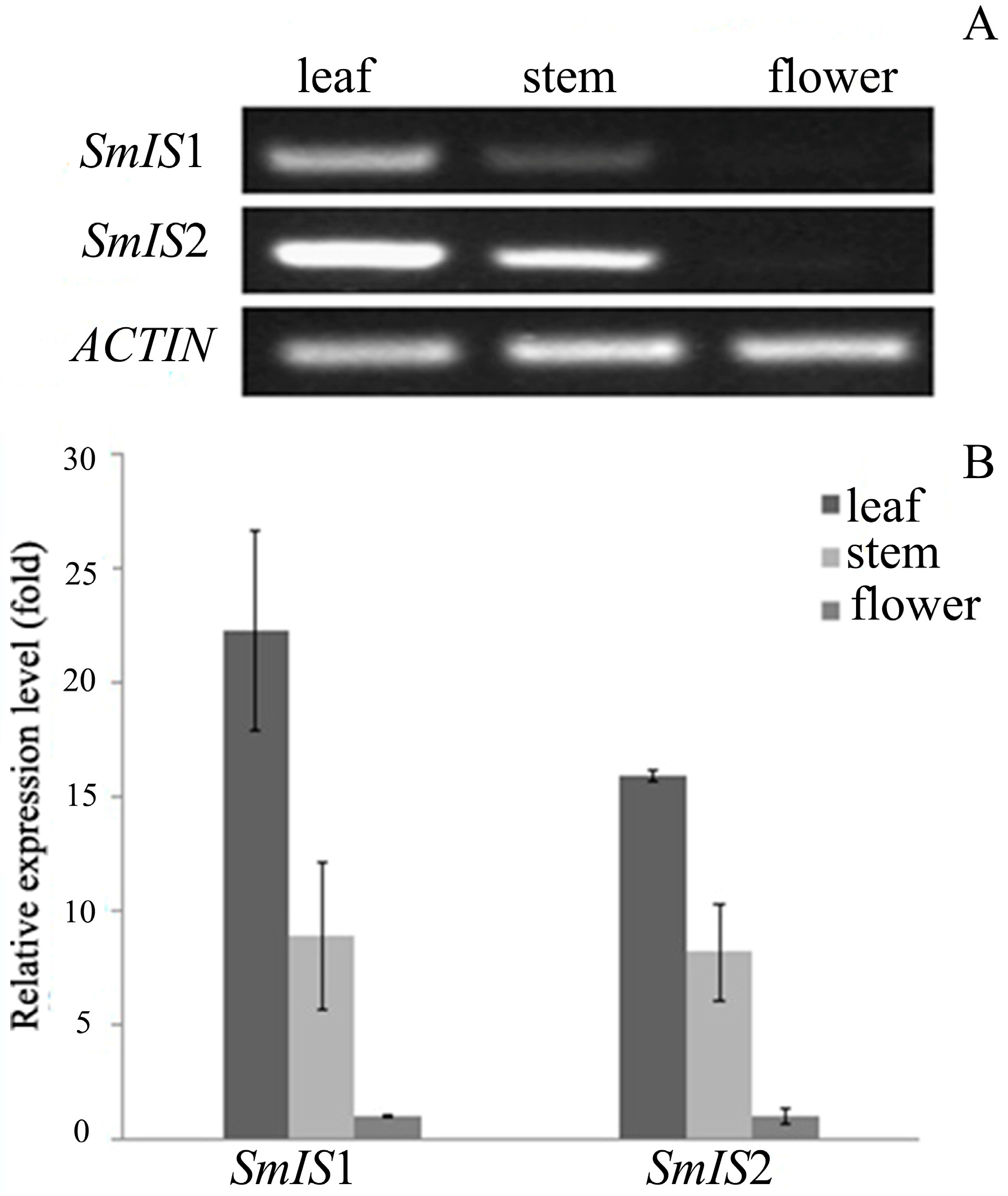
2.3. Tissue Profile of SmIS1 and SmIS2 Accumulation
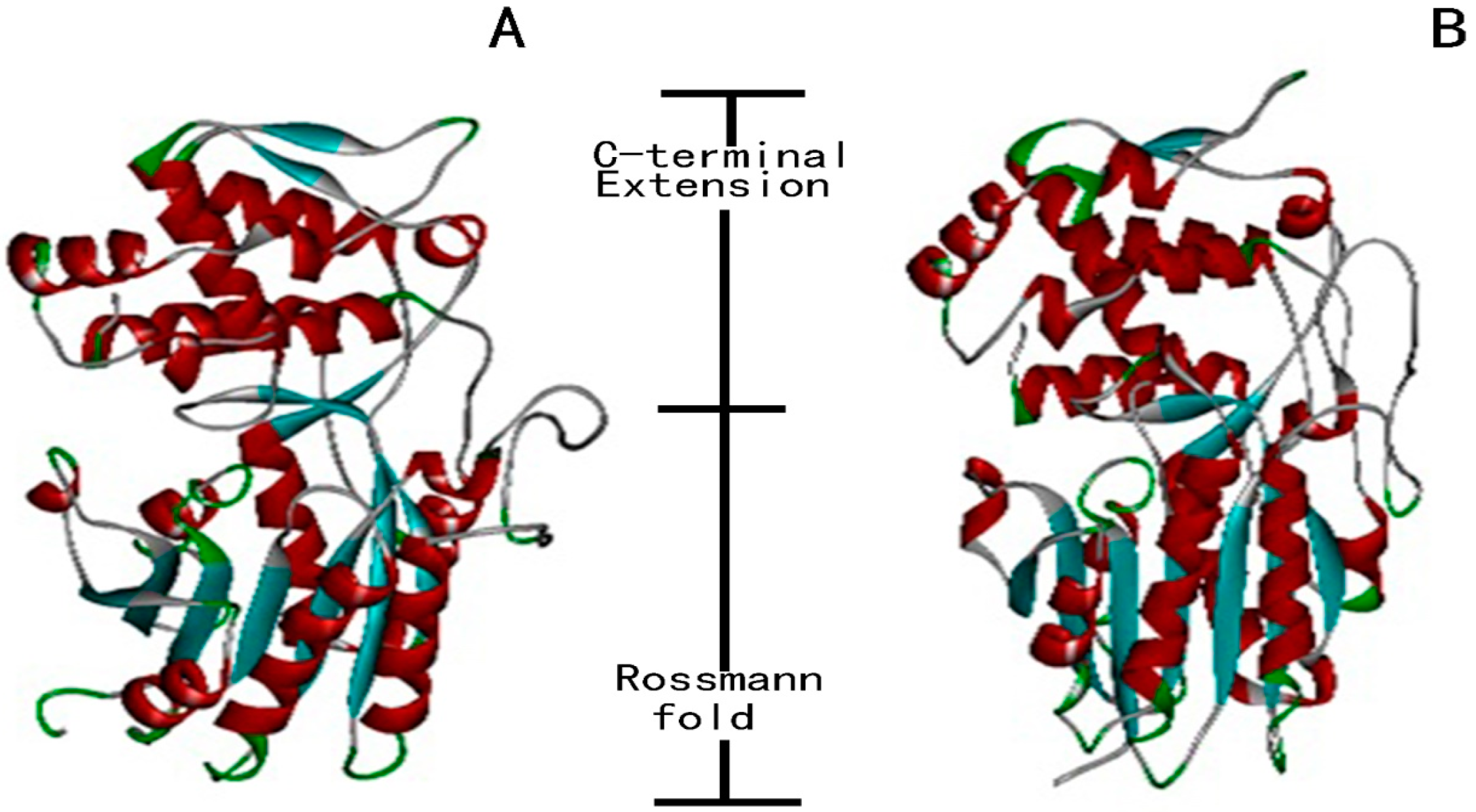
2.4. Prediction of Subcellular Localization of SmIS1 and SmIS2
3. Discussion
4. Materials and Methods
4.1. Plant Materials
4.2. Cloning and Sequencing of S. mussotii SmIS1 and SmIS2 Genes
4.3. Sequence Alignment, Phylogenetic Analysis and Homology Modelling
4.4. Heterologous Expression
4.5. GC-MS-Based Assays
4.6. Enzyme Kinetics of SmIS1 and SmIS2
4.7. Transcript Analysis
4.8. Subcellular Localization Prediction
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Xiang, B.; Li, X.; Qian, J.; Wang, L.; Ma, L.; Tian, X.; Wang, Y. The complete chloroplast genome sequence of the medicinal plant Swertia mussotii using the PacBio RS II platform. Molecules 2016, 21. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Liu, Y.; Cai, Y.; Zhang, F.; Xia, G.; Xiang, F. Cloning and functional analysis of geraniol 10-Hydroxylase, a cytochrome P450 from Franch. Biosci. Biotechnol. Biochem. 2010, 74, 1583–1590. [Google Scholar] [CrossRef] [PubMed]
- Jing, L.; Zhang, Y.J.; Chen, Z.; University, Q.N. Research progress on chemical components and pharmacological actions of Swertia mussotil. Sci. Technol. Qinghai Agric. For. 2014, 5, 55–57. [Google Scholar]
- Ji, L.; Bao, Y.; Chen, G.; Zhao, M.; Sun, H. Determination of active constituents in fifteen species Swertia of genus by high performance liquid chromatography. Acta Bot. Boreali-Occident. Sin. 2004, 24, 1298–1302. [Google Scholar]
- Chang-Liao, W.L.; Chien, C.F.; Lin, L.C.; Tsai, T.H. Isolation of gentiopicroside from Gentianae Radix and its pharmacokinetics on liver ischemia/reperfusion rats. J. Ethnopharmacol. 2012, 141, 668–673. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.B.; Ma, L.; Guo, H.J.; Feng, B.; Guo, Y.Y.; Li, X.Q.; Sun, W.J.; Zheng, L.H.; Zhao, M.G. Gentiopicroside attenuates morphine rewarding effect through down regulation of GluN2B receptors in nucleus accumbens. CNS Neurosci. Ther. 2012, 18, 652–658. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhao, C.; Chang, L.; Xia, G.; Xiang, F. Introgression of Swertia mussotii gene into Bupleurum scorzonerifolium via somatic hybridization. BMC Plant Biol. 2011, 11, 71. [Google Scholar] [CrossRef] [PubMed]
- Kavimani, S.; Manisenthlkumar, K.T. Effect of methanolic extract of Enicostemma littorale on Dalton’s ascitic lymphoma. J. Ethnopharmacol. 2000, 71, 349–352. [Google Scholar] [CrossRef]
- Vaidya, H.; Goyal, R.K.; Cheema, S.K. Anti-diabetic activity of swertiamarin is due to an active metabolite, gentianine, that upregulates PPAR-γ gene expression in 3T3-L1 cells. Phytother. Res. PTR 2013, 27, 624–627. [Google Scholar] [CrossRef] [PubMed]
- Saravanan, S.; Islam, V.I.H.; Babu, N.P.; Pandikumar, P.; Thirugnanasambantham, K.; Chellappandian, M.; Raj, C.S.D.; Paulraj, M.G.; Ignacimuthu, S. Swertiamarin attenuates inflammation mediators via modulating NF-κB/I κB and JAK2/STAT3 transcription factors in adjuvant induced arthritis. Eur. J. Pharm. Sci. Off. J. Eur. Fed. Pharm. Sci. 2014, 56, 70–86. [Google Scholar] [CrossRef] [PubMed]
- Padhan, J.K.; Kumar, V.; Sood, H.; Singh, T.R.; Chauhan, R.S. Contents of therapeutic metabolites in Swertia chirayita correlate with the expression profiles of multiple genes in corresponding biosynthesis pathways. Phytochemistry 2015, 116, 38–47. [Google Scholar] [CrossRef] [PubMed]
- Miettinen, K.; Dong, L.; Navrot, N.; Schneider, T.; Burlat, V.; Pollier, J.; Woittiez, L.; van der Krol, S.; Lugan, R.; Ilc, T.; et al. The seco-iridoid pathway from Catharanthus roseus. Nat. Commun. 2014, 5. [Google Scholar] [CrossRef] [PubMed]
- Geuflores, F.; Sherden, N.H.; Courdavault, V.; Burlat, V.; Glenn, W.S.; Wu, C.; Nims, E.; Cui, Y.; O’Connor, S.E. An alternative route to cyclic terpenes by reductive cyclization in iridoid biosynthesis. Nature 2012, 492, 138–142. [Google Scholar] [CrossRef] [PubMed]
- Lindner, S.; Geuflores, F.; Bräse, S.; Sherden, N.H.; O’Connor, S.E. Conversion of substrate analogs suggests a Michael cyclization in iridoid biosynthesis. Chem. Biol. 2014, 21, 1452–1456. [Google Scholar]
- Munkert, J.; Pollier, J.; Miettinen, K.; van Moerkercke, A.; Payne, R.; Muller-Uri, F.; Burlat, V.; O’Connor, S.E.; Memelink, J.; Kreis, W.; et al. Iridoid synthase activity is common among the plant progesterone 5β-reductase family. Mol. Plant 2015, 8, 136–152. [Google Scholar] [CrossRef] [PubMed]
- Thorn, A.; Egerersieber, C.; Jäger, C.M.; Herl, V.; Mülleruri, F.; Kreis, W.; Muller, Y.A. The crystal structure of progesterone 5beta-reductase from Digitalis lanata defines a novel class of short chain dehydrogenases/reductases. J. Bio. Chem. 2008, 283, 17260–17269. [Google Scholar] [CrossRef] [PubMed]
- Alagna, F.; Geuflores, F.; Kries, H.; Panara, F.; Baldoni, L.; O’Connor, S.E.; Osbourn, A. Identification and characterization of the iridoid synthase involved in oleuropein biosynthesis in olive (Olea europaea) fruits. J. Biol. Chem. 2016, 291, 5542–5554. [Google Scholar] [CrossRef] [PubMed]
- Sadre, R.; Magallaneslundback, M.; Pradhan, S.; Salim, V.; Mesberg, A.; Jones, A.D.; Dellapenna, D. Metabolite diversity in alkaloid biosynthesis: A multi-lane (diastereomer) highway for camptothecin synthesis in Camptotheca acuminata. Plant Cell 2016, 28, 1926–1944. [Google Scholar] [CrossRef] [PubMed]
- Rao, S.T.; Rossmann, M.G. Comparison of super-secondary structures in proteins. J. Mol. Biol. 1973, 76, 241–256. [Google Scholar] [CrossRef]
- Qin, L.; Yun, Z.; Ding, Z.; Zhang, X.; Sheng, Y.; Zhang, R. Structure of iridoid synthase in complex with NADP + /8-oxogeranial reveals the structural basis of its substrate specificity. J. Struct. Biol. 2016, 194, 224–230. [Google Scholar] [CrossRef] [PubMed]
- Xiang, B.; Li, X.; Wang, Y.; Tian, X.; Yang, L.; Ma, L. Cloning and expression of geranyl pyrophosphate synthase gene in Swertia mussotii. Chin. Tradit. Herb. Drugs 2017, 48, 962–970. [Google Scholar]
- Ma, Y.; Chen, G.; Ji, W.; Lu, X. RP-HPLC determination of three medicinal bioactive components in different parts of Swertia mussotii Franch. Chin. J. Pharm. Anal. 2005, 25, 1079–1081. [Google Scholar]
- Shitan, N.; Morita, M.; Yazaki, K. Identification of a nicotine transporter in leaf vacuoles of Nicotiana tabacum. Plant Signal Behav. 2009, 4, 530–532. [Google Scholar] [CrossRef] [PubMed]
- Nour-Eldin, H.H.; Andersen, T.G.; Burow, M.; Madsen, S.R.; Jørgensen, M.E.; Olsen, C.E.; Dreyer, I.; Hedrich, R.; Geiger, D.; Halkier, B.A. NRT/PTR transporters are essential for translocation of glucosinolate defence compounds to seeds. Nature 2012, 488, 531–534. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Xu, M.; Luo, Q.; Wang, J.; Li, H. De novo transcriptome analysis of Liriodendron chinense petals and leaves by Illumina sequencing. Gene 2014, 534, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular evolutionary genetics analysis Version 7.0 for bigger datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef] [PubMed]
- Saitou, N.N.M.; Nei, M.C. The neighbor-joining method—A new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 1987, 4, 406–425. [Google Scholar] [PubMed]
- Jones, D.T.; Taylor, W.R.; Thornton, J.M. The rapid generation of mutation data matrices from protein sequences. Bioinformatics 1992, 8, 275–282. [Google Scholar] [CrossRef]
- Arnold, K.; Bordoli, L.; Schwede, T. The SWISS-MODEL workspace: A web-based environment for protein structure homology modelling. Bioinformatics 2006, 22, 195–201. [Google Scholar] [CrossRef] [PubMed]
- Gavidia, I.; Tarrío, R.; Rodrígueztrelles, F.; Pérezbermúdez, P.; Seitz, H.U. Plant progesterone 5beta-reductase is not homologous to the animal enzyme. Molecular evolutionary characterization of P5betaR from Digitalis purpurea. Phytochemistry 2007, 68, 853–864. [Google Scholar] [CrossRef] [PubMed]
- Horton, P.; Park, K.J.; Obayashi, T.; Fujita, N.; Harada, H.; Adamscollier, C.J.; Nakai, K. WoLF PSORT: Protein localization predictor. Nucleic Acids Res. 2007, 35, 585–587. [Google Scholar] [CrossRef] [PubMed]
- Lan, J.; Guo, D.; Hu, W.; Niu, X. The prediction of a pathogenesis-related secretome of Puccinia helianthithrough high-throughput transcriptome analysis. Bmc. Bioinform. 2017, 18. [Google Scholar] [CrossRef]
- Lee, S.S.; Park, H.J.; Jung, W.Y.; Lee, A.; Yoon, D.H.; You, Y.N.; Kim, H.S.; Kim, B.G.; Ahn, J.C.; Cho, H.S. OsCYP21-4, a novel Golgi-resident cyclophilin, increases oxidative stress tolerance in rice. Front. Plant Sci. 2015, 6. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the Swertia mussotii are available from the authors. |
| Enzyme | Substrate | |||||
|---|---|---|---|---|---|---|
| 8-Oxogeranial | NADPH | |||||
| Km | Kcat | Kcat/Km | Km | Kcat | Kcat/Km | |
| (μM) | (s−1) | (s−1M−1) | (μM) | (s−1) | (s−1M−1) | |
| SmIS1 | 7.29 ± 1.59 | 0.11 ± 0.007 | 15,559.6 ± 4354.3 | 77.94 ± 6.03 | 0.135 ± 0.01 | 1731.7 ± 11.3 |
| SmIS2 | 54.37 ± 4.29 | 0.034 ± 0.001 | 628.6 ± 75.9 | 41.83 ± 7.2 | 0.026 ± 0.01 | 628 ± 104.7 |
| Enzyme | Subcellular Localization Prediction Methods | |||||
|---|---|---|---|---|---|---|
| WOLF PSORT | Predotar | ProtComp | ||||
| SmIS1 | ER | 6 | Mito | 0.09 | Cyto | 8.44 |
| Chlo | 5 | Plastid | 0.01 | ER | 0.13 | |
| Mito | 1 | ER | 0 | Chlo | 0.84 | |
| Plastid | 1 | Else | 0.9 | Vacuolar | 0.38 | |
| SmIS2 | Cyto | 9 | Mito | 0.01 | Cyto | 8.32 |
| Cysk | 3 | Plastid | 0.01 | ER | 0.27 | |
| Plastid | 1 | ER | 0 | Chlo | 0.82 | |
| Else | 0.98 | Vacuolar | 0.43 | |||
| Primer usage | Gene | Forward (5′-3′) | Reverse (5′-3′) |
|---|---|---|---|
| ORF Cloning | SmIS1 | gcggatccATGAGCTGGTGG | cccaagcttCTATGGGATAAATTTG |
| TGGAAAAG | AAGTCTCTC | ||
| SmIS2 | ggaattccatATGAGCTGGTG | ccctcgagAGGAACAATTTTATGA | |
| GTGGACTGGT | GCTTTCATT | ||
| RT-PCR | SmIS1 | AAGCATGAAAAAAAGC | CAATATATCCCACCATTTCCAT |
| CATTAGT | |||
| SmIS2 | AAGCATGAAAATGTTCC | ATTCTCGTCGAATTCCACGTA | |
| TTTCAC | |||
| Actin | ACTGGTGTTATGGTTGG | TCGGTGAGAAGTATAGGGTGCGG | |
| TATTGG | |||
| qRT-PCR | SmIS1 | AAGCATGAAAAAAAGC | CAATATATCCCACCATTTCCAT |
| CATTAGT | |||
| SmIS2 | AAGCATGAAAATGTTCC | ATTCTCGTCGAATTCCACGTA | |
| TTTCAC | |||
| Actin | ACTGGTGTTATGGTTGG | TCGGTGAGAAGTATAGGGTGC | |
| TATGG |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiang, B.; Li, X.; Wang, Y.; Tian, X.; Yang, Z.; Ma, L.; Liu, X.; Wang, Y. Cloning and Characterization of Two Iridoid Synthase Homologs from Swertia Mussotii. Molecules 2017, 22, 1387. https://doi.org/10.3390/molecules22081387
Xiang B, Li X, Wang Y, Tian X, Yang Z, Ma L, Liu X, Wang Y. Cloning and Characterization of Two Iridoid Synthase Homologs from Swertia Mussotii. Molecules. 2017; 22(8):1387. https://doi.org/10.3390/molecules22081387
Chicago/Turabian StyleXiang, Beibei, Xiaoxue Li, Yan Wang, Xiaoxuan Tian, Zhen Yang, Lin Ma, Xia Liu, and Yong Wang. 2017. "Cloning and Characterization of Two Iridoid Synthase Homologs from Swertia Mussotii" Molecules 22, no. 8: 1387. https://doi.org/10.3390/molecules22081387
APA StyleXiang, B., Li, X., Wang, Y., Tian, X., Yang, Z., Ma, L., Liu, X., & Wang, Y. (2017). Cloning and Characterization of Two Iridoid Synthase Homologs from Swertia Mussotii. Molecules, 22(8), 1387. https://doi.org/10.3390/molecules22081387







