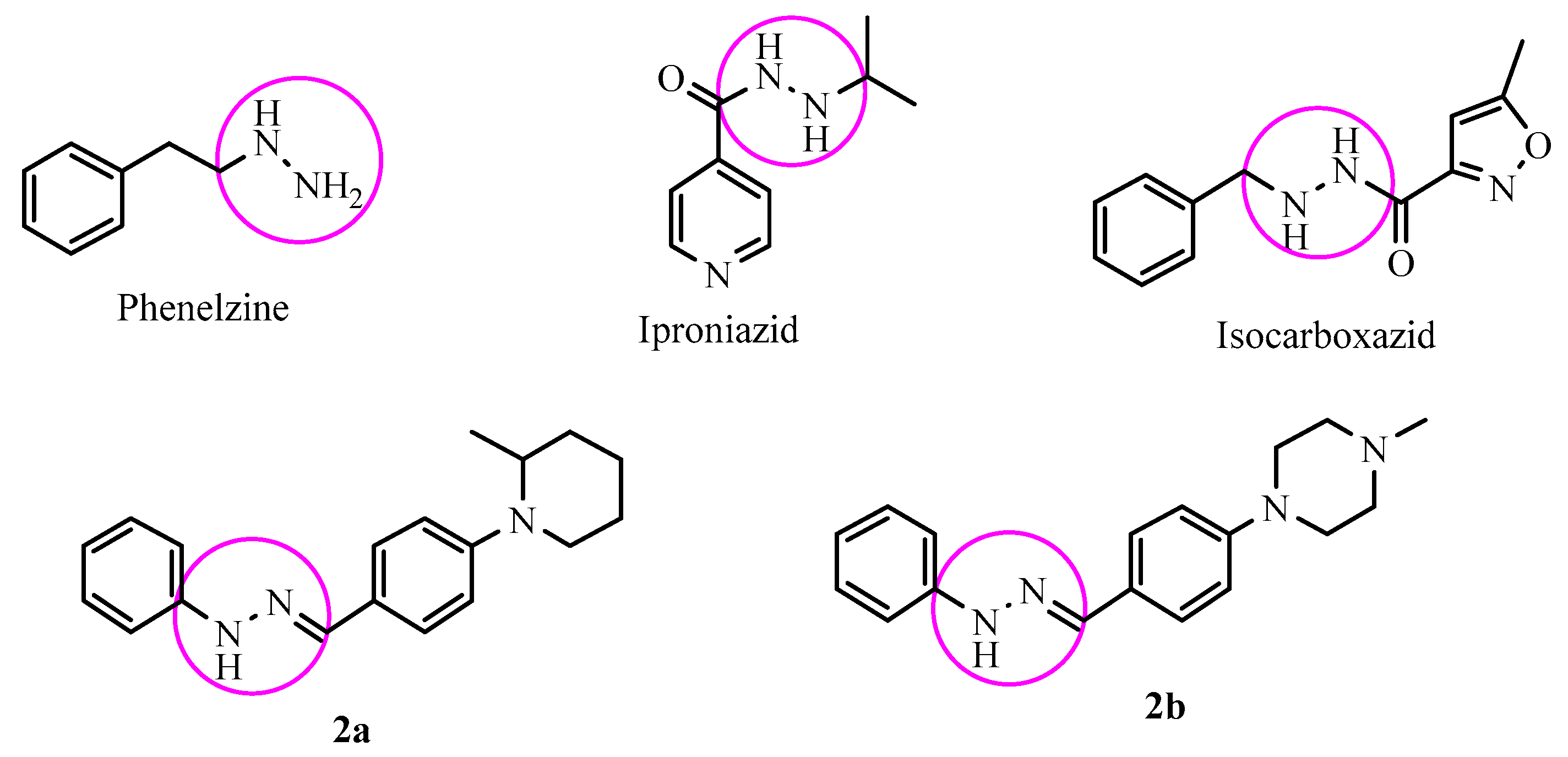
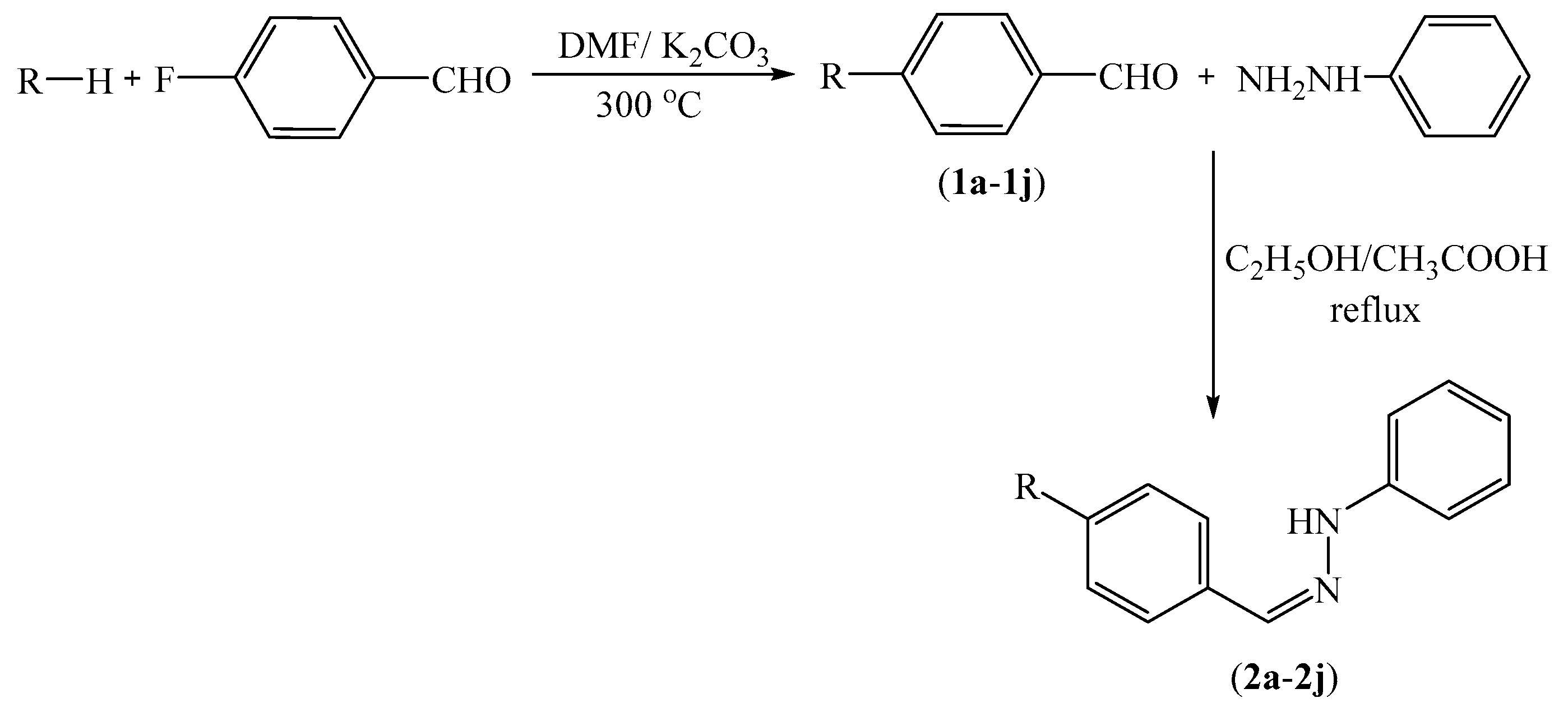
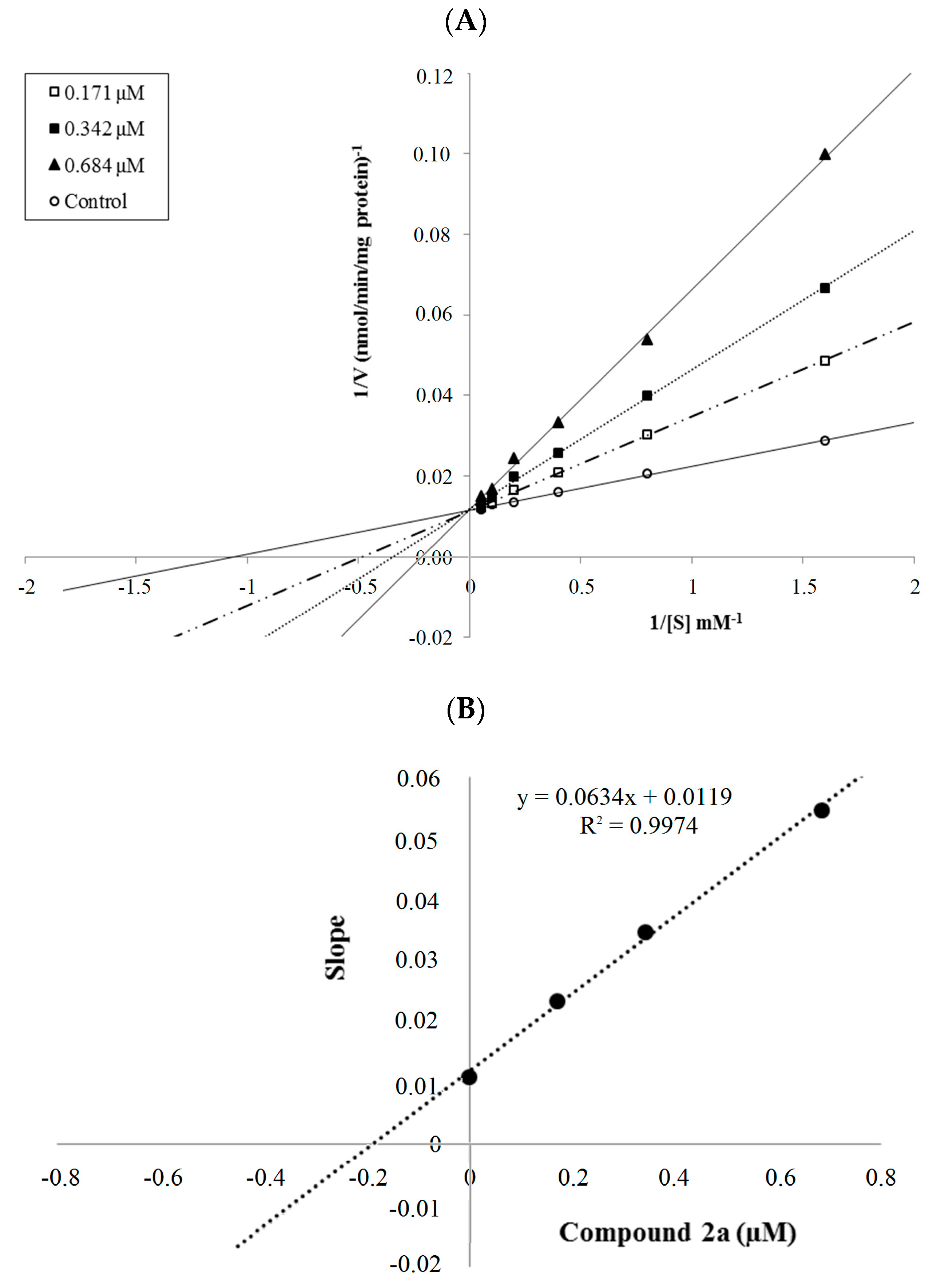
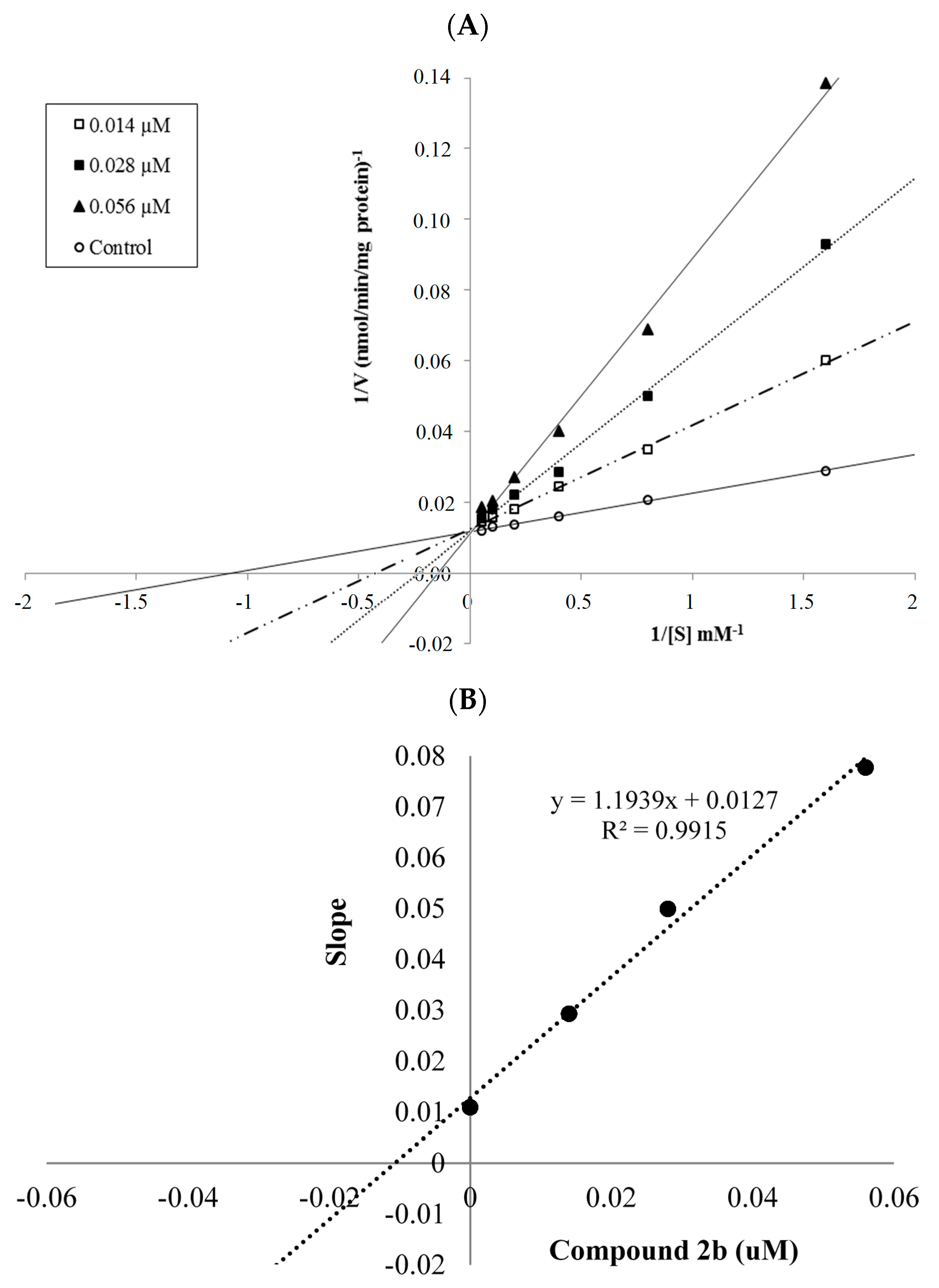
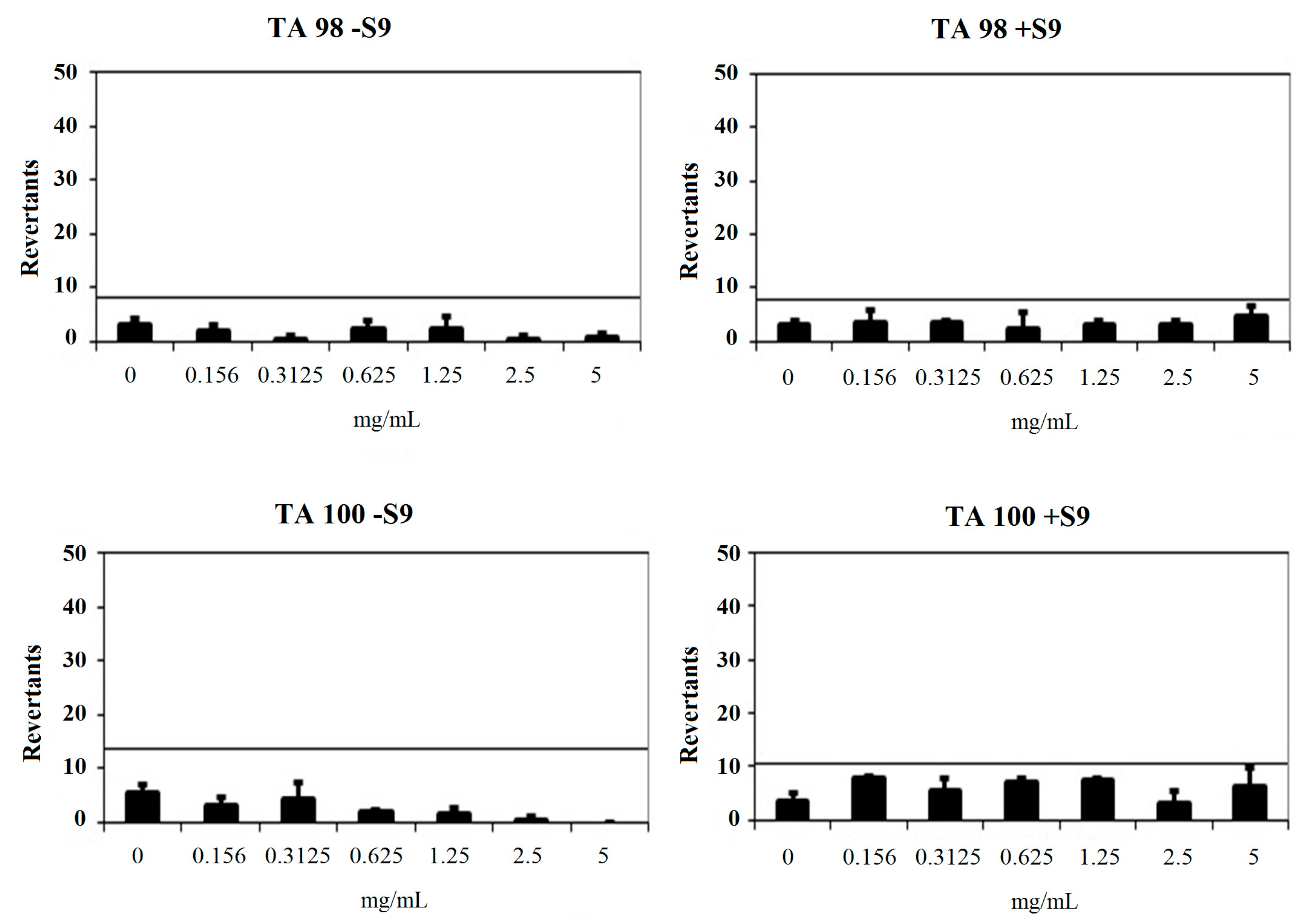
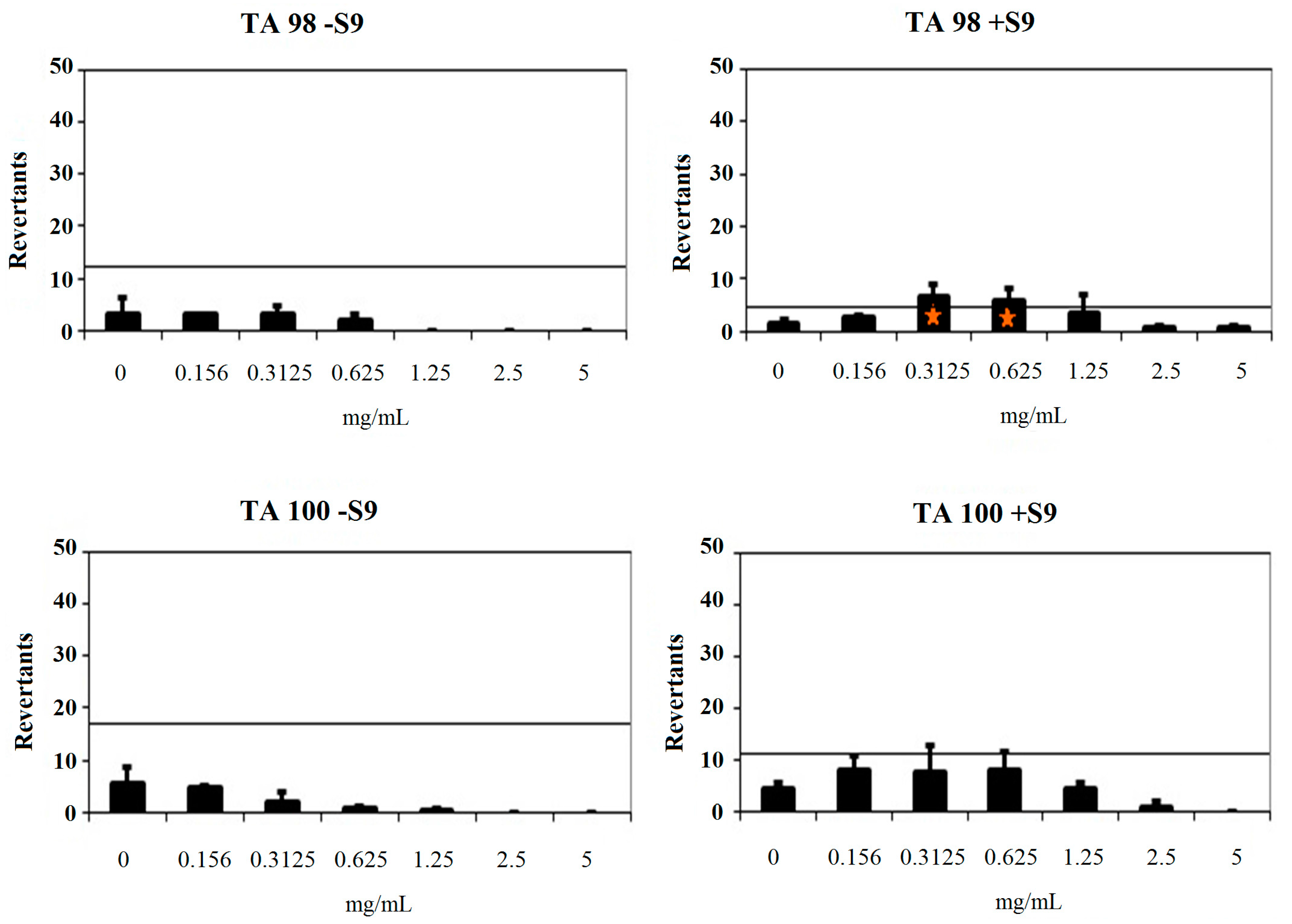
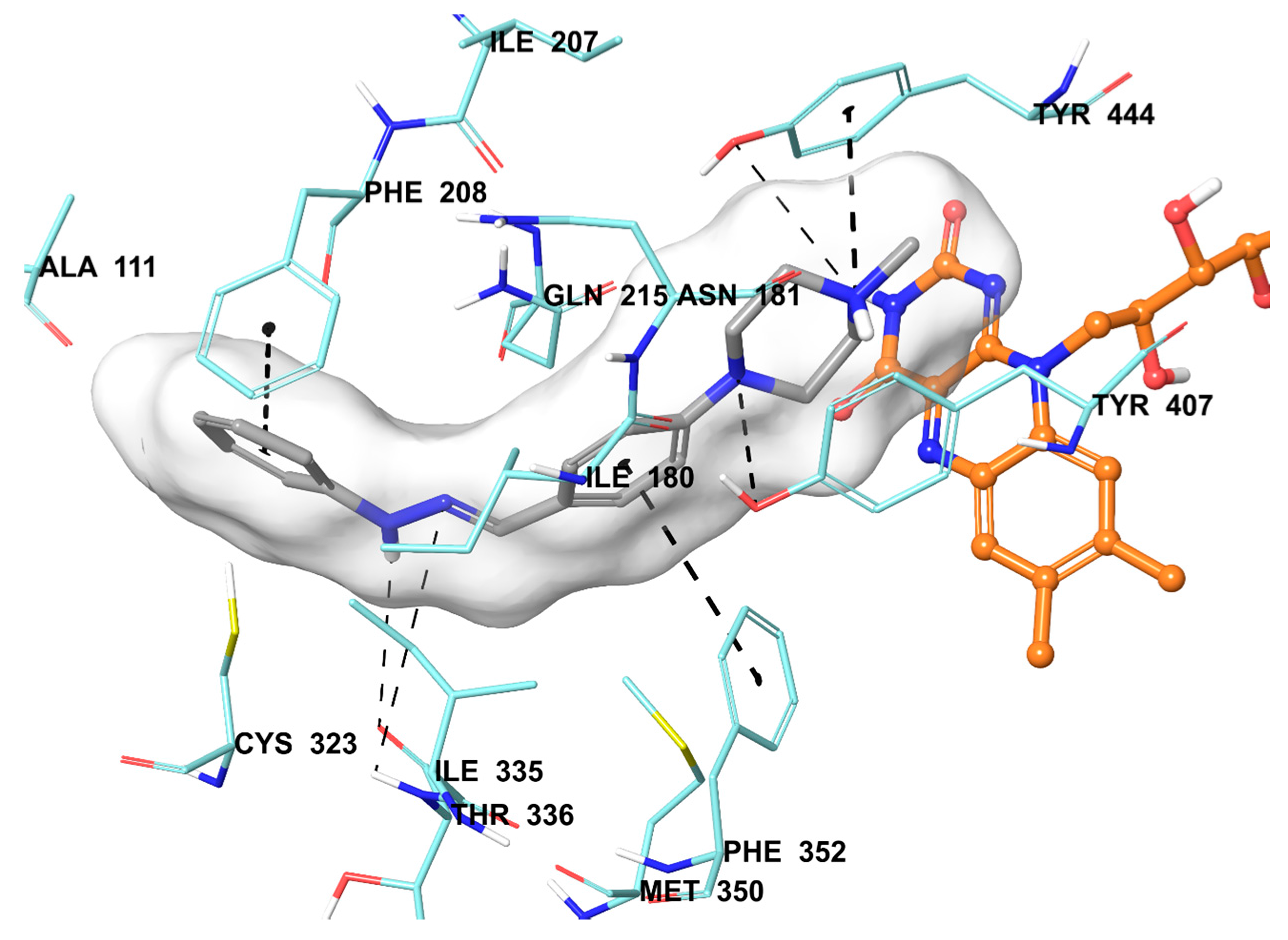
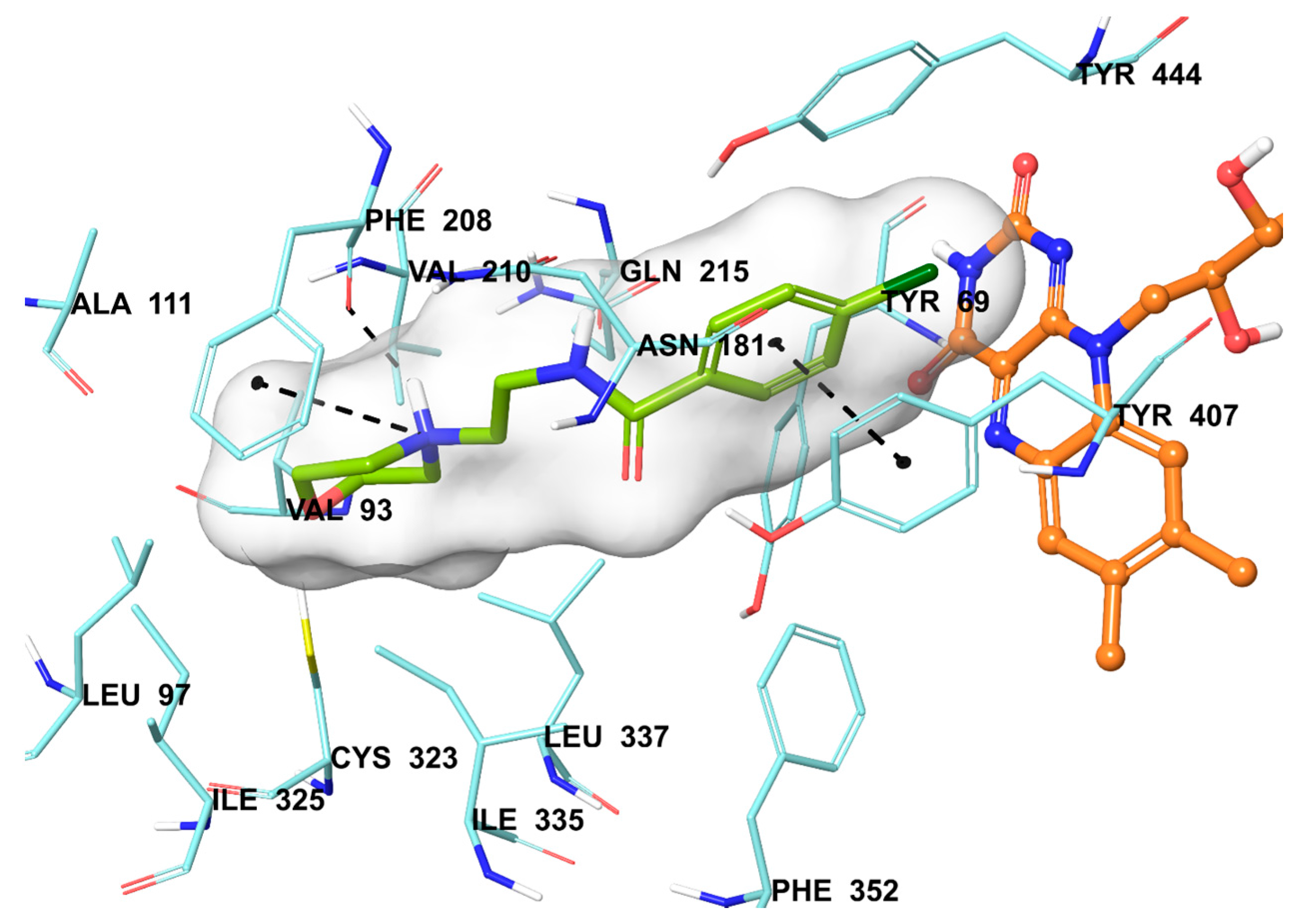
3.2.2. General Procedure for the Synthesis of Target Compounds 2a–2n
Phenylhydrazine, the appropriate 4-substituted benzaldehyde derivative 1a–1n and catalytic quantity of acetic acid were refluxed in EtOH for 2 h. The mixture was cooled, precipitated product was filtered, dried, and recrystallized from EtOH.
1-(4-(2-Methypiperidin-1-yl)benzylidene)-2-phenylhydrazine (2a). Yield: 85%, M.P. = 180.1–185.2 °C, FTIR (ATR, cm−1): 3269 (N-H), 2933 (C-H), 1251 (C-N), 823, 748. 1H-NMR: δ = 0.97 (3H, d, J = 6.63 Hz, -CH3), 1.54–1.59 (4H, m, piperidine), 1.71–1.74 (4H, m, piperidine), 2.83–2.89 (1H, m, -CH-), 6.68 (1H, t, J = 7.26 Hz, monosubstituted benzene H4), 6.89 (2H, d, J = 8.85 Hz, disubstituted benzene CH), 7.01 (2H, d, J = 7.53 Hz, monosubstituted benzene H2,2’), 7.18 (2H, t, J = 7.89 Hz, monosubstituted benzene H3,3’), 7.46 (2H, d, J = 8.85 Hz, disubstituted benzene CH), 7.76 (1H, s, -CH=N-), 10.02 (1H, s, NH). 13C-NMR: δ = 13.32, 18.92, 25.94, 31.06, 42.56, 49.69, 112.12, 115.80, 118.43, 125.72, 127.22, 129.47, 137.83, 146.23, 150.99. HRMS (m/z): [M + H]+ calcd for C19H23N3: 294.1965; found: 294.1966.
1-(4-(4-Methylpiperazine-1-yl)benzylidene)-2-phenylhydrazine (2b). Yield: 83%, M.P. = 166.8–180.9 °C, FTIR (ATR, cm−1): 3313 (N-H), 2933 (C-H), 1253 (C-N), 815, 759. 1H-NMR: δ = 2.77 (3H, s, -CH3), 3.27 (4H, br.s, piperazine), 3.49 (4H, br.s, piperazine), 6.70 (1H, t, J = 7.23 Hz, monosubstituted benzene H4), 6.99–7.05 (4H, m, monosubstituted benzene H2,2’, disubstituted benzene CH), 7.19 (2H, t, J = 7.29 Hz, monosubstituted benzene H3,3’), 7.53 (2H, d, J = 8.76 Hz, disubstituted benzene CH), 7.83 (1H, s, -CH=N-), 10.25 (1H, s, NH). 13C-NMR: δ = 42.46, 45.60, 52.43, 112.22, 116.19, 118.65, 127.15, 127.92, 129.49, 137.19, 146.10, 149.78. HRMS (m/z): [M + H]+ calcd for C18H22N4: 295.1917; found: 295.1924.
1-(4-(4-Phenylpiperazine-1-yl)benzylidene)-2-phenylhydrazine (2c). Yield: 86%, M.P. = 149.4–160.9 °C, FTIR (ATR, cm−1): 3313 (N-H), 2933 (C-H), 1253 (C-N), 815, 7.59. 1H-NMR: δ = 3.26-3.27 (4H, m, piperazine), 3.31–3.33 (4H, m, piperazine), 6.71 (1H, t, J = 7.20 Hz, monosubstituted benzene H4), 6.81 (1H, t, J = 7.23 Hz, monosubstituted benzene H4), 6.97–7.06 (6H, m, monosubstituted benzene CH, disubstituted benzene CH), 7.18–7.26 (4H, m, monosubstituted benzene CH), 7.53 (2H, d, J = 8.79 Hz, disubstituted benzene CH), 7.80 (1H, s, -CH=N-), 10.08 (1H, s, NH). 13C-NMR: δ = 48.32, 48.70, 112.20, 115.76, 116.14, 118.60, 119.63, 127.04, 127.17, 129.45, 129.52, 137.55, 146.15, 151.12, 151.35. HRMS (m/z): [M + H]+ calcd for C23H24N4: 357.2074; found: 357.2057.
1-(4-(4-(4-Methoxyphenyl)piperazine-1-yl)benzylidene)-2-phenylhydrazine (2d). Yield: 81%, M.P. = 141.2–150.4 °C, FTIR (ATR, cm−1): 3302 (N-H), 2951 (C-H), 1273 (C-N), 819, 750. 1H-NMR: δ = 3.14–3.16 (4H, m, piperazine), 3.31–3.33 (4H, m, piperazine), 3.69 (3H, s, -OCH3), 6.70 (1H, t, J = 7.23 Hz, monosubstituted benzene H4), 6.84 (2H, d, J = 9.10 Hz, methoxyphenyl CH), 6.96 (2H, d, J = 9.10 Hz, methoxyphenyl CH), 6.99–7.03 (4H, m, monosubstituted benzene H2,2’, disubstituted benzene CH), 7.19 (2H, t, J = 8.37 Hz, monosubstituted benzene H3,3’), 7.52 (2H, d, J = 8.76 Hz, disubstituted benzene CH), 7.79 (1H, s, -CH=N-), 10.06 (1H, s, NH). 13C-NMR: δ = 48.44, 50.18, 55.66, 112.18, 114.75, 115.73, 118.20, 118.59, 126.98, 127.15, 129.51, 137.56, 145.72, 146.14, 151.17, 151.63. HRMS (m/z): [M + H]+ calcd for C24H26N4O: 387.2179; found: 387.2166.
1-(4-(4-Methoxyphenoxy)benzylidene)-2-phenylhydrazine (2e). Yield: 83%, M.P. = 170.9–174.9 °C, FTIR (ATR, cm−1): 3302 (N-H), 2924 (C-H), 1255 (C-N), 831, 744. 1H-NMR: δ = 3.76 (3H, s, -OCH3), 6.72 (1H, t, J = 7.23 Hz, monosubstituted benzene H4), 6.93 (2H, d, J = 8.64 Hz, methoxyphenyl CH), 6.96–7.05 (6H, m, monosubstituted benzene H2,2’, disubstituted benzene CH, methoxyphenyl CH), 7.20 (2H, t, J = 8.10 Hz, monosubstituted benzene H3,3’), 7.61 (2H, d, J = 8.67 Hz, Disubstituted benzene CH), 7.83 (1H, s, -CH=N-), 10.23 (1H, s, NH). 13C-NMR: δ = 55.89, 112.33, 115.58, 117.87, 118.98, 121.29, 127.67, 129.54, 130.89, 136.49, 145.87, 149.60, 156.22, 158.40. HRMS (m/z): [M + H]+ calcd for C20H18N2O2: 319.1441; found: 319.1448
1-(4-((4-Methoxyphenyl)thio)benzylidene)-2-phenylhydrazine (2f). Yield: 85%, M.P. = 178.1–184.3 °C, FTIR (ATR, cm−1): 3300 (N-H), 2956 (C-H), 1244 (C-N), 815, 742. 1H-NMR: δ = 3.79 (3H, s, -OCH3), 6.74 (1H, t, J = 7.26 Hz, monosubstituted benzene H4), 6.99–7.06 (4H, m, monosubstituted benzene H2,2’, disubstituted benzene CH), 7.13 (2H, d, J = 8.43 Hz, methoxyphenyl CH), 7.20 (2H, t, J = 7.32 Hz, monosubstituted benzene H3,3’), 7.43 (2H, d, J = 8.85 Hz, disubstituted benzene CH), 7.56 (2H, d, J = 8.46 Hz, methoxyphenyl CH), 7.80 (1H, s, -CH=N-), 10.33 (1H, s, NH). 13C-NMR: δ = 55.80, 112.44, 115.87, 119.24, 123.42, 126.79, 128.50, 129.56, 134.28, 135.64, 136.14, 137.73, 145.64, 160.19. HRMS (m/z): [M + H]+ calcd for C20H18N2OS: 335.1213; found: 335.1207.
1-(4-(4-Fluorophenoxy)benzylidene)-2-phenylhydrazine (2g). Yield: 81%, M.P. = 121.3–124.9 °C, FTIR (ATR, cm−1): 3315 (N-H), 2951 (C-H), 1251 (C-N), 821, 752. 1H-NMR: δ = 6.73 (1H, t, J = 7.23 Hz, monosubstituted benzene H4), 6.99 (2H, d, J = 8.73 Hz, monosubstituted benzene H2,2’), 7.03–7.12 (4H, m, disubstituted benzene CH, fluorophenyl CH), 7.18–7.28 (4H, m, monosubstituted benzene H3,3’, fluorophenyl CH), 7.65 (2H, d, J = 8.76 Hz, disubstituted benzene CH), 7.85 (1H, s, -CH=N-), 10.27 (1H, s, NH). 13C-NMR: δ = 112.36, 116.94, 117.25, 118.88 (2JCF = 26.34 Hz), 121.28 (3JCF = 8.54 Hz), 127.76, 129.55, 131.62, 136.30, 145.82, 152.82, 157.42, 158.78 (1JCF = 238.19 Hz). HRMS (m/z): [M + H]+ calcd for C19H15FN2O: 307.1241; found: 307.1235.
1-(4-((4-Fluorophenyl)thio)benzylidene)-2-phenylhydrazine (2h). Yield: 80%, M.P. = 132.3–133.9 °C, FTIR (ATR, cm−1): 3325 (N-H), 2958 (C-H), 1257 (C-N), 831, 752. 1H-NMR: δ = 6.75 (1H, t, J = 7.23 Hz, monosubstituted benzene H4), 7.06 (2H, d, J = 8.00 Hz, monosubstituted benzene H2,2’), 7.20 (2H, d, J = 7.32 Hz, disubstituted benzene CH), 7.23–7.29 (4H, m, monosubstituted benzene H3,3’, fluorophenyl CH), 7.42–7.47 (2H, m, fluorophenyl CH), 7.62 (2H, d, J = 8.00 Hz, disubstituted benzene CH), 7.82 (1H, s, -CH=N-), 10.40 (1H, s, NH). 13C-NMR: δ = 112.49, 117.08, 117.32, 119.36, 126.99, 129.59, 130.42 (2JCF = 26.74 Hz), 134.33 (3JCF = 8.34 Hz), 135.20, 135.34, 135.87, 145.56, 162.29 (1JCF = 244.11 Hz). HRMS (m/z): [M + H]+ calcd for C19H15FN2S: 323.1013; found: 323.1001.
1-(4-(1-Imidazolyl)benzylidene)-2-phenylhydrazine (2i). Yield: 85%, M.P. = 201.9–208.9 °C, FTIR (ATR, cm−1): 3350 (N-H), 2951 (C-H), 1269 (C-N), 906, 759. 1H-NMR: δ = 6.77 (1H, t, J = 7.17 Hz, monosubstituted benzene H4), 7.12 (2H, d, J = 7.50 Hz, monosubstituted benzene H3,3’), 7.22 (2H, t, J = 7.29 Hz, monosubstituted benzene H2,2’), 7.54 (1H, s, imidazole CH), 7.75 (2H, d, J = 8.76 Hz, disubstituted benzene CH), 7.82 (2H, d, J = 8.76 Hz, disubstituted benzene CH), 7.95 (1H, s, -CH=N-) 8.07 (1H, m, imidazole CH), 9.08 (1H, s, imidazole CH), 10.68 (1H, s, NH). 13C-NMR: δ = 112.61, 119.48, 119.80, 121.84, 125.67, 127.19, 129.59, 135.26, 135.33, 135.38, 136.33, 145.55. HRMS (m/z): [M + H]+ calcd for C16H14N4: 263.1291; found: 263.1295.
1-(4-(1H-1,2,4-triazole-1-yl)benzylidene)-2-phenylhydrazine (2j). Yield: 79%, M.P. = 127.4–132.2 °C, FTIR (ATR, cm−1): 3319 (N-H), 2951 (C-H), 1228 (C-N), 829, 756. 1H-NMR: δ = 6.77 (1H, t, J = 7.23 Hz, monosubstituted benzene H4), 7.10 (2H, d, J = 7.53 Hz, monosubstituted benzene H3,3’), 7.23 (2H, t, J = 7.26 Hz, monosubstituted benzene H2,2’), 7.26 (1H, s, triazole CH), 7.81 (2H, d, J = 8.76 Hz, disubstituted benzene CH), 7.88 (2H, d, J = 9.15 Hz, disubstituted benzene CH), 8.25 (1H, s, -CH=N-), 9.33 (1H, s, triazole CH), 10.47 (1H, s, NH). 13C-NMR: δ =112.61, 119.48, 119.80, 121.84, 125.67, 127.19, 129.59, 135.26, 135.38, 136.33, 145.55. HRMS (m/z): [M + H]+ calcd for C15H13N5: 264.1244; found: 264.1230.
1-(4-((4-Chlorophenyl)thio)benzylidene)-2-phenylhydrazine (2k). Yield: 85%, M.P. = 164.4–165.7 °C, FTIR (ATR, cm−1): 3223 (N-H), 2912 (C-H), 1255 (C-N), 827, 744. 1H-NMR: δ = 6.76 (1H, t, J = 7.26 Hz, monosubstituted benzene H4), 7.07 (2H, d, J = 7.59 Hz, monosubstituted benzene H2,2’), 7.19–7.24 (2H, m, monosubstituted benzene H3,3’), 7.32 (2H, d, J = 8.64 Hz, disubstituted benzene CH), 7.36 (2H, d, J = 8.40 Hz, disubstituted benzene CH), 7.43 (2H, d, J = 8.64 Hz, disubstituted benzene CH), 7.66 (2H, d, J = 8.40 Hz, disubstituted benzene CH), 7.85 (1H, s, -CH=N-), 10.43 (1H, s, NH). 13C-NMR: δ = 112.55, 119.44, 127.13, 129.59, 129.59, 129.96, 132.12, 132.18, 132.40, 133.38, 134.94, 135.76, 136.07, 145.53. ESI-MS (M+H): C19H15ClN2S: 339.10.
1-(4-(Benzylpiperidine)benzylidene)-2-phenylhydrazine (2l). Yield: 85%, M.P. = 175.4–178.3 °C, FTIR (ATR, cm−1): 3223 (N-H), 2816 (C-H), 1247 (C-N), 817, 740. 1H-NMR: δ = 1.24–1.28 (2H, m, piperidine), 1.61–1.65 (3H, m, piperidine), 2.51–2.54 (2H, m, piperidine), 2.59–2.67 (2H, m, piperidine), 3.70–3.74 (2H, d, J = 12.57 Hz, -CH2-), 6.69 (1H, t, J = 7.23 Hz, monosubstituted benzene H4), 6.90 (2H, d, J = 8.85 Hz, disubstituted benzene CH), 7.02 (2H, d, J = 7.56 Hz, monosubstituted benzene H2,2’), 7.16–7.21 (5H, m), 7.26–7.31 (2H, m), 7.46 (2H, d, J = 8.79 Hz, disubstituted benzene CH), 7.77 (1H, s, -CH=N-), 9.99 (1H, s, NH). 13C-NMR): δ = 31.66, 37.74, 42.73, 48.71, 112.17, 115.71, 118.51, 126.13, 126.24, 127.16, 128.61, 129.48, 129.49, 137.77, 140.66, 146.21, 151.44. ESI-MS (M + H): C25H27N3: 370.30.
1-(4-(2-Dimethylaminoethyl)piperazine)benzylidene)-2-phenylhydrazine (2m). Yield: 87%, M.P. = 140.7–143.7 °C, FTIR (ATR, cm−1): 3223 (N-H), 2823 (C-H), 1253 (C-N), 821, 744. 1H-NMR: δ = 2.15 (6H, s, -CH3), 2.35-2.39 (2H, m, -CH2-), 2.41-2.46 (2H, m, -CH2-), 2.51–2.55 (4H, m, piperazine), 3.15–3.18 (4H, m, piperazine), 6.69 (1H, t, J = 7.23 Hz, monosubstituted benzene H4), 6.93 (2H, d, J = 8.76 Hz, disubstituted benzene CH), 7.01 (2H, d, J = 7.65 Hz, monosubstituted benzene H2,2’), 7.18 (2H, t, J = 7.82 Hz, monosubstituted benzene H3,3’), 7.48 (2H, d, J = 8.76 Hz, disubstituted benzene CH), 7.77 (1H, s, -CH=N-), 10.01 (1H, s, NH). 13C-NMR: δ = 46.04, 48.24, 53.47, 56.34, 57.15, 112.19, 115.41, 118.57, 126.65, 127.12, 129.50, 137.68, 146.18, 151.29. ESI-MS (M + H): C21H29N5: 352.35.
1-(4-(3-Dimethylaminopropyl)piperazine)benzylidene)-2-phenylhydrazine (2n). Yield: 84%, M.P. = 142.6–145.5 °C, FTIR (ATR, cm−1): 3217 (N-H), 2823 (C-H), 1267 (C-N), 821, 744. 1H-NMR: δ = 1.59 (2H, p, J = 7.56 Hz, -CH2-), 2.15 (6H, s, -CH3), 2.22–2.35 (4H, m, -CH2-), 2.47-2.49 (4H, m, piperazine), 3.16–3.19 (4H, m, piperazine), 6.69 (1H, t, J = 7.23 Hz, monosubstituted benzene H4), 6.93 (2H, d, J = 8.76 Hz, disubstituted benzene CH), 7.01 (2H, d, J = 7.59 Hz, monosubstituted benzene H2,2’), 7.18 (2H, t, J = 7.38 Hz, monosubstituted benzene H3,3’), 7.48 (2H, d, J = 8.76 Hz, disubstituted benzene CH), 7.78 (1H, s, -CH=N-), 10.04 (1H, s, NH). 13C-NMR: δ = 24.75, 45.50, 48.22, 53.16, 56.41, 57.67, 112.18, 115.41, 118.54, 126.66, 127.11, 129.48, 137.65, 146.17, 151.27. ESI-MS (M + H): C22H31N5: 366.35.