Tissue-Specific Accumulation of Sulfur Compounds and Saponins in Different Parts of Garlic Cloves from Purple and White Ecotypes
Abstract
1. Introduction
2. Results and Discussion
3. Materials and Methods
3.1. Plant Materials
3.2. HPLC-MS
3.3. Bioinformatics
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Dixon, R.A.; Strack, D. Phytochemistry meets genome analysis, and beyond. Phytochemistry 2003, 62, 815–816. [Google Scholar] [CrossRef]
- Lewinsohn, E.; Gijzen, M. Phytochemical diversity: The sounds of silent metabolism. Plant Sci. 2009, 176, 161–169. [Google Scholar] [CrossRef]
- Rivlin, R.S. Historical perspective on the use of garlic. J. Nutr. 2001, 131, 951S–954S. [Google Scholar] [PubMed]
- Qidwai, W.; Ashfaq, T. Role of garlic usage in cardiovascular disease prevention: An evidence-based approach. Evid. Based Complement. Alternat. Med. 2013, 2013, 125649. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.Y.; Tian, F.M.; Hu, W.N.; Zhang, J.H.; Cai, H.F.; Li, N. Apoptosis of human gastric cancer cells line SGC 7901 induced by garlic-derived compound S-allylmercaptocysteine (SAMC). Eur. Rev. Med. Pharmacol. Sci. 2013, 17, 745–751. [Google Scholar] [PubMed]
- Chen, J.; Cheong, K.; Song, Z.; Shi, Y.; Huang, X. Structure and protective effect on UVB-induced keratinocyte damage of fructan from white garlic. Carbohydr. Polym. 2013, 92, 200–205. [Google Scholar] [CrossRef] [PubMed]
- Berginc, K.; Milisav, I.; Kristl, A. Garlic flavonoids and organosulfur compounds: Impact on the hepatic pharmacokinetics of saquinavir and darunavir. Drug Metab. Pharmacokinet. 2010, 25, 521–530. [Google Scholar] [CrossRef] [PubMed]
- Patra, A.K.; Yu, Z. Effects of garlic oil, nitrate, saponin and their combinations supplemented to different substrates on in vitro fermentation, ruminal methanogenesis, and abundance and diversity of microbial populations. J. Appl. Microbiol. 2015, 119, 127–138. [Google Scholar] [CrossRef] [PubMed]
- Amagase, H. Clarifying the real bioactive constituents of garlic. J. Nutr. 2006, 136 (Suppl. 3), 716S–725S. [Google Scholar] [PubMed]
- Martins, N.; Petropoulos, S.; Ferreira, I.C. Chemical composition and bioactive compounds of garlic (Allium sativum L.) as affected by pre-and post-harvest conditions: A review. Food Chem. 2016, 211, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Lanzotti, V.; Scala, F.; Bonanomi, G. Compounds from Allium species with cytotoxic and antimicrobial activity. Phytochem. Rev. 2014, 13, 769–791. [Google Scholar] [CrossRef]
- Borlinghaus, J.; Albrecht, F.; Gruhlke, M.C.; Nwachukwu, I.D.; Slusarenko, A.J. Allicin: Chemistry and biological properties. Molecules 2014, 19, 12591–12618. [Google Scholar] [CrossRef] [PubMed]
- Kopec, A.; Piatkowska, E.; Leszczynska, T.; Sikora, E. Healthy properties of garlic. Curr. Nutr. Food Sci. 2013, 9, 59–64. [Google Scholar]
- Chung, L.Y. The antioxidant properties of garlic compounds: Allyl cysteine, alliin, allicin, and allyl disulfide. J. Med. Food 2006, 9, 205–213. [Google Scholar] [CrossRef] [PubMed]
- Slusarenko, A.J.; Patel, A.; Portz, D. Control of plant diseases by natural products: Allicin from garlic as a case study. Eur. J. Plant Pathol. 2008, 121, 313–322. [Google Scholar] [CrossRef]
- Hunter, R.; Caira, M.; Stellenboom, N. Thiolsulfinate allicin from garlic: Inspiration for a new antimicrobial agent. Ann. N. Y. Acad. Sci. 2005, 1056, 234–241. [Google Scholar] [CrossRef] [PubMed]
- Rose, P.; Whiteman, M.; Moore, P.K.; Zhu, Y.Z. Bioactive S-alk (en) yl cysteine sulfoxide metabolites in the genus Allium: The chemistry of potential therapeutic agents. Nat. Prod. Rep. 2005, 22, 351–368. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Yang, C.S. Effects of cytochrome P450 2E1 modulators on the pharmacokinetics of chlorzoxazone and 6-hydroxychlorzoxazone in rats. Life Sci. 1996, 58, 1575–1585. [Google Scholar] [CrossRef]
- Park, S.; Kim, M.-Y.; Lee, D.H.; Lee, S.H.; Baik, E.J.; Moon, C.-H.; Park, S.W.; Ko, E.Y.; Oh, S.-R.; Jung, Y.-S. Methanolic extract of onion (Allium cepa) attenuates ischemia/hypoxia-induced apoptosis in cardiomyocytes via antioxidant effect. Eur. J. Nutr. 2009, 48, 235–242. [Google Scholar] [CrossRef] [PubMed]
- Lanzotti, V. Bioactive polar natural compounds from garlic and onions. Phytochem. Rev. 2012, 11, 179–196. [Google Scholar] [CrossRef]
- Matsuura, H. Saponins in garlic as modifiers of the risk of cardiovascular disease. J. Nutr. 2001, 131, 1000S–1005S. [Google Scholar] [PubMed]
- Sobolewska, D.; Michalska, K.; Podolak, I.; Grabowska, K. Steroidal saponins from the genus Allium. Phytochem. Rev. 2016, 15, 1–35. [Google Scholar] [CrossRef] [PubMed]
- Sobolewska, D.; Janeczko, Z.; Podolak, I.; Szerłomska, A. Densitometric analysis of diosgenin in methanolic extracts of Allium ursinum collected at different times during plant development. JPC J. Planta Chromatogr. Mod. TLC 2009, 22, 305–307. [Google Scholar] [CrossRef]
- Mostafa, A.; Sudisha, J.; El-Sayed, M.; Ito, S.-I.; Ikeda, T.; Yamauchi, N.; Shigyo, M. Aginoside saponin, a potent antifungal compound, and secondary metabolite analyses from Allium nigrum L. Phytochem. Lett. 2013, 6, 274–280. [Google Scholar] [CrossRef]
- Akhov, L.; Musienko, M.; Piacente, S.; Pizza, C.; Oleszek, W. Structure of steroidal saponins from underground parts of Allium nutans L. J. Agric. Food chem. 1999, 47, 3193–3196. [Google Scholar] [CrossRef] [PubMed]
- Barile, E.; Capasso, R.; Izzo, A.A.; Lanzotti, V.; Sajjadi, S.E.; Zolfaghari, B. Structure-activity relationships for saponins from Allium hirtifolium and Allium elburzense and their antispasmodic activity. Planta Med. 2005, 71, 1010–1018. [Google Scholar] [CrossRef] [PubMed]
- Matsuura, H.; Ushiroguchi, T.; Itakura, Y.; Hayashi, N.; Fuwa, T. A furostanol glycoside from garlic, bulbs of Allium sativum L. Chem. Pharm. Bull. 1988, 36, 3659–3663. [Google Scholar] [CrossRef]
- Yu, Z.; Zhang, T.; Zhou, F.; Xiao, X.; Ding, X.; He, H.; Rang, J.; Quan, M.; Wang, T.; Zuo, M. Anticancer activity of saponins from Allium chinense against the B16 melanoma and 4T1 breast carcinoma cell. Evid. Based Complement. Alternat. Med. 2015, 2015. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, D.; Maity, A.; Jha, T.; Mondal, N.B. Spermicidal and contraceptive potential of desgalactotigonin: A prospective alternative of nonoxynol-9. PLoS ONE 2014, 9, e107164. [Google Scholar] [CrossRef] [PubMed]
- Lanzotti, V.; Barile, E.; Antignani, V.; Bonanomi, G.; Scala, F. Antifungal saponins from bulbs of garlic, Allium sativum L. var. Voghiera. Phytochemistry 2012, 78, 126–134. [Google Scholar] [CrossRef] [PubMed]
- Lanzotti, V.; Bonanomi, G.; Scala, F. What makes Allium species effective against pathogenic microbes? Phytochem. Rev. 2013, 12, 751–772. [Google Scholar] [CrossRef]
- Palmero, D.; De Cara, M.; Nosir, W.; GálvEz, L.; Cruz, A.; WooDWarD, S.; GoNzálEz-JaéN, M.T.; TEllo, J.C. Fusarium proliferatum isolated from garlic in Spain: Identification, toxigenic potential and pathogenicity on related Allium species. Phytopathol. Med. 2012, 51, 207–218. [Google Scholar]
- Rambla, J.L.; Trapero-Mozos, A.; Diretto, G.; Rubio-Moraga, A.; Granell, A.; Gómez-Gómez, L.; Ahrazem, O. Gene-metabolite networks of volatile metabolism in airen and tempranillo grape cultivars revealed a distinct mechanism of aroma bouquet production. Front. Plant Sci. 2016, 7. [Google Scholar] [CrossRef] [PubMed]
- Fasano, C.; Diretto, G.; Aversano, R.; D’Agostino, N.; Di Matteo, A.; Frusciante, L.; Giuliano, G.; Carputo, D. Transcriptome and metabolome of synthetic Solanum autotetraploids reveal key genomic stress events following polyploidization. New Phytol. 2016, 210, 1382–1394. [Google Scholar] [CrossRef] [PubMed]
- Alboresi, A.; Perin, G.; Vitulo, N.; Diretto, G.; Block, M.A.; Jouhet, J.; Meneghesso, A.; Valle, G.; Giuliano, G.; Maréchal, E. Light remodels lipid biosynthesis in nannochloropsis gaditana by modulating carbon partitioning between organelles. Plant Physiol. 2016. [CrossRef] [PubMed]
- Hammer, Ø.; Harper, D.; Ryan, P. PAST-Palaeontological STatistics, ver. 1.89. Palaeontol. Electron. 2001, 4, 1–9. [Google Scholar]
- Gómez-Gómez, L.; Parra-Vega, V.; Rivas-Sendra, A.; Seguí-Simarro, J.M.; Molina, R.V.; Pallotti, C.; Rubio-Moraga, Á.; Diretto, G.; Prieto, A.; Ahrazem, O. Unraveling massive crocins transport and accumulation through proteome and microscopy tools during the development of saffron stigma. Int. J. Mol. Sci. 2017, 18, 76. [Google Scholar] [CrossRef] [PubMed]
- D’Esposito, D.; Ferriello, F.; Dal Molin, A.; Diretto, G.; Sacco, A.; Minio, A.; Barone, A.; Di Monaco, R.; Cavella, S.; Tardella, L. Unraveling the complexity of transcriptomic, metabolomic and quality environmental response of tomato fruit. BMC Plant Biol. 2017, 17, 66. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are not available from the authors. |
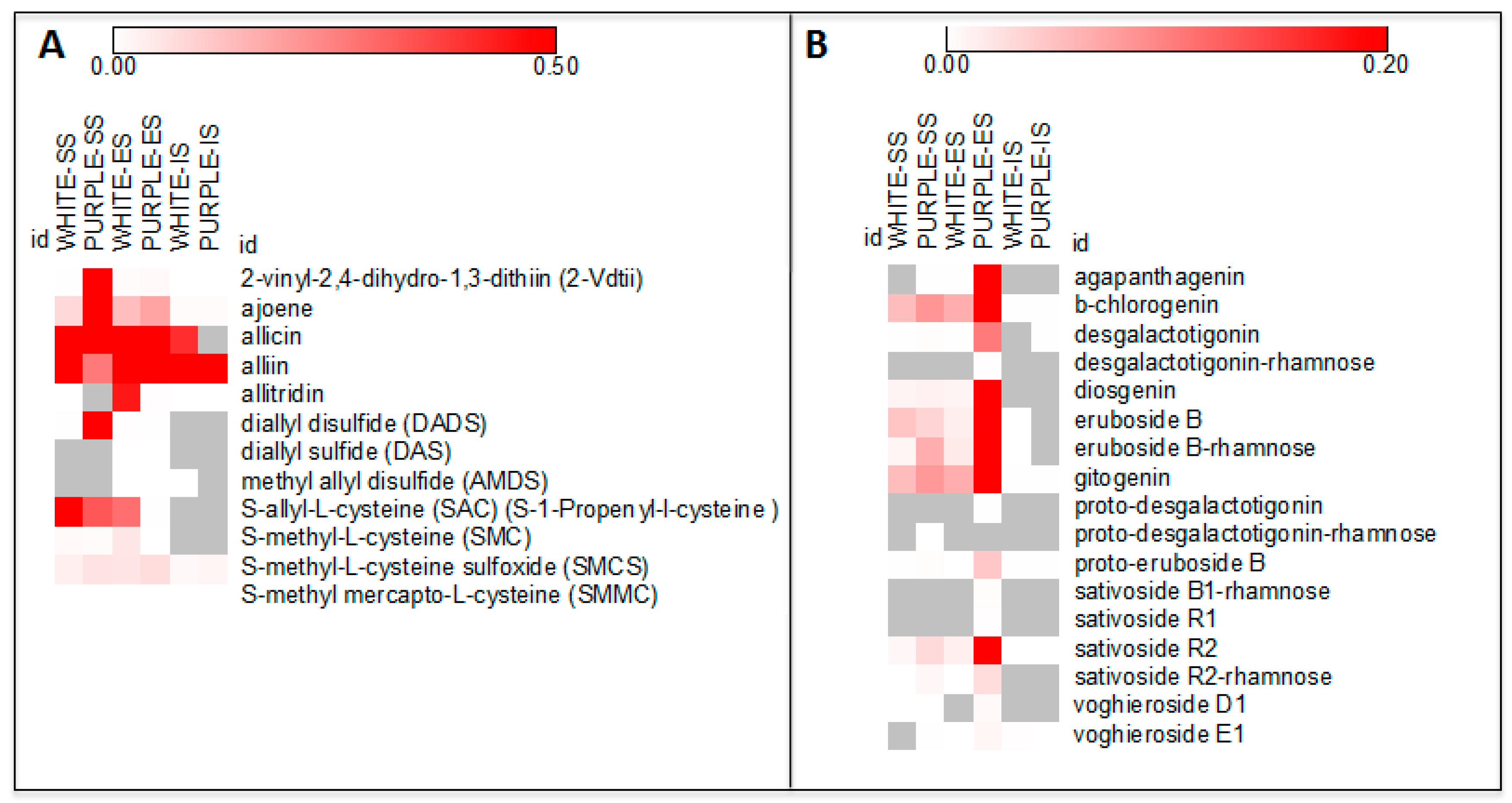
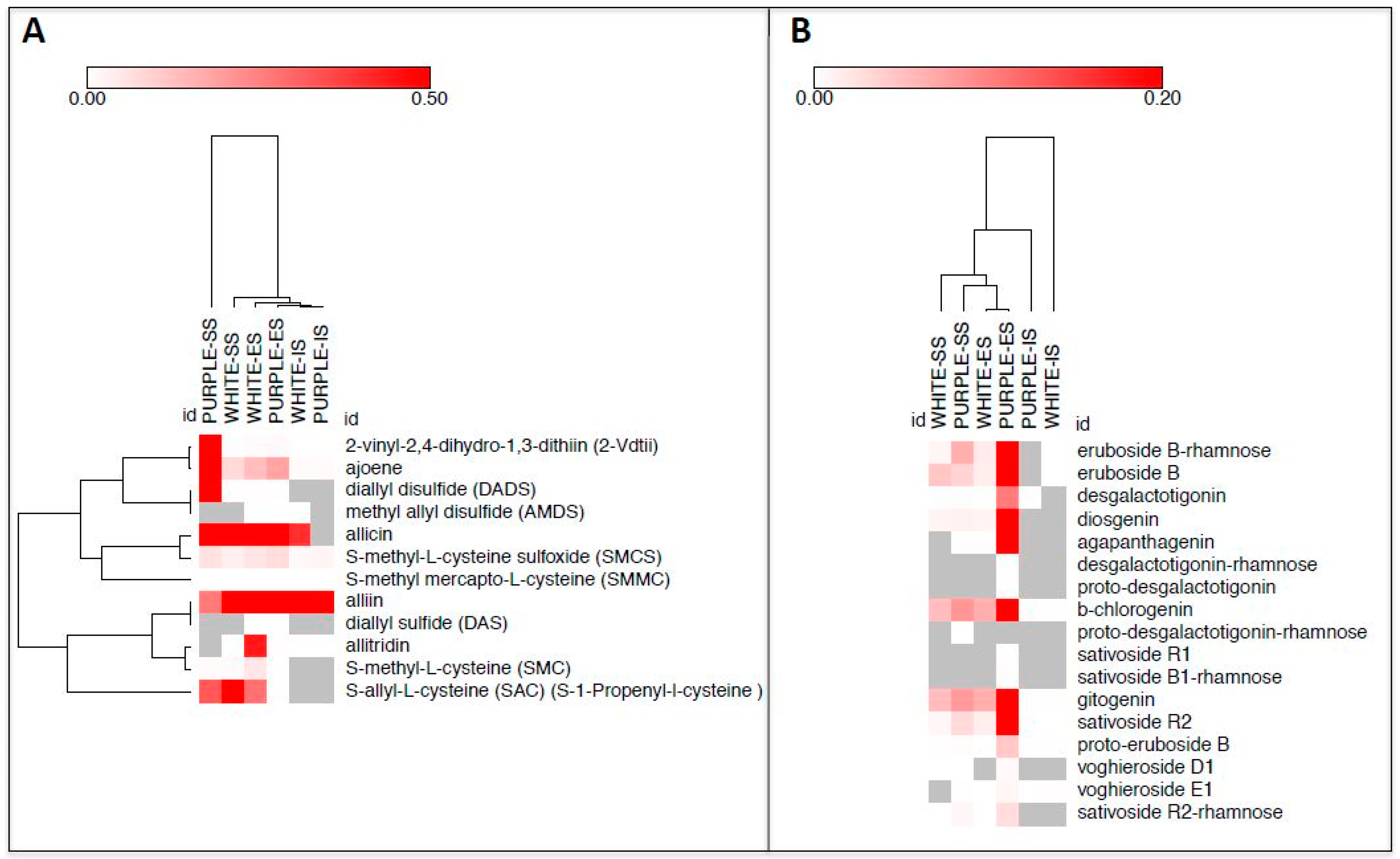
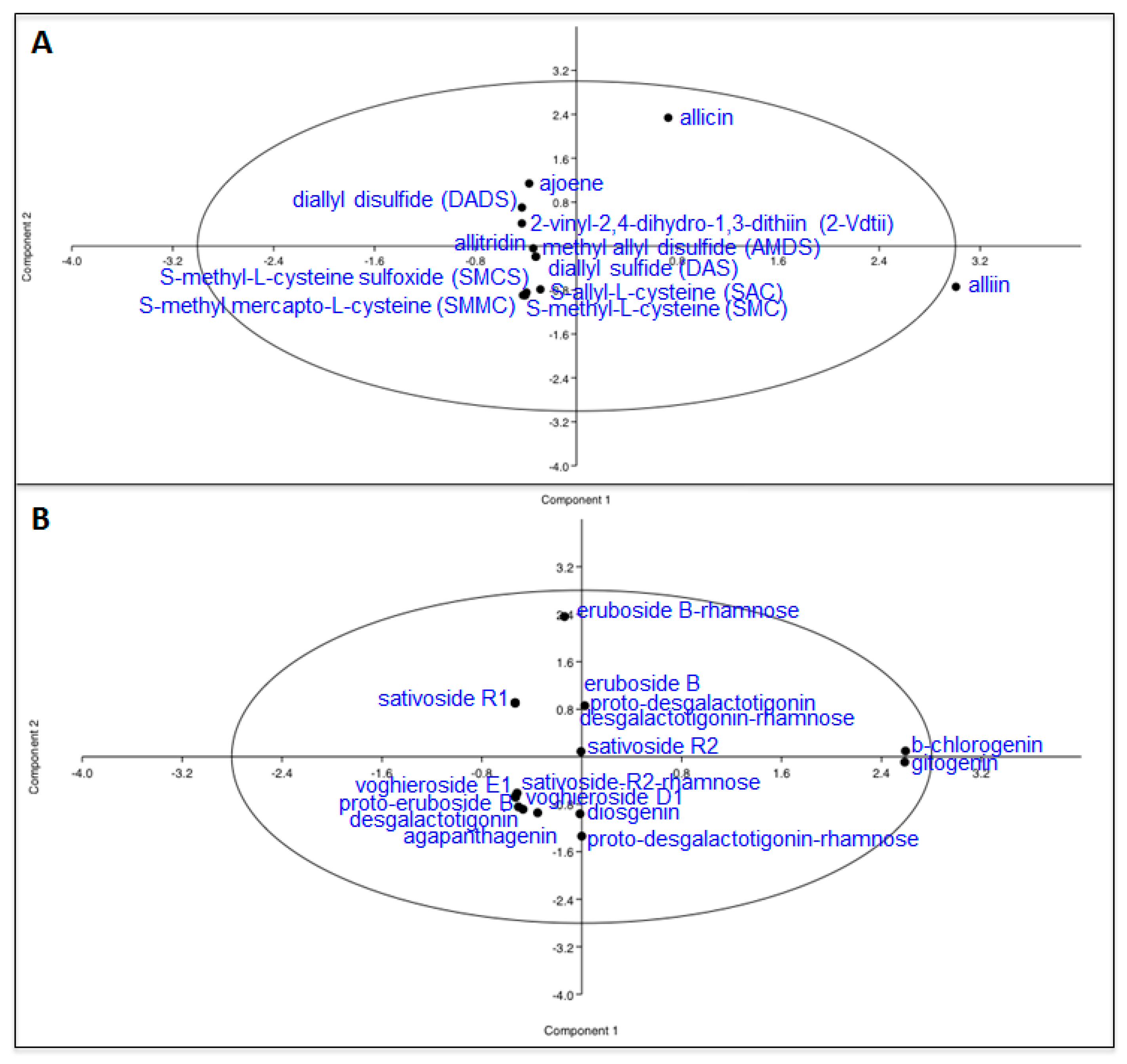
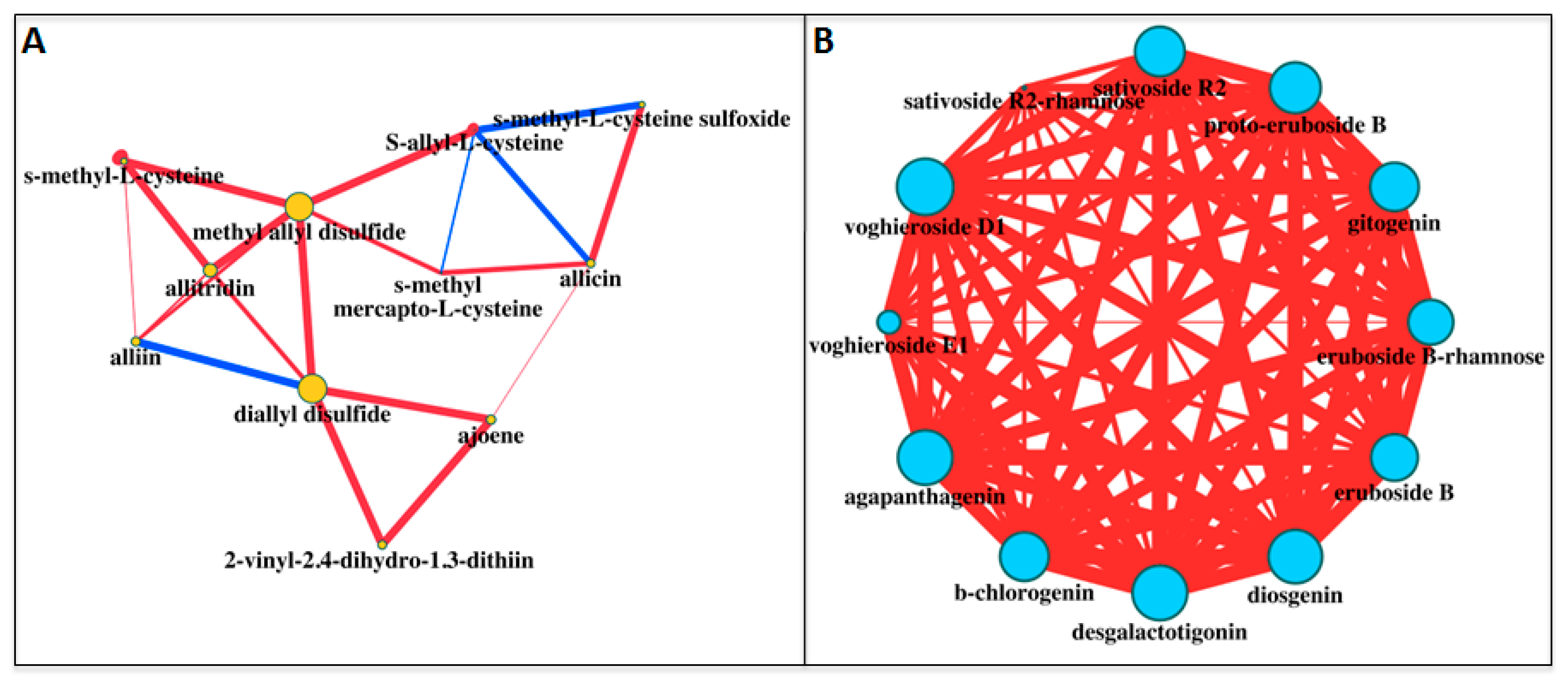
| Sulfur Compounds | White | Purple | White/Purple | Purple/White |
| 2-vinyl-2,4-dihydro-1,3-dithiin (2-Vdtii) | 0.0115 ± 0.0016 | 1.8250 ± 0.0011 *** | 0.0063 | 158.7012 |
| Ajoene | 0.2148 ± 0.0089 | 2.9764 ± 0.0161 *** | 0.0722 | 13.8574 |
| Allicin | 3.1560 ± 0.1577 | 7.1073 ± 0.3014 *** | 0.4440 | 2.2520 |
| Alliin | 14.3933 ± 0.3834 *** | 6.8875 ± 0.1465 | 2.0898 | 0.4785 |
| Allitridin | 0.4451 ± 0.0002 *** | 0.0040 ± 0.0005 | 111.7082 | 0.0090 |
| Diallyl disulfide (DADS) | 0.0095 ± 0.0001 | 2.2228 ± 0.0002 *** | 0.0043 | 234.9036 |
| Diallyl sulfide (DAS) | 0.0007 ± 0.0001 | 0.0005 ± 0.0001 | 1.3896 | 0.7196 |
| Methyl allyl disulfide | 0.0002 ± 0.0001 | 0.0001 ± 0.0001 | 1.2389 | 0.8071 |
| S-Allyl-l-cysteine (SAC) (S-1-Propenyl-l-cysteine) | 0.8031 ± 0.1176 *** | 0.3293 ± 0.1009 | 2.4388 | 0.4100 |
| S-Methyl-l-cysteine (SMC) | 0.0630 ± 0.018 *** | 0.0073 ± 0.009 | 8.6301 | 0.1159 |
| S-Methyl-l-cysteine sulfoxide (SMCS) | 0.1006 ± 0.0146 | 0.1450 ± 0.0096 | 0.6941 | 1.4406 |
| S-Methyl mercapto-l-cysteine | 0.0010 ± 0.0001 | 0.0019 ± 0.0001 * | 0.5412 | 1.8477 |
| Total | 19.1960 ± 0.5887 | 21.5063 ± 0.4919 * | 0.8926 | 1.1204 |
| Saponins | White | Purple | White/Purple | Purple/White |
| Agapanthagenin | 0.0009 ± 0.0002 | 0.2938 ± 0.0212 *** | 0.0031 | 321.3416 |
| β-chlorogenin | 0.1201 ± 0.0279 | 5.1217 ± 0.5347 *** | 0.0234 | 42.6560 |
| Desgalactotigonin | 0.0015 ± 0.0003 | 0.1077 ± 0.0120 *** | 0.0144 | 69.4736 |
| Desgalactotigonin-rhamnose | nd | 0.0018 ± 0.0001 | 0.0000 | - |
| Diosgenin | 0.0174 ± 0.0039 | 0.8497 ± 0.0515 *** | 0.0204 | 48.9731 |
| Eruboside B | 0.0604 ± 0.0103 | 0.9349 ± 0.1731 *** | 0.0646 | 15.4892 |
| Eruboside B-rhamnose | 0.0249 ± 0.0036 | 0.7043 ± 0.1750 *** | 0.0353 | 28.2983 |
| Gitogenin | 0.1199 ± 0.0279 | 5.1086 ± 0.5335 *** | 0.0235 | 42.6249 |
| Proto-desgalactotigonin | 0.0001 ± 0.0001 | 0.0004 ± 0.0001 * | 0.1634 | 6.1215 |
| Proto-desgalactotigonin-rhamnose | nd | 0.0003 ± 0.0001 | 0.0000 | - |
| Proto-eruboside B | 0.0016 ± 0.0001 | 0.0475 ± 0.0115 *** | 0.0327 | 30.5637 |
| Sativoside B1-rhamnose | nd | 0.0026 ± 0.0002 | 0.0000 | - |
| Sativoside R1 | nd | 0.0014 ± 0.0004 | 0.0000 | - |
| Sativoside R2 | 0.0199 ± 0.0022 | 0.8814 ± 0.2130 *** | 0.0226 | 44.2229 |
| Sativoside R2-rhamnose | 0.0004 ± 0.0001 | 0.0337 ± 0.0079 *** | 0.0121 | 82.5336 |
| Voghieroside D1 | 0.0001 ± 0.0001 | 0.0049 ± 0.0008 *** | 0.0118 | 84.6999 |
| Voghieroside E1 | 0.0017 ± 0.0001 | 0.0082 ± 0.0022 ** | 0.2042 | 4.8961 |
| Total | 0.3687 ± 0.0765 | 14.1029 ± 1.7371 *** | 0.0261 | 38.2539 |
| SS | ES | IS | ||||
| Sulfur Compounds | White/Purple | Purple/White | White/Purple | Purple/White | White/Purple | Purple/White |
| 2-vinyl-2,4-dihydro-1,3-dithiin (2-Vdtii) | 0.0016 | 638.6639 | 0.6930 | 1.4431 | 0.5953 | 1.6798 |
| Ajoene | 0.0271 | 36.8755 | 0.7260 | 1.3774 | 0.8480 | 1.1793 |
| Allicin | 0.1559 | 6.4160 | 0.5931 | 1.6861 | - | - |
| Alliin | 22.5323 | 0.0444 | 1.2892 | 0.7757 | 1.1267 | 0.8875 |
| Allitridin | - | - | 115.0373 | 0.0087 | 0.5913 | 1.6913 |
| Diallyl disulfide (DADS) | 0.0013 | 758.0864 | 2.4338 | 0.4109 | 4.7702 | 0.2096 |
| Diallyl sulfide (DAS) | - | - | 1.3896 | 0.7196 | - | - |
| Methyl allyl disulfide (AMS) | - | - | 1.1860 | 0.8431 | - | - |
| S-Allyl-l-cysteine (SAC) (S-1-Propenyl-l-cysteine) | 1.5925 | 0.6280 | 128.2727 | 0.0078 | - | - |
| S-Methyl-l-cysteine (SMC) | 1.7681 | 0.5656 | 127.0000 | 0.0079 | - | - |
| S-Methyl-l-cysteine sulfoxide (SMCS) | 0.5857 | 1.7074 | 0.7755 | 1.2896 | 0.7361 | 1.3585 |
| S-Methyl mercapto-l-cysteine (SMMC) | 0.2545 | 3.9290 | 1.2378 | 0.8079 | 0.3569 | 2.8016 |
| Total | 0.6308 | 1.5852 | 1.0545 | 0.9483 | 1.6271 | 0.6146 |
| SS | ES | IS | ||||
| Saponins | White/Purple | Purple/White | White/Purple | Purple/White | White/Purple | Purple/White |
| Agapanthagenin | - | - | 0.0031 | 320.2470 | - | - |
| β-Chlorogenin | 0.6367 | 1.5705 | 0.0130 | 76.9899 | 1.0887 | 0.9185 |
| Desgalactotigonin | 0.1966 | 5.0872 | 0.0101 | 98.5283 | - | - |
| Desgalactotigonin-rhamnose | - | - | - | - | - | - |
| Diosgenin | 0.7670 | 1.3037 | 0.0105 | 95.3023 | - | - |
| Eruboside B | 1.3198 | 0.7577 | 0.0156 | 64.0878 | - | - |
| Eruboside B-rhamnose | 0.1158 | 8.6331 | 0.0267 | 37.3852 | - | - |
| Gitogenin | 0.6642 | 1.5056 | 0.0130 | 77.0948 | 1.0887 | 0.9185 |
| Proto-desgalactotigonin | - | - | - | - | - | - |
| Proto-desgalactotigonin-rhamnose | - | - | - | - | - | - |
| Proto-eruboside B | 0.1705 | 5.8666 | 0.0034 | 293.7059 | 2.0534 | 0.4870 |
| Sativoside B1-rhamnose | - | - | - | - | - | - |
| Sativoside R1 | - | - | - | - | - | - |
| Sativoside R2 | 0.2160 | 4.6288 | 0.0157 | 63.5507 | 0.9527 | 1.0497 |
| Sativoside R2-rhamnose | 0.0145 | 69.0498 | 0.0116 | 86.4215 | - | - |
| Voghieroside D1 | 0.2191 | 4.5643 | - | - | - | - |
| Voghieroside E1 | - | - | 0.0147 | 67.9012 | 5.1114 | 0.1956 |
| Total | 0.5540 | 1.8051 | 0.0135 | 73.8849 | 1.4165 | 0.7059 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Diretto, G.; Rubio-Moraga, A.; Argandoña, J.; Castillo, P.; Gómez-Gómez, L.; Ahrazem, O. Tissue-Specific Accumulation of Sulfur Compounds and Saponins in Different Parts of Garlic Cloves from Purple and White Ecotypes. Molecules 2017, 22, 1359. https://doi.org/10.3390/molecules22081359
Diretto G, Rubio-Moraga A, Argandoña J, Castillo P, Gómez-Gómez L, Ahrazem O. Tissue-Specific Accumulation of Sulfur Compounds and Saponins in Different Parts of Garlic Cloves from Purple and White Ecotypes. Molecules. 2017; 22(8):1359. https://doi.org/10.3390/molecules22081359
Chicago/Turabian StyleDiretto, Gianfranco, Angela Rubio-Moraga, Javier Argandoña, Purificación Castillo, Lourdes Gómez-Gómez, and Oussama Ahrazem. 2017. "Tissue-Specific Accumulation of Sulfur Compounds and Saponins in Different Parts of Garlic Cloves from Purple and White Ecotypes" Molecules 22, no. 8: 1359. https://doi.org/10.3390/molecules22081359
APA StyleDiretto, G., Rubio-Moraga, A., Argandoña, J., Castillo, P., Gómez-Gómez, L., & Ahrazem, O. (2017). Tissue-Specific Accumulation of Sulfur Compounds and Saponins in Different Parts of Garlic Cloves from Purple and White Ecotypes. Molecules, 22(8), 1359. https://doi.org/10.3390/molecules22081359







