Spirulina maxima Extract Prevents Neurotoxicity via Promoting Activation of BDNF/CREB Signaling Pathways in Neuronal Cells and Mice
Abstract
:1. Introduction
2. Results
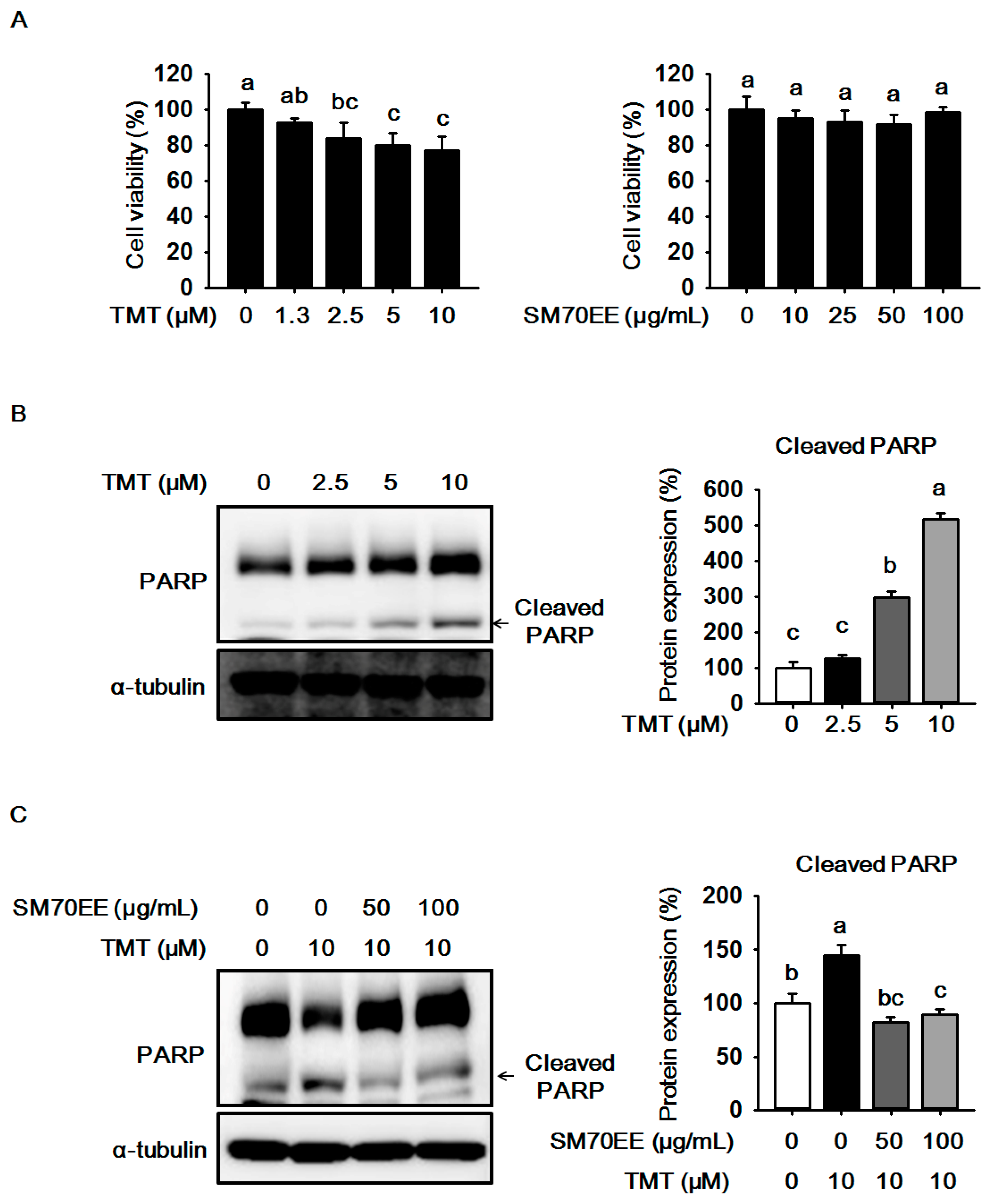
2.1. The Effects of SM70EE on Cell Viability and Neuronal Death in HT-22 Cells
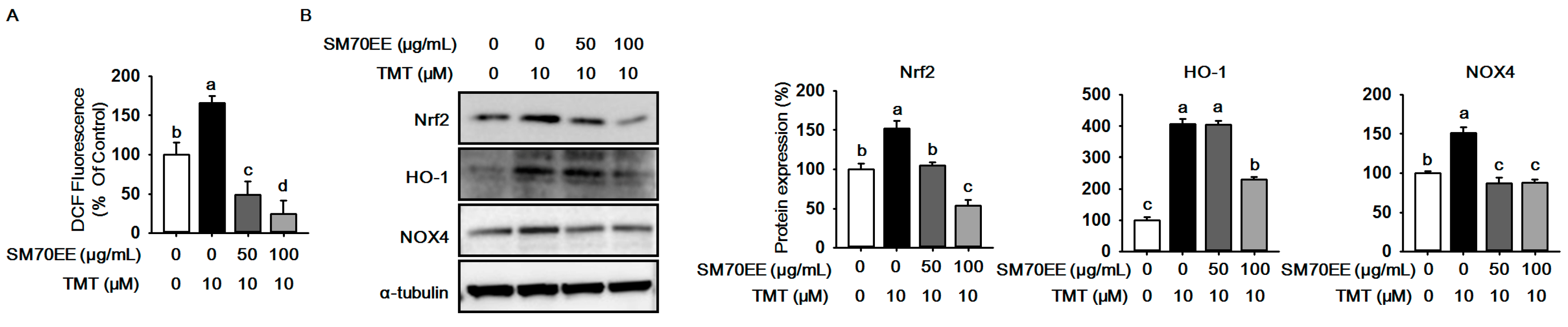
2.2. SM70EE Suppresses ROS Production against TMT-Induced Neurotoxicity in HT-22 Cells
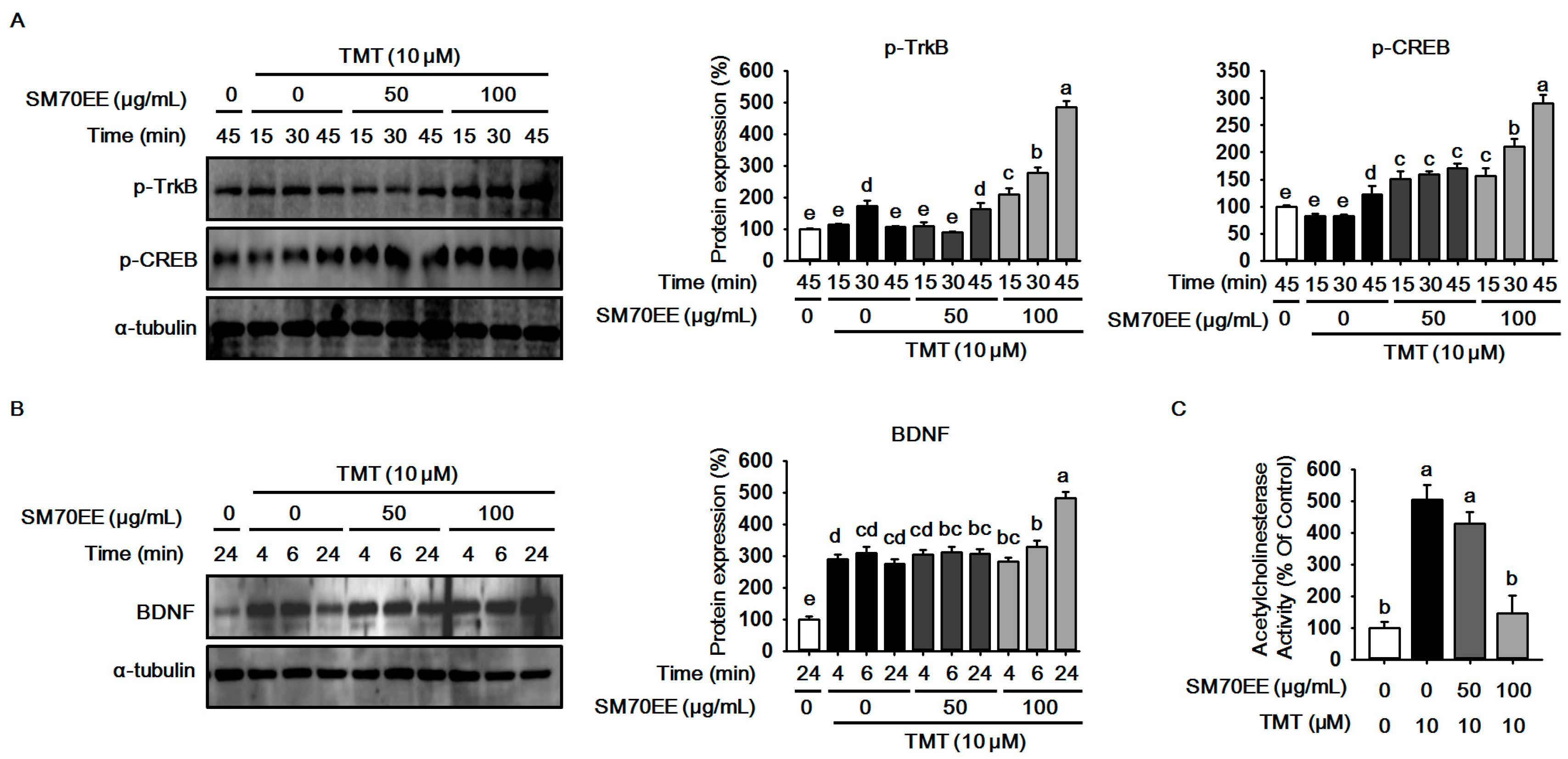
2.3. SM70EE Prevents Neuronal Cell Damage Caused in HT-22 Cells by TMT-Induced Neurotoxicity via Enhancing BDNF/CREB Signaling Pathways and Suppressing AChE Activity
2.4. SM70EE Prevents Learning and Memory Impairment against Scopolamine-Induced Neurotoxicity in Mice
3. Discussion
4. Materials and Methods
4.1. Preparation of SM70EE
4.2. Materials
4.3. Animals
4.4. Passive Avoidance Test
4.5. Morris Water Maze Test
4.6. Cell Culture
4.7. MTT Assay
4.8. Measurement of Intracellular ROS Production
4.9. Acetylcholinesterase Activity
4.10. Western Blotting
4.11. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Conflicts of Interest
References
- Vajda, F.J. Neuroprotection and neurodegenerative disease. J. Clin. Neurosci. 2002, 9, 4–8. [Google Scholar] [CrossRef] [PubMed]
- Corvino, V.; Marchese, E.; Michetti, F.; Geloso, M.C. Neuroprotective strategies in hippocampal neurodegeneration induced by the neurotoxicant trimethyltin. Neurochem. Res. 2013, 38, 240–253. [Google Scholar] [CrossRef] [PubMed]
- Tao, L.; Xie, J.; Wang, Y.; Wang, S.; Wu, S.; Wang, Q.; Ding, H. Protective effects of aloe-emodin on scopolamine-induced memory impairment in mice and H(2)O(2)-induced cytotoxicity in PC12 cells. Bioorg. Med. Chem. Lett. 2014, 24, 5385–5389. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Yang, M.; Kim, J.; Kang, S.; Kim, J.; Kim, J.C.; Jung, C.; Shin, T.; Kim, S.H.; Moon, C. Trimethyltin-induced hippocampal neurodegeneration: A mechanism-based review. Brain Res. Bull. 2016, 125, 187–199. [Google Scholar] [CrossRef] [PubMed]
- Babic, T. The cholinergic hypothesis of alzheimer’s disease: A review of progress. J. Neurol. Neurosurg. Psychiatry 1999, 67, 558. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.S.; Kim, H.G.; Lee, H.W.; Han, J.M.; Lee, S.K.; Kim, D.W.; Saravanakumar, A.; Son, C.G. Hippocampal memory enhancing activity of pine needle extract against scopolamine-induced amnesia in a mouse model. Sci. Rep. 2015, 5, 9651. [Google Scholar] [CrossRef] [PubMed]
- Terry, A.V., Jr.; Buccafusco, J.J. The cholinergic hypothesis of age and alzheimer’s disease-related cognitive deficits: Recent challenges and their implications for novel drug development. J. Pharmacol. Exp. Ther. 2003, 306, 821–827. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, A.; Ramasamy, K.; Jaafar, S.M.; Majeed, A.B.; Mani, V. Total isoflavones from soybean and tempeh reversed scopolamine-induced amnesia, improved cholinergic activities and reduced neuroinflammation in brain. Food Chem. Toxicol. 2014, 65, 120–128. [Google Scholar] [CrossRef] [PubMed]
- Kwon, S.H.; Lee, H.K.; Kim, J.A.; Hong, S.I.; Kim, H.C.; Jo, T.H.; Park, Y.I.; Lee, C.K.; Kim, Y.B.; Lee, S.Y.; et al. Neuroprotective effects of chlorogenic acid on scopolamine-induced amnesia via anti-acetylcholinesterase and anti-oxidative activities in mice. Eur. J. Pharmacol. 2010, 649, 210–217. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, S.M.; Barone, S. The neurotoxicant trimethyltin induces apoptosis via caspase activation, p38 protein kinase, and oxidative stress in PC12 cells. Toxicol. Lett. 2004, 147, 63–72. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.F.; LeBel, C.P.; Bondy, S.C. Reactive oxygen species formation as a biomarker of methylmercury and trimethyltin neurotoxicity. Neurotoxicology 1992, 13, 637–648. [Google Scholar] [PubMed]
- Ewing, J.F.; Maines, M.D. Rapid induction of heme oxygenase 1 mrna and protein by hyperthermia in rat brain: Heme oxygenase 2 is not a heat shock protein. Proc. Natl. Acad. Sci. USA 1991, 88, 5364–5368. [Google Scholar] [CrossRef] [PubMed]
- Vanella, L.; Sodhi, K.; Kim, D.H.; Puri, N.; Maheshwari, M.; Hinds, T.D.; Bellner, L.; Goldstein, D.; Peterson, S.J.; Shapiro, J.I.; et al. Increased heme-oxygenase 1 expression in mesenchymal stem cell-derived adipocytes decreases differentiation and lipid accumulation via upregulation of the canonical wnt signaling cascade. Stem Cell Res. Ther. 2013, 4, 28. [Google Scholar] [CrossRef] [PubMed]
- Catino, S.; Paciello, F.; Miceli, F.; Rolesi, R.; Troiani, D.; Calabrese, V.; Santangelo, R.; Mancuso, C. Ferulic acid regulates the nrf2/heme oxygenase-1 system and counteracts trimethyltin-induced neuronal damage in the human neuroblastoma cell line sh-sy5y. Front. Pharmacol. 2015, 6, 305. [Google Scholar] [CrossRef] [PubMed]
- Chen, A.; Xiong, L.J.; Tong, Y.; Mao, M. The neuroprotective roles of bdnf in hypoxic ischemic brain injury. Biomed. Rep. 2013, 1, 167–176. [Google Scholar] [PubMed]
- Soreq, H.; Seidman, S. Acetylcholinesterase—New roles for an old actor. Nat. Rev. Neurosci. 2001, 2, 294–302. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.K.; Bae, H.; Kim, M.J.; Choi, S.J.; Cho, H.Y.; Hwang, H.J.; Kim, Y.J.; Lim, S.T.; Kim, E.K.; Kim, H.K.; et al. Inhibitory effect of poncirus trifoliate on acetylcholinesterase and attenuating activity against trimethyltin-induced learning and memory impairment. Biosci. Biotechnol. Biochem. 2009, 73, 1105–1112. [Google Scholar] [CrossRef] [PubMed]
- Kay, R.A. Microalgae as food and supplement. Crit. Rev. Food Sci. Nutr. 1991, 30, 555–573. [Google Scholar] [CrossRef] [PubMed]
- Abd El-Baky, H.H.; El-Baroty, G.S. Characterization and bioactivity of phycocyanin isolated from Spirulina maxima grown under salt stress. Food Funct. 2012, 3, 381–388. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Hernandez, A.; Ble-Castillo, J.L.; Juarez-Oropeza, M.A.; Diaz-Zagoya, J.C. Spirulina maxima prevents fatty liver formation in cd-1 male and female mice with experimental diabetes. Life Sci. 2001, 69, 1029–1037. [Google Scholar] [CrossRef]
- Deng, R.; Chow, T.J. Hypolipidemic, antioxidant, and antiinflammatory activities of microalgae spirulina. Cardiovasc. Ther. 2010, 28, e33–45. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Chua, C.C.; Kong, J.; Kostrzewa, R.M.; Kumaraguru, U.; Hamdy, R.C.; Chua, B.H. Necrostatin-1 protects against glutamate-induced glutathione depletion and caspase-independent cell death in ht-22 cells. J. Neurochem. 2007, 103, 2004–2014. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Ren, X.; Simpkins, J.W. Sequential upregulation of superoxide dismutase 2 and heme oxygenase 1 by tert-butylhydroquinone protects mitochondria during oxidative stress. Mol. Pharmacol. 2015, 88, 437–449. [Google Scholar] [CrossRef] [PubMed]
- Lipp, H.P.; Wolfer, D.P. Genetically modified mice and cognition. Curr. Opin. Neurobiol. 1998, 8, 272–280. [Google Scholar] [CrossRef]
- Bedard, K.; Krause, K.H. The nox family of ros-generating nadph oxidases: Physiology and pathophysiology. Physiol. Rev. 2007, 87, 245–313. [Google Scholar] [CrossRef] [PubMed]
- Numakawa, T.; Matsumoto, T.; Numakawa, Y.; Richards, M.; Yamawaki, S.; Kunugi, H. Protective action of neurotrophic factors and estrogen against oxidative stress-mediated neurodegeneration. J. Toxicol. 2011, 2011, 405194. [Google Scholar] [CrossRef] [PubMed]
- Wu, A.; Ying, Z.; Gomez-Pinilla, F. Dietary omega-3 fatty acids normalize bdnf levels, reduce oxidative damage, and counteract learning disability after traumatic brain injury in rats. J. Neurotrauma. 2004, 21, 1457–1467. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.; Cao, R.; Choi, Y.S.; Cho, H.Y.; Rhee, A.D.; Hah, C.K.; Hoyt, K.R.; Obrietan, K. The creb/cre transcriptional pathway: Protection against oxidative stress-mediated neuronal cell death. J. Neurochem. 2009, 108, 1251–1265. [Google Scholar] [CrossRef] [PubMed]
- Walton, M.R.; Dragunow, I. Is creb a key to neuronal survival? Trends Neurosci. 2000, 23, 48–53. [Google Scholar] [CrossRef]
- Abreu-Villaca, Y.; Filgueiras, C.C.; Manhaes, A.C. Developmental aspects of the cholinergic system. Behav. Brain Res. 2011, 221, 367–378. [Google Scholar] [CrossRef] [PubMed]
- Lapchak, P.A.; Araujo, D.M.; Hefti, F. Cholinergic regulation of hippocampal brain-derived neurotrophic factor mrna expression: Evidence from lesion and chronic cholinergic drug treatment studies. Neuroscience 1993, 52, 575–585. [Google Scholar] [CrossRef]
- Berchtold, N.C.; Kesslak, J.P.; Cotman, C.W. Hippocampal brain-derived neurotrophic factor gene regulation by exercise and the medial septum. J. Neurosci. Res. 2002, 68, 511–521. [Google Scholar] [CrossRef] [PubMed]
- Weon, J.B.; Yun, B.R.; Lee, J.; Eom, M.R.; Kim, J.S.; Lee, H.Y.; Park, D.S.; Chung, H.C.; Chung, J.Y.; Ma, C.J. The ameliorating effect of steamed and fermented codonopsis lanceolata on scopolamine-induced memory impairment in mice. Evid. Based Complement. Altern. Med. 2013, 2013, 464576. [Google Scholar] [CrossRef] [PubMed]
- Vorhees, C.V.; Williams, M.T. Morris water maze: Procedures for assessing spatial and related forms of learning and memory. Nat. Protoc. 2006, 1, 848–858. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are not available from the authors. |

© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Koh, E.-J.; Seo, Y.-J.; Choi, J.; Lee, H.Y.; Kang, D.-H.; Kim, K.-J.; Lee, B.-Y. Spirulina maxima Extract Prevents Neurotoxicity via Promoting Activation of BDNF/CREB Signaling Pathways in Neuronal Cells and Mice. Molecules 2017, 22, 1363. https://doi.org/10.3390/molecules22081363
Koh E-J, Seo Y-J, Choi J, Lee HY, Kang D-H, Kim K-J, Lee B-Y. Spirulina maxima Extract Prevents Neurotoxicity via Promoting Activation of BDNF/CREB Signaling Pathways in Neuronal Cells and Mice. Molecules. 2017; 22(8):1363. https://doi.org/10.3390/molecules22081363
Chicago/Turabian StyleKoh, Eun-Jeong, Young-Jin Seo, Jia Choi, Hyeon Yong Lee, Do-Hyung Kang, Kui-Jin Kim, and Boo-Yong Lee. 2017. "Spirulina maxima Extract Prevents Neurotoxicity via Promoting Activation of BDNF/CREB Signaling Pathways in Neuronal Cells and Mice" Molecules 22, no. 8: 1363. https://doi.org/10.3390/molecules22081363
APA StyleKoh, E.-J., Seo, Y.-J., Choi, J., Lee, H. Y., Kang, D.-H., Kim, K.-J., & Lee, B.-Y. (2017). Spirulina maxima Extract Prevents Neurotoxicity via Promoting Activation of BDNF/CREB Signaling Pathways in Neuronal Cells and Mice. Molecules, 22(8), 1363. https://doi.org/10.3390/molecules22081363




