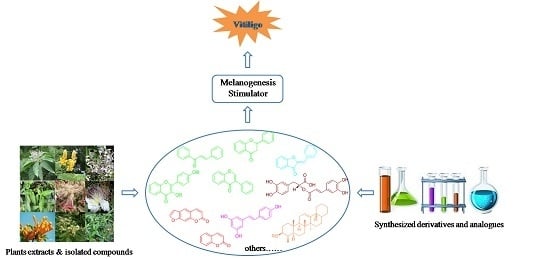
Upregulation of Melanogenesis and Tyrosinase Activity: Potential Agents for Vitiligo
Abstract
:1. Introduction
2. Melanogenesis Stimulators from Different Sources
- Plant extracts/crude drug extracts (mixtures);
- Active natural products/synthesized derivatives (single compounds).
2.1. Plant Extracts/Crude Drug Extracts (Mixtures)
2.1.1. Daphne gnidium
2.1.2. Moricandia arvensis
2.1.3. Ecliptae herba, Polygoni multiflori radixpraeparata and Rehmanniae radix praeparata
2.1.4. Cassia alata and Cassia occidentalis
2.1.5. Pyrostegia venusta
2.1.6. Vernonia anthelmintica
2.1.7. Melissa officinalis
2.1.8. Melia azedarach
2.1.9. Capparis spinosa and Erica multiflora
2.1.10. Citrus paradisi, Citrus grandis, Fructus aurantii immaturus and Fructus aurantii
2.1.11. Bee Venom
2.2. Active Natural Products/Synthesized Derivatives (Single Compounds)
2.2.1. Flavonoids
Flavanones
Chalcones
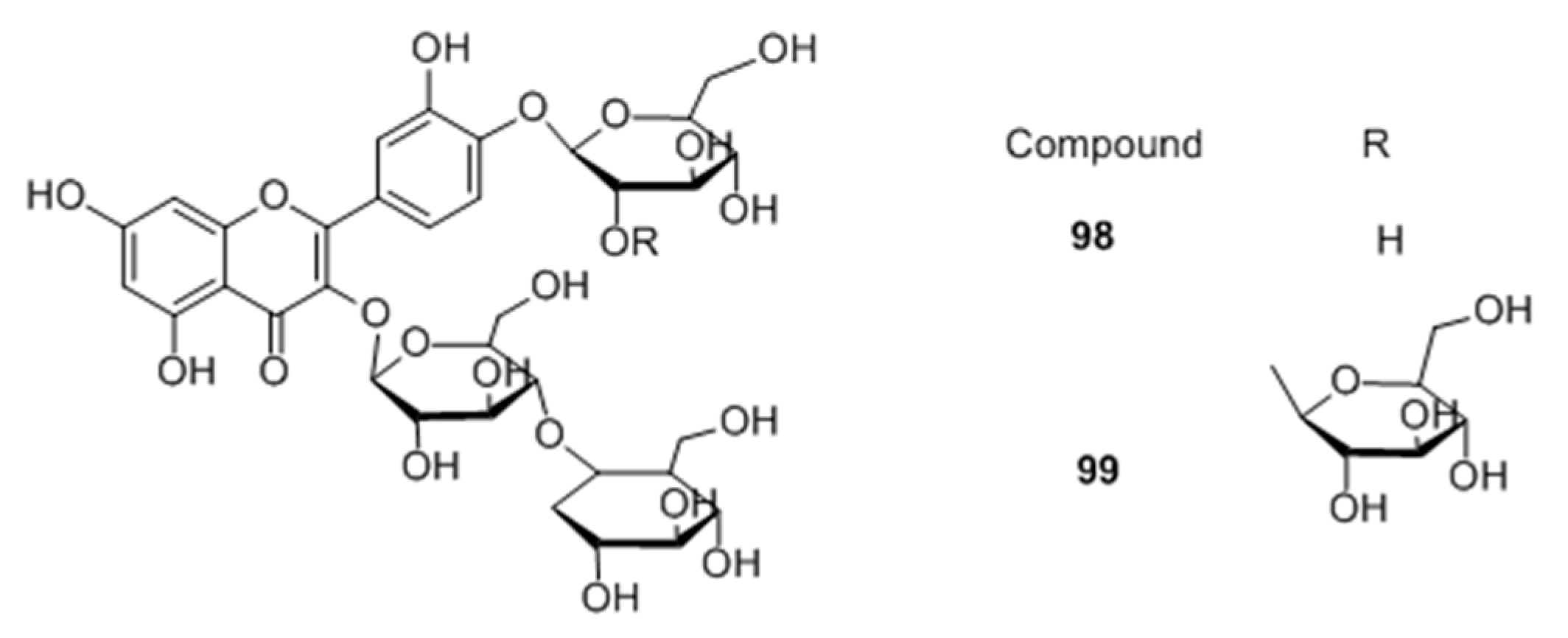
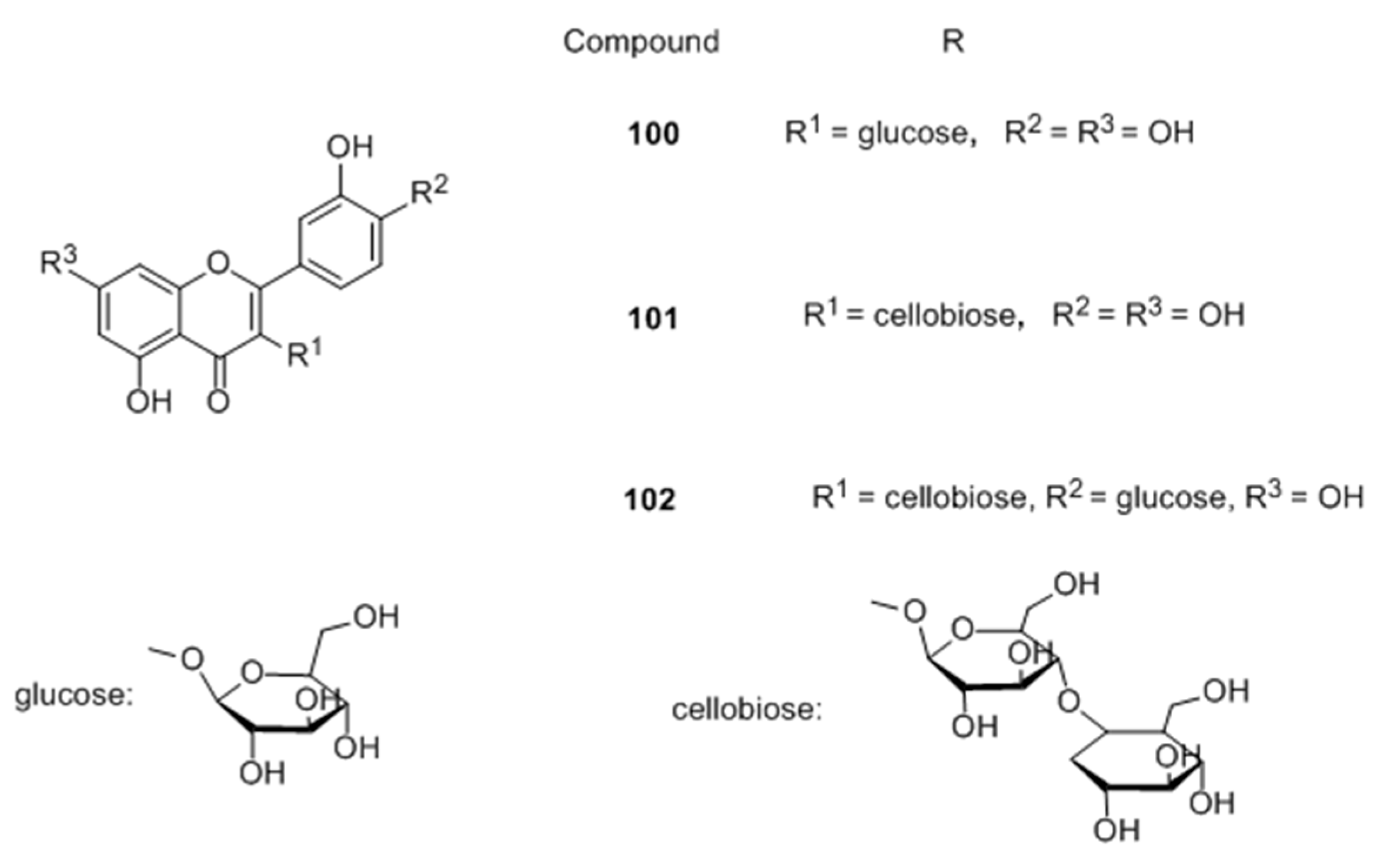
Flavonoid glycosides
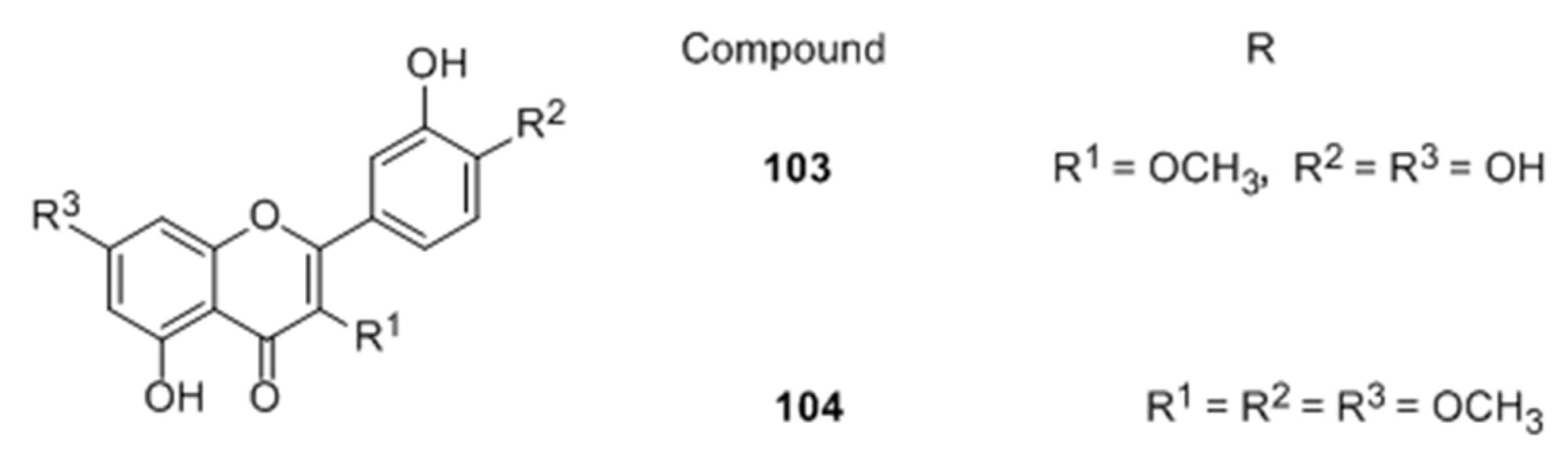
Flavones
Isoflavones
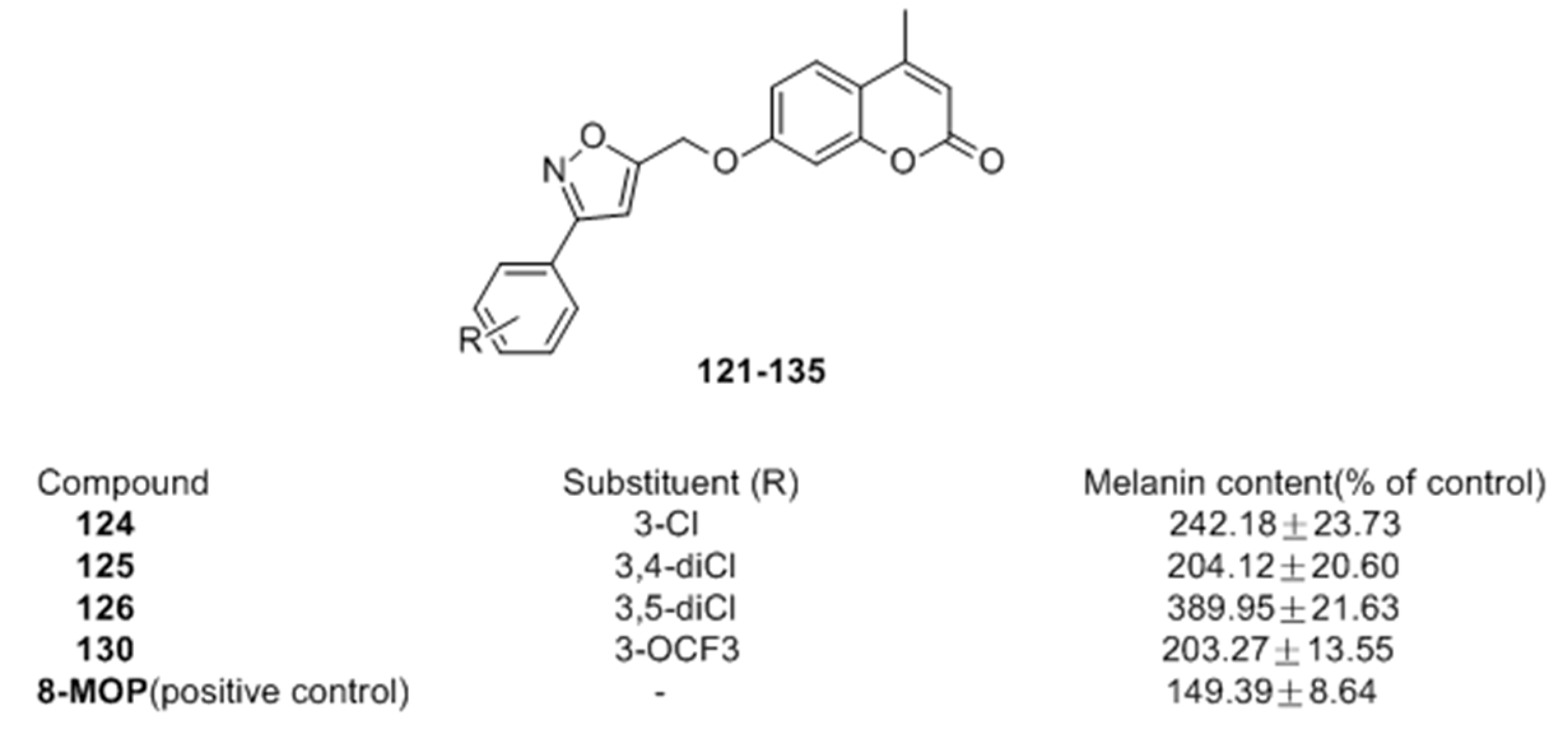
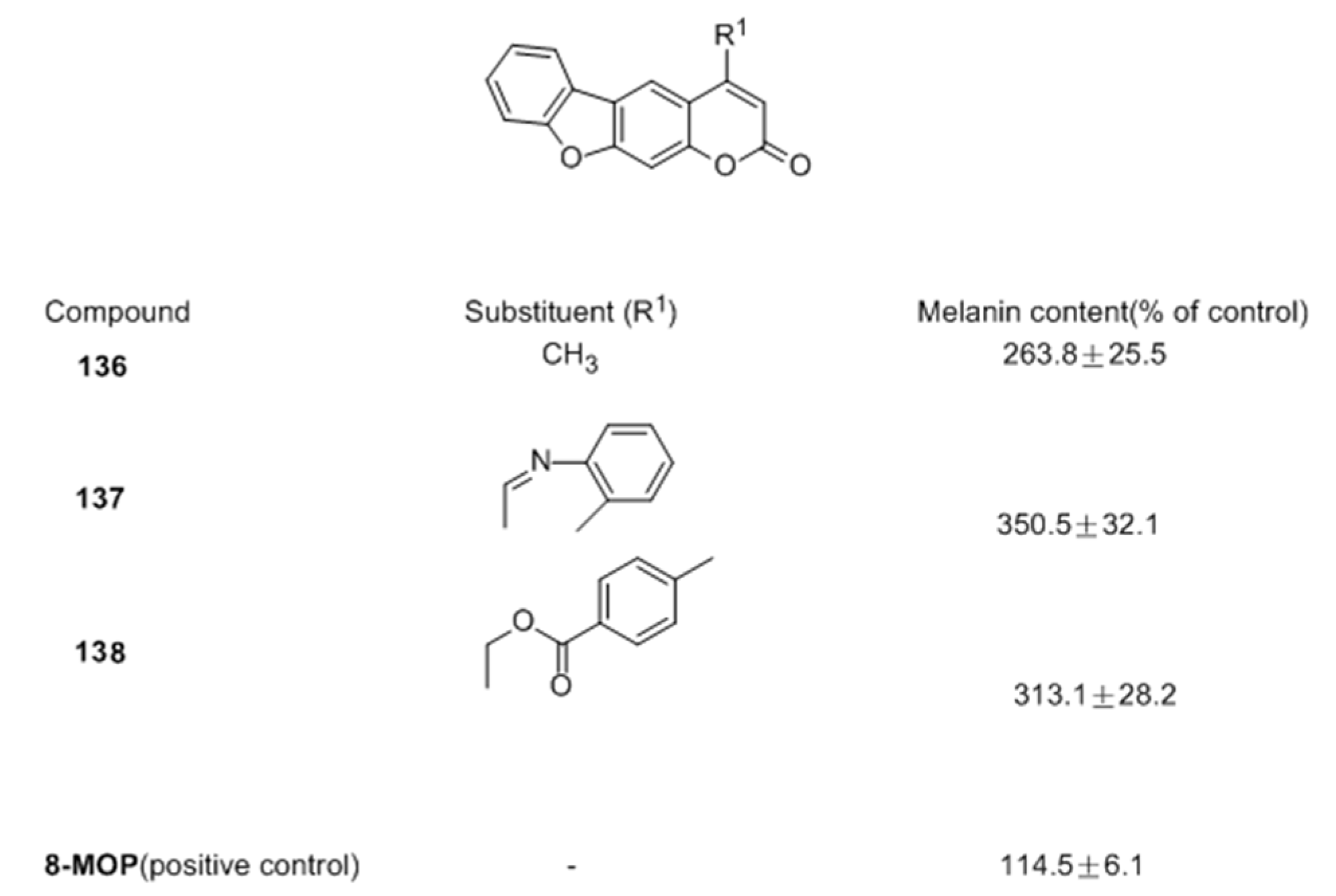
2.2.2. Coumarins
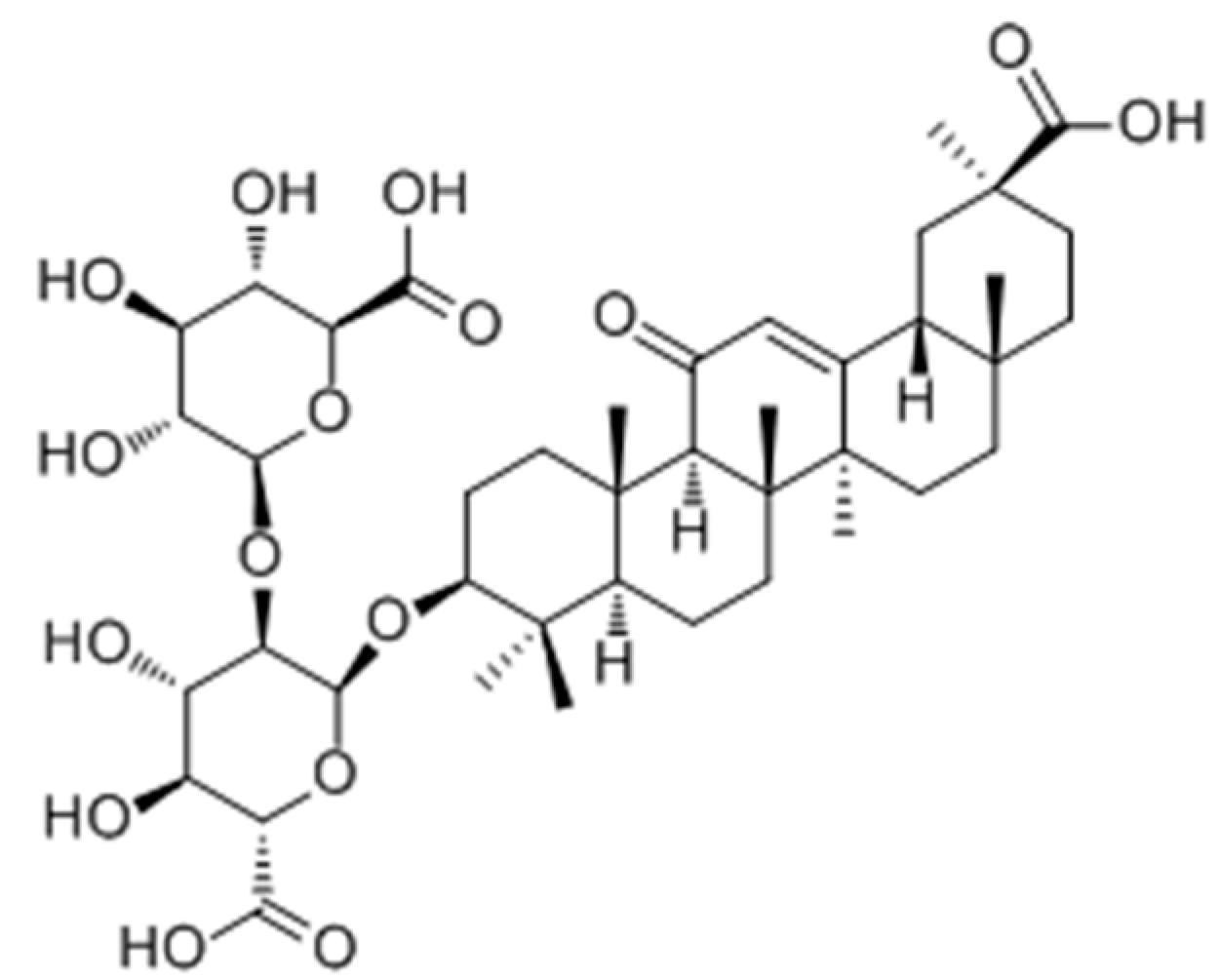
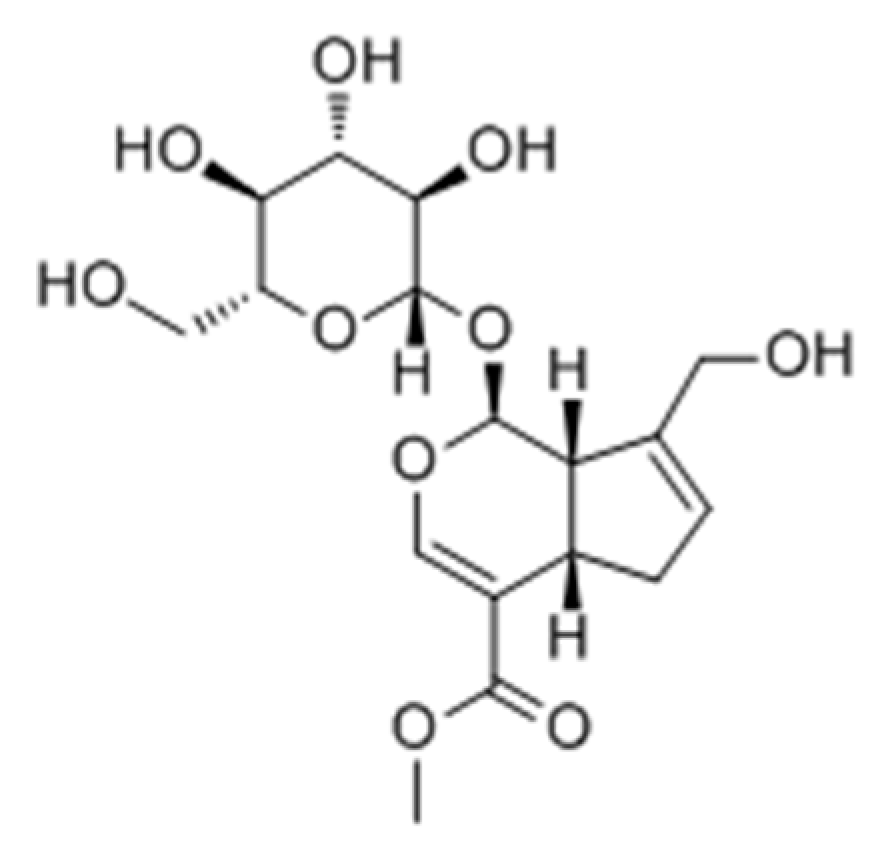
2.2.3. Terpenoids
2.2.4. Resveratrols
2.2.5. Aurones
2.2.6. Polyphenols
2.2.7. Other Compounds
3. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| UV | Ultra-violet |
| α-MSH | α-Melanocyte-stimulating hormone |
| SCF | Stem cell factor |
| ET-1 | Endothelin-1 |
| NO | Nitric oxide |
| ACTH | Adrenocorticotropic hormone |
| MITF | Microphthalmia-associated transcription factor |
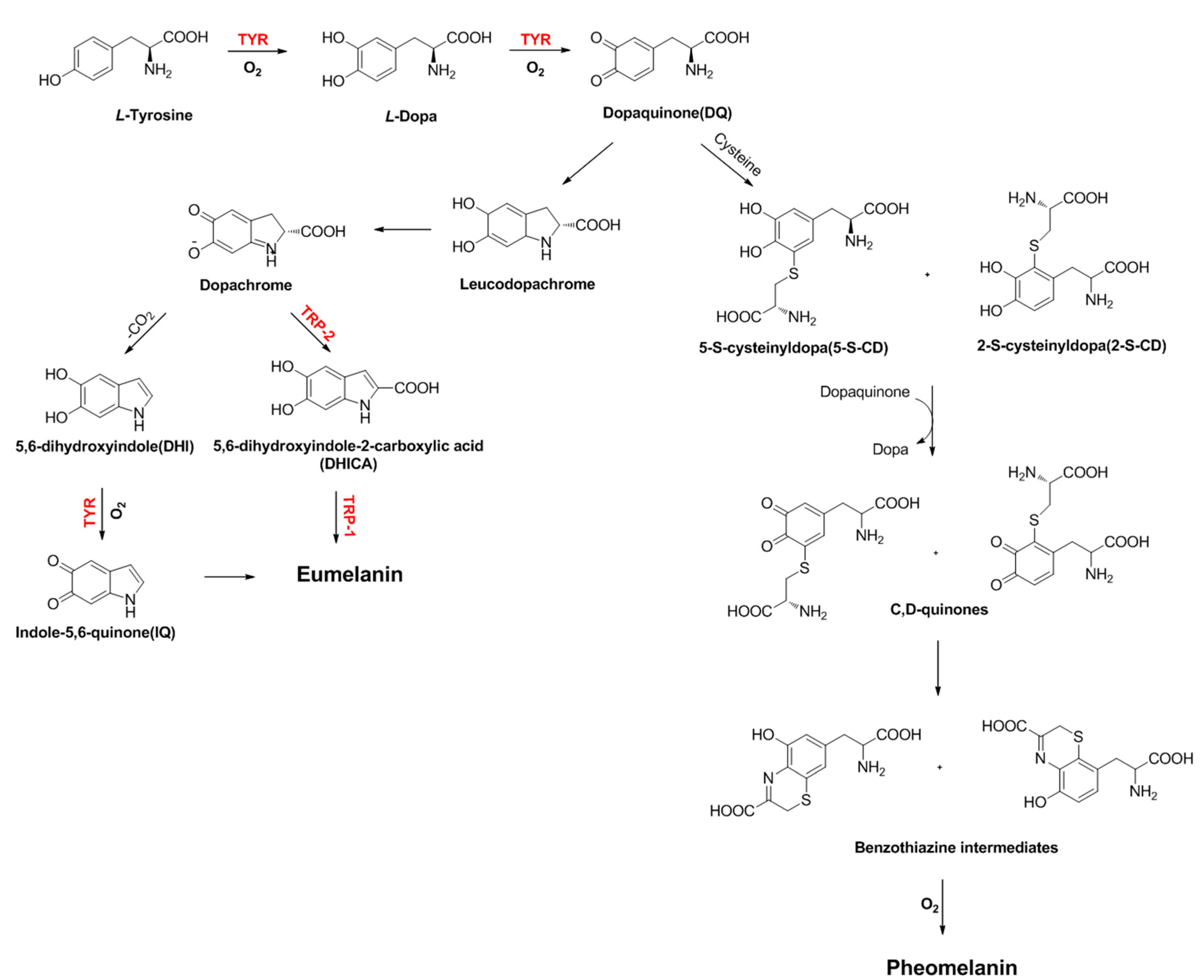
| TYR | Tyrosinase |
| TRP-1 | Tyrosinase-related protein 1 |
| TRP-2 | Tyrosinase-related protein 2 |
| Dct | Dopachrome tautomerase |
| L-DOPA | 3,4-Dihydroxyphenylalanine |
| DQ | Dopaquinone |
| DHI | 5,6-Dihydroxyindole |
| DHICA | 5,6-Dihydroxyindole-2-carboxylic acid |
| IQ | Indole-5,6-quinone |
| 5-S-CD | 5-S-cysteinyldopa |
| 2-S-CD | 2-S-cysteinyldopa |
| p38 MAPK | p38 Mitogen-activated protein kinase |
| 8-MOP | 8-Methoxypsoralen |
| PMRP | Polygoni multiflori radix praeparata |
| EH | Ecliptae herba |
| RRP | Rehmanniae radix praeparata |
| TNF-α | Tumor necrosis factor |
| CGA | Chlorogenic acid |
| RA | Rosmarinic acid |
| BV | Bee venom |
| GSK3β | Glycogen synthase kinase-3β |
| PI3K | Phosphatidylinositol 3-kinase |
| MAPKs | Mitogen-activated protein kinases |
| EDCI | 1-Ethyl-3-(3-dimethylaminopropyl)carbodiimide hydrochloride |
| HOBt | 1-Hydroxybenzotriazole |
| SAR | Structure-activity relationship |
| CREB | cAMP response element binding protein |
| ERK | Extracellular signal-regulated kinases |
| AP-1 | Activator protein-1 |
| PKA | Protein kinase AA |
References
- Nair, B.K. Vitiligo-a retrospect. Int. J. Dermatol. 1978, 17, 755–757. [Google Scholar] [CrossRef] [PubMed]
- Gauthier, Y.; Benzekri, L. Historical aspects. In Vitiligo; Picardo, M., Taieb, A., Eds.; Springer Verlag: Heidelberg/Berlin, Germany, 2010; pp. 3–9. [Google Scholar]
- Sheth, V.M.; Gunasekera, N.S.; Silwal, S.; Qureshi, A.A. Development and pilot testing of a vitiligo screening tool. Arch. Dermatol. Res. 2015, 307, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Ezzedine, K.; Sheth, V.; Rodrigues, M.; Eleftheriadou, V.; Harris, J.E.; Hamzavi, I.H.; Pandya, A.G. Vitiligo is not a cosmetic disease. J. Am. Acad. Dermatol. 2015, 73, 883–885. [Google Scholar] [CrossRef] [PubMed]
- Ezzedine, K.; Lim, H.W.; Suzuki, T.; Katayama, I.; Hamzavi, I.; Lan, C.C.E.; Goh, B.K.; Anbar, T.; Silva de Castro, C.; Lee, A.Y.; et al. Revised classification/nomenclature of vitiligo and related issues: The Vitiligo Global Issues Consensus Conference. Pigment Cell Melanoma Res. 2012, 25, E1–E13. [Google Scholar] [CrossRef] [PubMed]
- Halder, R.M.; Chappell, J.L. Vitiligo update. Semin. Cutan. Med. Surg. 2009, 28, 86–92. [Google Scholar] [CrossRef] [PubMed]
- Namazi, M.R. Neurogenic dysregulation, oxidative stress, autoimmunity, and melanocytorrhagy in vitiligo: Can they be interconnected? Pigment Cell Melanoma Res. 2007, 20, 360–363. [Google Scholar] [CrossRef] [PubMed]
- Schallreuter, K.U.; Bahadoran, P.; Picardo, M.; Slominski, A.; Elassiuty, Y.E.; Kemp, E.; Giachino, C.; Liu, J.B.; Luiten, R.M.; Lambe, T.; et al. Vitiligo pathogenesis: Autoimmune disease, genetic defect, excessive reactive oxygen species, calcium imbalance, or what else? Exp. Dermatol. 2008, 17, 139–140. [Google Scholar] [PubMed]
- Schallreuter, K.U.; Kothari, S.; Chavan, B.; Spencer, J.D. Regulation of Melanogenesis-controversies and new concepts. Exp. Dermatol. 2008, 17, 395–404. [Google Scholar] [CrossRef] [PubMed]
- Hachiya, A.; Kobayashi, A.; Yoshida, Y.; Kitahara, T.; Takema, Y.; Imokawa, G. Biphasic expression of two paracrine melanogenic cytokines, stem cell factor and endothelin-1, in ultraviolet B-induced human melanogenesis. Am. J. Pathol. 2004, 165, 2099–2109. [Google Scholar] [CrossRef]
- Suzuki, I.; Cone, R.D.; Im, S.; Nordlund, J.; Abdel-Malek, Z.A. Binding of melanotropic hormones to the melanocortin receptor MC1R on human melanocytes stimulates proliferation and melanogenesis. Endocrinology 1996, 137, 1627–1633. [Google Scholar] [CrossRef] [PubMed]
- Imokawa, G.; Miyagishi, M.; Yada, Y. Endothelin-1 as a new melanogen: Coordinated expression of its gene and the tyrosinase gene in UVB-exposed human epidermis. J. Investig. Dermatol. 1995, 105, 32–37. [Google Scholar] [CrossRef] [PubMed]
- Schauer, E.; Trautinger, F.; Köck, A.; Schwarz, A.; Bhardwaj, R.; Simon, M.; Ansel, J.C.; Schwarz, T.; Luger, T.A. Proopiomelanocortin-derived peptides are synthesized and released by human keratinocytes. J. Clin. Investig. 1994, 93, 2258–2262. [Google Scholar] [CrossRef] [PubMed]
- Thody, A.J.; Graham, A. Does alpha-MSH have a role in regulating skin pigmentation in humans? Pigment Cell Res. 1998, 11, 265–274. [Google Scholar] [CrossRef] [PubMed]
- Nordlund, J.J.; Collins, C.E.; Rheins, L.A. Prostaglandin E2 and D2 but not MSH stimulate the proliferation of pigment cells in the pinnal epidermis of the DBA/2 mouse. J. Investig. Dermatol. 1986, 86, 433–437. [Google Scholar] [CrossRef] [PubMed]
- Eller, M.S.; Yaar, M.; Gilchrest, B.A. DNA damage and melanogenesis. Nature 1994, 372, 413–414. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, M.; Takahashi, Y.; Inoue, S. Histamine induces melanogenesis and morphologic changes by protein kinase A activation via H2 receptors in human normal melanocytes. J. Investig. Dermatol. 2000, 14, 334–342. [Google Scholar] [CrossRef] [PubMed]
- Pillaiyar, T.; Manickam, M.; Jung, S.H. Downregulation of melanogenesis: Drug discovery and therapeutic options. Drug Discov. Today 2017, 22, 282–298. [Google Scholar] [CrossRef] [PubMed]
- Vachtenheim, J.; Borovanský, J. Transcription physiology of pigment formation in melanocytes: Central role of MITF. Exp. Dermatol. 2010, 19, 617–627. [Google Scholar] [CrossRef] [PubMed]
- DelMarmol, V.; Beermann, F. Tyrosinase and related proteins in mammalian pigmentation. FEBS Lett. 1996, 381, 165–168. [Google Scholar] [CrossRef]
- Liu, J.J.; Fisher, D.E. Lighting a path to pigmentation: Mechanisms of MITF induction by UV. Pigment Cell Melanoma Res. 2010, 23, 741–745. [Google Scholar] [CrossRef] [PubMed]
- Park, S.H.; Kim, D.S.; Kim, W.G.; Ryoo, I.J.; Lee, D.H.; Huh, C.H.; Youn, S.W.; Yoo, I.D.; Park, K.C. Terrein: A new melanogenesis inhibitor and its mechanism. Cell Mol. Life Sci. 2004, 61, 2878–2885. [Google Scholar] [CrossRef] [PubMed]
- Pillaiyar, T.; Manickam, M.; Jung, S.H. Inhibitors of melanogenesis: A patent review (2009–2014). Expert Opin. Ther. Pat. 2015, 25, 775–788. [Google Scholar] [CrossRef] [PubMed]
- Schiaffino, M.V. Signaling pathways in melanosome biogenesis and pathology. Int. J. Biochem. Cell Biol. 2010, 42, 1094–1104. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Ferrer, A.; Rodríguez-López, J.N.; García-Cánovas, F.; García-Carmina, F. Tyrosinase: A comprehensive review of its mechanism. Biochim. Biophys. Acta 1995, 1247, 1–11. [Google Scholar] [CrossRef]
- Matoba, Y.; Kumagai, T.; Yamamoto, A.; Yoshitsu, H.; Sugiyama, M. Crystallographic evidence that the dinuclear copper center of tyrosinase is flexible during catalysis. J. Biol. Chem. 2008, 281, 8981–8990. [Google Scholar] [CrossRef] [PubMed]
- Decker, H.; Tuczek, F. Tyrosinase/catecholoxidase activity of hemocyanins: Structural basis and molecular mechanism. Trends Biochem. Sci. 2000, 25, 392–397. [Google Scholar] [CrossRef]
- Jiménezatiénzar, M.; Escribano, J.; Cabanes, J.; Gandíaherrero, F.; Garcíacarmona, F. Oxidation of the flavonoid eriodictyol by tyrosinase. Plant. Physiol. Biochem. 2005, 43, 866–873. [Google Scholar] [CrossRef] [PubMed]
- Pillaiyar, T.; Manickam, M.; Namasivayam, V. Skin whitening agents: Medicinal chemistryperspective of tyrosinase inhibitors. J. Enzym. Inhib. Med. Chem. 2017, 32, 403–425. [Google Scholar] [CrossRef] [PubMed]
- Tessari, I.; Bisaglia, M.; Valle, F.; Samori, B.; Bergantino, E.; Mammi, S.; Bubacco, L. The reaction of alpha-synuclein with tyrosinase: Possible implications for Parkinsondisease. J. Biol. Chem. 2008, 283, 16808–16817. [Google Scholar] [CrossRef] [PubMed]
- Hasegawa, T. Tyrosinase-expressing neuronal cell line as invitro model of Parkinson’s disease. Int. J. Mol. Sci. 2010, 11, 1082–1089. [Google Scholar] [CrossRef] [PubMed]
- Yi, W.; Cao, R.H.; Peng, W.L.; Wen, H.; Yan, Q.; Zhou, B.; Ma, L.; Song, H.C. Synthesis and biological evaluationof novel 4-hydroxybenzaldehyde derivatives as tyrosinaseinhibitors. Eur. J. Med. Chem. 2010, 45, 639–646. [Google Scholar]
- Friedman, M. Food browning and its prevention: An overview. J. Agric. Food Chem. 1996, 44, 631–653. [Google Scholar] [CrossRef]
- Liu, S.H.; Pan, I.H.; Chu, I.M. Inhibitory effect of p-hydroxybenzylalcohol on tyrosinase activity and melanogenesis. Biol. Pharm. Bull. 2007, 30, 1135–1139. [Google Scholar] [CrossRef] [PubMed]
- Passeron, T.; Mantoux, F.; Ortonne, J.P. Genetic disorders of pigmentation. Clin. Dermatol. 2005, 23, 56–67. [Google Scholar] [CrossRef] [PubMed]
- Teulings, H.E.; Lommerts, J.E.; Wolkerstorfer, A.; Nieuweboer-Krobotova, L.; Luiten, R.M.; Bekkenk, M.W.; Van der Veen, J.P. Vitiligo-like depigmentations as the first sign of melanoma: A retrospective case series from a tertiary vitiligo centre. Br. J. Dermatol. 2017, 176, 503–506. [Google Scholar] [CrossRef] [PubMed]
- Chaabane, F.; Pinon, A.; Simon, A.; Ghedira, K.; Chekir-Ghedira, L. Phytochemical potential of Daphne gnidium in inhibiting growth of melanoma cells and enhancing melanogenesis of B16-F0 melanoma. Cell Biochem. Funct. 2013, 31, 460–467. [Google Scholar] [CrossRef] [PubMed]
- Chaabane, F.; Pinon, A.; Simon, A.; Ghedira, K.; Chekir-Ghedira, L. Chloroform leaf extract of Daphne gnidium inhibits growth of melanoma cells and enhances melanogenesis of B16-F0 melanoma. S. Afr. J. Bot. 2014, 90, 80–86. [Google Scholar] [CrossRef]
- Skandrani, I.; Pinon, A.; Simon, A.; Ghedira, K.; Chekir-Ghedira, L. Chloroform extract from Moricandia arvensis inhibits growth of B16-F0 melanoma cells and promotes differentiation in vitro. Cell Prolif. 2010, 43, 471–479. [Google Scholar] [CrossRef] [PubMed]
- Xu, P.; Su, S.L.; Tan, C.; Lai, R.S.; Min, Z.S. Effects of aqueous extracts of Ecliptae herba, Polygoni multiflori radix praeparata and Rehmanniae radix praeparata on melanogenesis and the migration of human melanocytes. J. Ethnopharmacol. 2017, 195, 89–95. [Google Scholar] [CrossRef] [PubMed]
- Babitha, S.; Nguyen, D.H.; Park, S.J.; Shin, J.H.; Reyes, G.A.; Caburian, A.; Kim, E.K. Potential of Cassia alata leaf extract in inducing differentiation and migration of mouse melanoblasts. Biotechnol. Bioproc. E 2010, 15, 1071–1076. [Google Scholar] [CrossRef]
- Babitha, S.; Shin, J.H.; Nguyen, D.H.; Park, S.J.; Reyes, G.A.; Caburian, A.; Kim, E.K. A stimulatory effect of Cassia occidentalis on melanoblast differentiation and migration. Arch. Dermatol. Res. 2011, 303, 211–216. [Google Scholar] [CrossRef] [PubMed]
- Moreira, C.G.; Horinouchi, C.D.S.; Souza-Filho, C.S.; Campos, F.R.; Barison, A.; Cabrini, D.A.; Otuki, M.F. Hyperpigmentant activity of leaves and flowers extracts of Pyrostegia venusta on murine B16-F10 melanoma. J. Ethnopharmacol. 2012, 141, 1005–1011. [Google Scholar] [CrossRef] [PubMed]
- Moreira, C.G.; Carrenho, L.Z.B.; Pawloski, P.L.; Soley, B.S.; Cabrini, D.A.; Otuki, M.F. Pre-clinicalevidencesof Pyrostegia venusta in thetreatment of vitiligo. J. Ethnopharmacol. 2015, 168, 315–325. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Shang, J.; Ping, F.F.; Zhao, G.R. Alcohol extract from Vernonia anthelmintica (L.) willd seed enhances melanin synthesis through activation of the p38 MAPK signaling pathway in B16-F10 cells and primary melanocytes. J. Ethnopharmacol. 2012, 143, 639–647. [Google Scholar] [CrossRef] [PubMed]
- Tuerxuntayi, A.; Liu, Y.Q.; Tulake, A.; Kabas, M.; Eblimit, A.; Aisa, H.A. Kaliziri extract upregulates tyrosinase, TRP-1, TRP-2 and MITF expression in murine B16 melanoma cells. BMC Complement. Altern. Med. 2014, 14, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Li, H. R.; Habasi, M.; Xie, L.Z.; Aisa, H.A. Effect of chlorogenic acid on melanogenesis of B16 melanoma cells. Molecules 2014, 19, 12940–12948. [Google Scholar] [CrossRef] [PubMed]
- Maimaiti, Z.; Turak, A.; Aisa, H.A. Two new compounds from the seeds of Vernonia anthelmintica. J. Asian Nat. Prod. Res. 2016, 25, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Sáncheza, A.; Barrajón-Catalána, E.; Herranz-Lópeza, M.; Castilloc, J.; Micola, V. Lemon balm extract (Melissa officinalis, L.) promotes melanogenesis and prevents UVB-induced oxidative stress and DNA damage in a skin cell model. J. Dermatol. Sci. 2016, 84, 169–177. [Google Scholar] [CrossRef] [PubMed]
- Yao, C.; Jin, C.L.; OH, I.G.; Park, C.H.; Chung, J.H. Melia azedarach extract stimulates melanogenesis through increase of tyrosinase-related protein 1 expression in B16-F10 mouse melanoma cells. Int. J. Mol. Med. 2015, 35, 1761–1766. [Google Scholar] [PubMed]
- Matsuyama, K.; Kawano, M.; Kchouk, M.E.; Shinmoto, H.; Isoda, H. Effect of Tunisian aromatic plant extracts on melanogenesis. In Cells and Culture; Noll, T., Ed.; Springer: Dordrecht, The Netherlands, 2010; pp. 735–738. [Google Scholar]
- Chiang, H.M.; Lin, J.W.; Hsiao, P.L.; Tsai, S.Y.; Wen, K.C. Hydrolysates of Citrus plants stimulate melanogenesis protecting against UV-induced dermal damage. Phytother. Res. 2011, 25, 569–576. [Google Scholar] [CrossRef] [PubMed]
- Jeon, S.; Kim, N.H.; Koo, B.S.; Lee, H.J.; Lee, A.Y. Bee venom stimulates human melanocyte proliferation, melanogenesis, dendricity and migration. Exp. Mol. Med. 2007, 39, 603–613. [Google Scholar] [CrossRef] [PubMed]
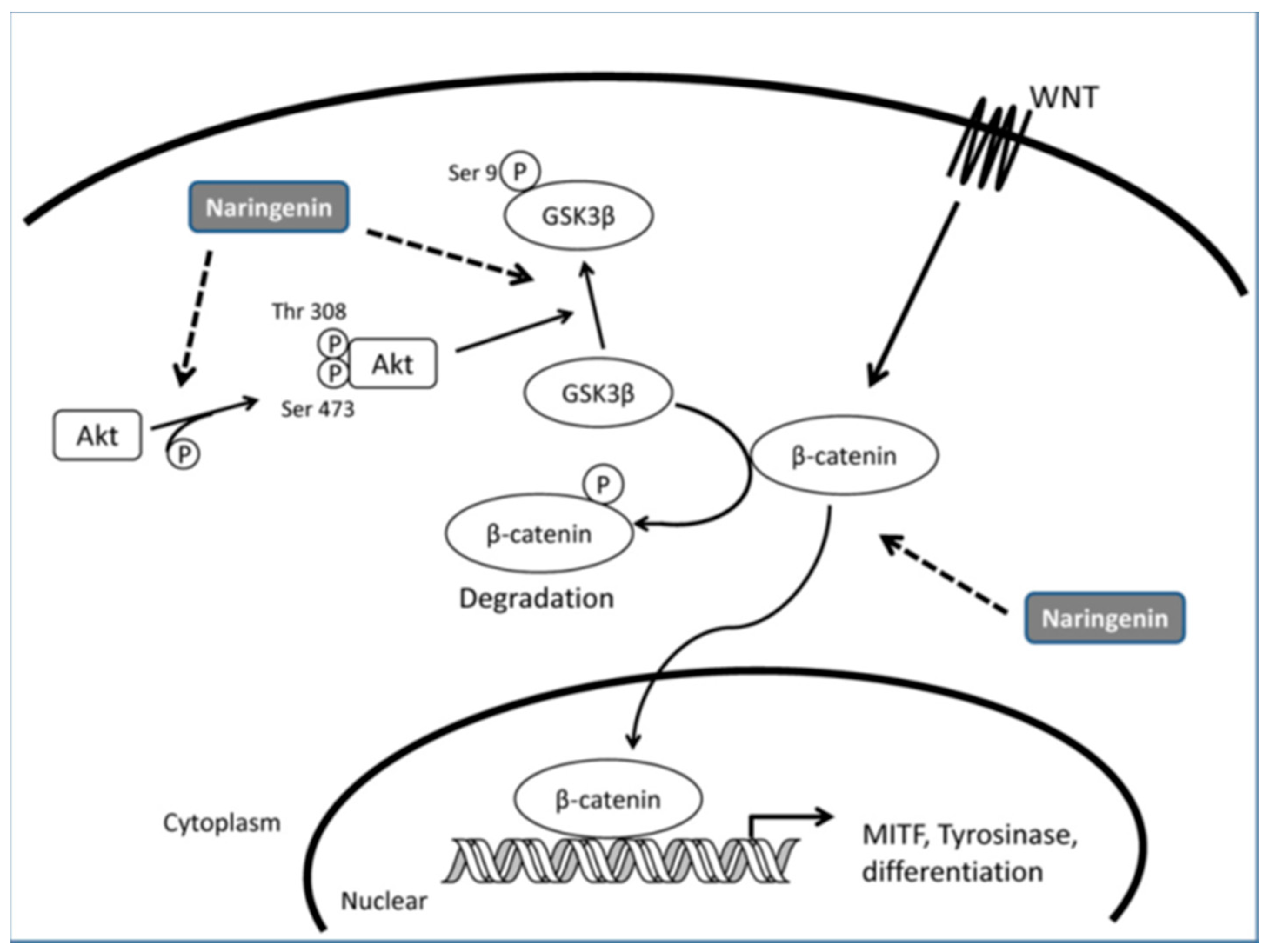
- Ohguchi, K.; Akao, Y.; Nozawa, Y. Stimulation of melanogenesis by the Citrus flavonoid naringenin in mouse B16 melanoma cells. Biosci. Biotechnol. Biochem. 2006, 70, 1499–1501. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.C.; Yang, C.H.; Chiou, Y.L. Citrus flavanone naringenin enhances melanogenesis through the activation of Wnt/β-catenin signalling in mouse melanoma cells. Phytomedicine 2011, 18, 1244–1249. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.C.; Liu, K.C.; Chiou, Y.L. Melanogenesis of murine melanoma cells induced by hesperetin, a Citrus hydrolysate-derived flavonoid. Food Chem. Toxicol. 2012, 50, 653–659. [Google Scholar] [CrossRef] [PubMed]
- Lv, J.P.; Zha, X. M.; Pang, S.L.; Jia, H.P.; Zhang, Y.; Shang, J. Synthesis and melanogenesis evaluation of 3′,4′,7-trihydroxyflavanone derivatives and characterization of flavanone-BODIPY. Bioorg. Med. Chem. Lett. 2015, 25, 1607–1610. [Google Scholar] [CrossRef] [PubMed]
- Garcez, F.R.; Garcez, W.S.; Santana, A.L.B.D.; Alves, M.M.; Matos, M.F.C.; Scaliante, A.M. Bioactive flavonoids and triterpenes from Terminalia fagifolia (Combretaceae). J. Braz. Chem. Soc. 2006, 17, 1223–1228. [Google Scholar] [CrossRef]
- Vanamala, J.; Reddivari, L.; Yoo, K.S.; Pike, L.M.; Patil, B.S. Variation in the content of bioactive flavonoids in different brands of orange and grapefruit juices. J. Food Compos. Anal. 2006, 19, 157–166. [Google Scholar] [CrossRef]
- De Sousa, J.P.; Bueno, P.C.; Gregório, L.E.; Da Silva Filho, A.A.; Furtado, N.A.; De Sousa, M.L.; Bastos, J.K. A reliable quantitative method for the analysis of phenolic compounds in Brazilian propolis by reverse phase high performance liquid chromatography. J. Sep. Sci. 2007, 30, 2656–2665. [Google Scholar] [CrossRef] [PubMed]
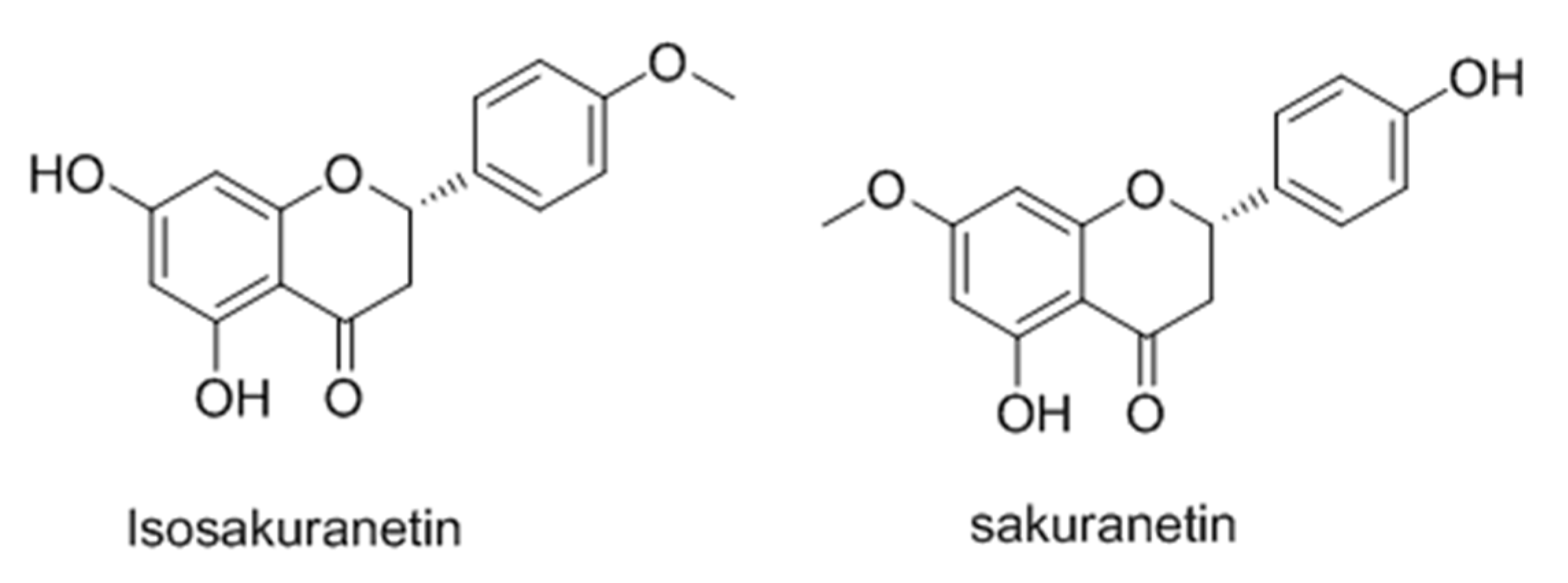
- Drira, R.; Sakamoto, K. Isosakuranetin, a 4′-O-methylated flavonoid, stimulates melanogenesis in B16-BL6 murine melanoma cells. Life Sci. 2015, 143, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Modzelewska, A.; Pettit, C.; Achanta, G.; Davidson, N.E.; Huang, P.; Khan, S.R. Anticancer activities of novel chalcone and bis-chalcone derivatives. Bioorg. Med. Chem. 2006, 14, 3491–3495. [Google Scholar] [CrossRef] [PubMed]
- Araico, A.; Terencio, M.C.; Alcaraz, M.J.; Dominguez, J.N.; Leon, C.; Ferrandiz, M.L. Evaluation of the anti-inflammatory and analgesic activity of Me-UCH9, a dual cyclooxygenase-2/5-lipoxygenase inhibitor. Life Sci. 2007, 80, 2108–2117. [Google Scholar] [CrossRef] [PubMed]
- Batovska, D.; Parushev, S.; Stamboliyska, B.; Tsvetkova, I.; Ninova, M.; Najdenski, H. Examination of growth inhibitory properties of synthetic chalcones for which antibacterial activity was predicted. Eur. J. Med. Chem. 2009, 44, 2211–2218. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Cruz, F.; Vazquez-Rodriguez, S.; Matos, M.J.; Herrera-Morales, A.; Villamena, F.A.; Das, A.; Gopalakrishnan, B.; Olea-Azar, C.; Santana, L.; Uriarte, E. Synthesis and electrochemical and biological studies of novel coumarin-chalcone hybrid compounds. J. Med. Chem. 2013, 56, 6136–6145. [Google Scholar] [CrossRef] [PubMed]
- Jun, N.; Gao, H.; Jun, K. Synthesis and evaluation of 2′,4′,6′-trihydroxychalcones as a new class of tyrosinase inhibitors. Bioorg. Med. Chem. 2007, 15, 2396–2402. [Google Scholar] [CrossRef] [PubMed]
- Thanigaimalai, P.; Lee, K.C.; Sharma, V.K.; Rao, E.V.; Roh, E.; Kim, Y.; Jung, S.H. Structural requirements of (E)-6-benzylidene-4a-methyl-4,4a,5,6,7,8-hexahydronaphthalen-2(3H)-one derivatives as novel melanogenesis inhibitors. Bioorg. Med. Chem. Lett. 2011, 21, 1922–1925. [Google Scholar] [CrossRef] [PubMed]
- Sonmez, F.; Sevmezler, S.; Atahan, A.; Ceylan, M.; Demir, D.; Gencer, N.; Arslan, O.; Kucukislamoglu, M. Evaluation of new chalcone derivatives as polyphenol oxidase inhibitors. Bioorg. Med. Chem. Lett. 2011, 21, 7479–7482. [Google Scholar] [CrossRef] [PubMed]
- Nixha, A.R.; Arslan, M.; Atalay, Y.; Gençer, N.; Ergün, A.; Arslan, O. Synthesis and theoretical calculations of carbazole substituted chalcone urea derivatives and studies their polyphenol oxidase enzyme activity. Enzym. Inhib. Med. Chem. 2013, 28, 808–815. [Google Scholar] [CrossRef] [PubMed]
- Niu, C.; Li, G.; Madina, K.; Aisa, H.A. Synthesis and activity on tyrosinase of novel chalcone derivatives. Chem. J. Chin. Univ. 2014, 35, 1204–1211. [Google Scholar]
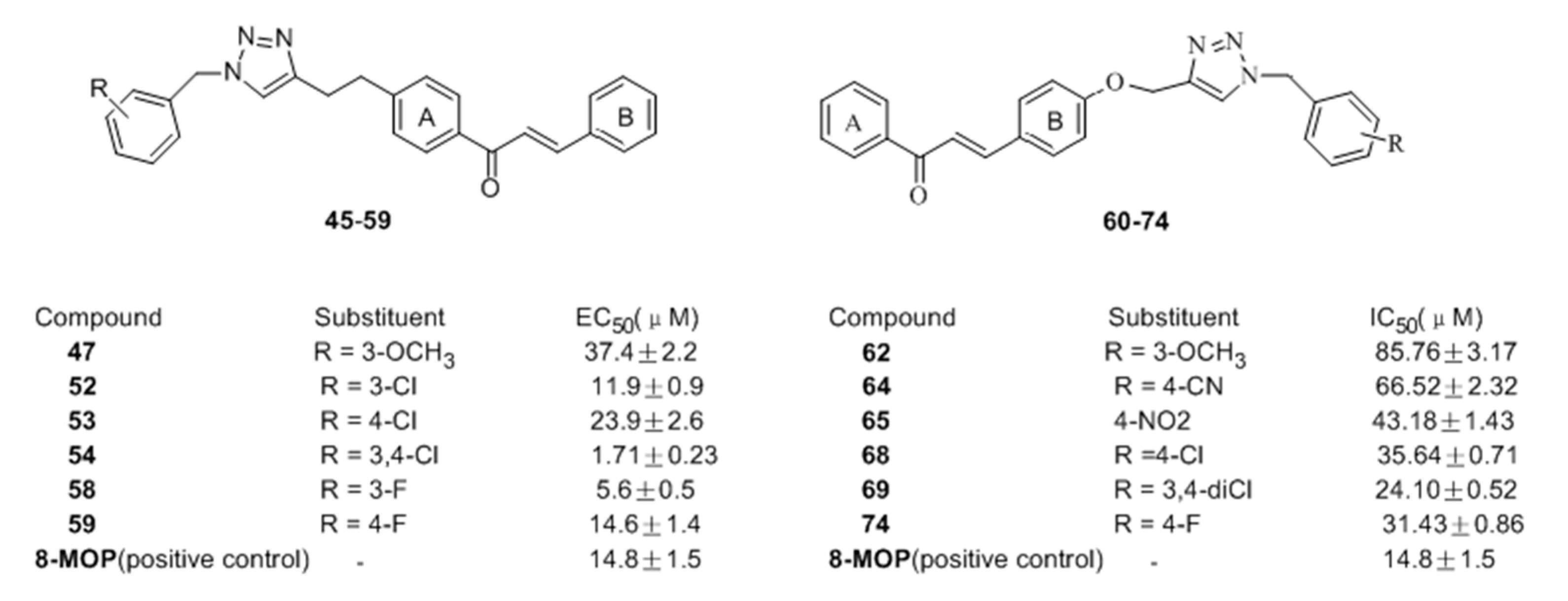
- Niu, C.; Li, G.; Tuerxuntayi, A.; Aisa, H.A. Synthesis and bioactivity of new chalcone derivatives as potential tyrosinase activator based on the click chemistry. Chin. J. Chem. 2015, 33, 486–494. [Google Scholar] [CrossRef]
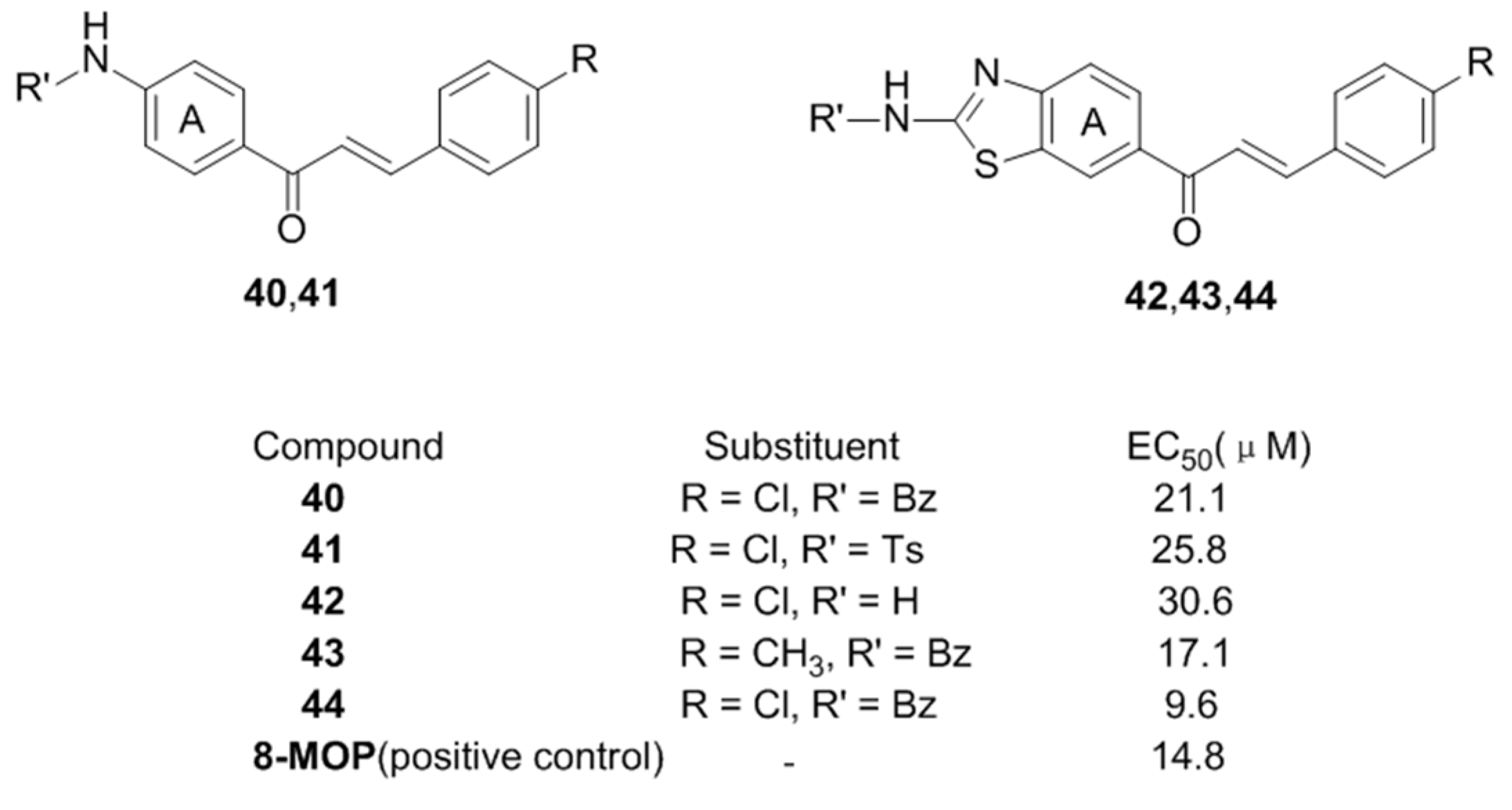
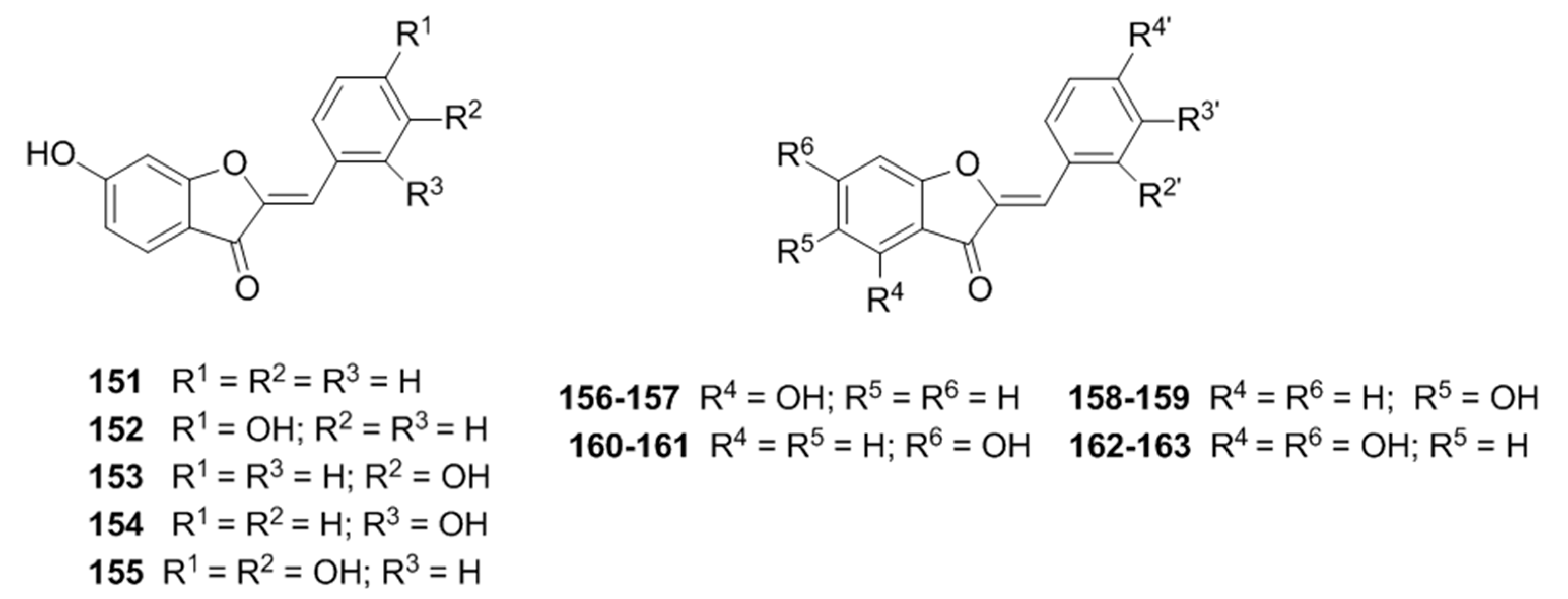
- Niu, C.; Yin, L.; Nie, L.F.; Dou, J.; Zhao, J.Y.; Li, G.; Aisa, H.A. Synthesis and bioactivity of novel isoxazole chalcone derivatives on tyrosinase and melanin synthesis in murine B16 cells for the treatment of vitiligo. Bioorg. Med. Chem. 2016, 24, 5440–5448. [Google Scholar] [CrossRef] [PubMed]
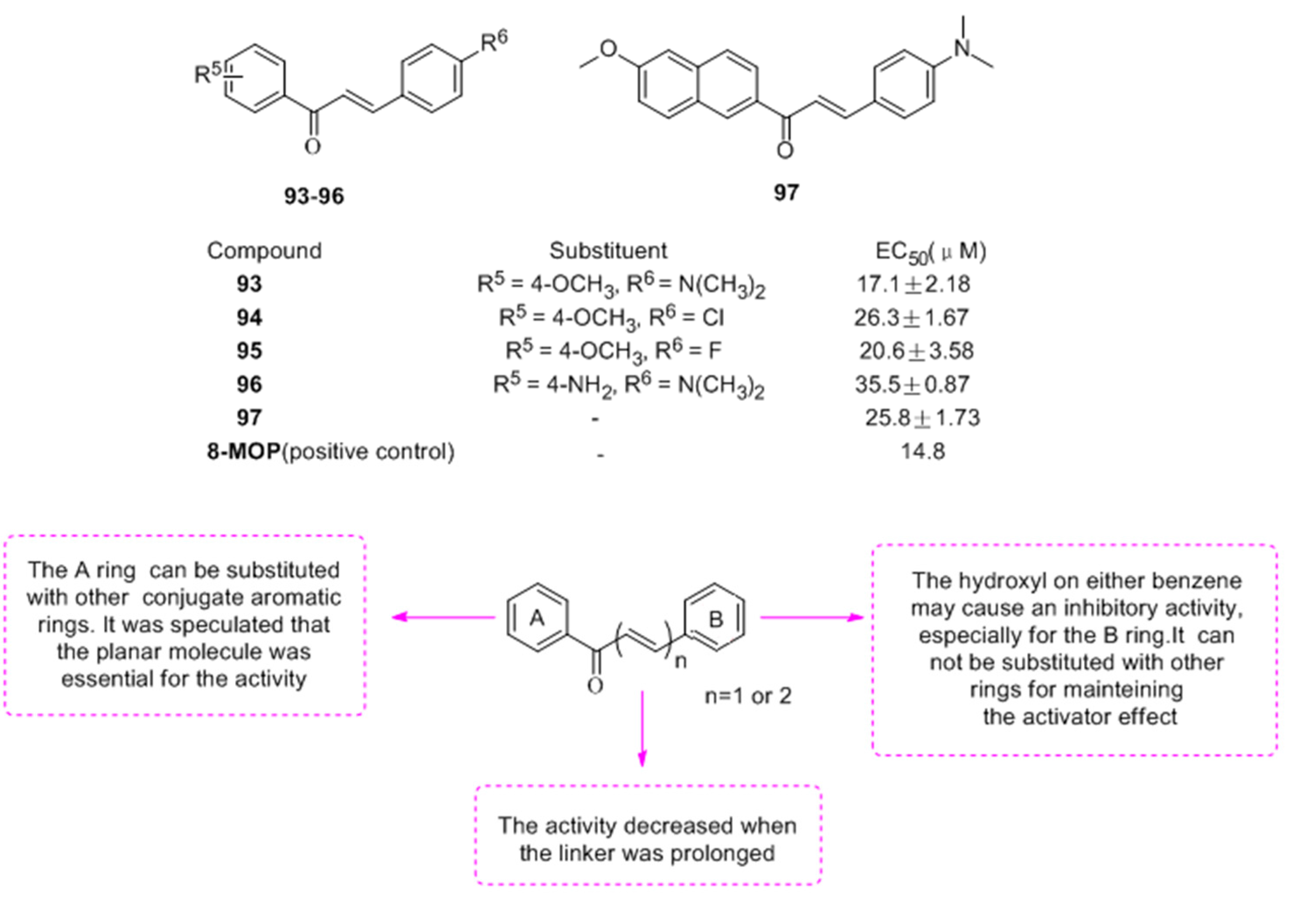
- Niu, C.; Tuerxuntayi, A.; Li, G.; Kabas, M.; Dong, C.Z.; Aisa, H.A. Design, synthesis and bioactivity of chalcones and its analogues. Chin. Chem. Lett. 2017, 28, 1533–1538. [Google Scholar] [CrossRef]
- Yamauchi, K.; Mitsunaga, T.; Batubara, I. Novel quercetin glucosides from Helminthostachys zeylanica root and acceleratory activity of melanin biosynthesis. J. Nat. Med. 2013, 67, 369–374. [Google Scholar] [CrossRef] [PubMed]
- Yamauchi, K.; Mitsunaga, T.; Batubara, I. Synthesis of quercetin glycosides and their melanogenesis stimulatory activity in B16 melanoma cells. Bioorg. Med. Chem. 2014, 22, 937–944. [Google Scholar] [CrossRef] [PubMed]
- Yamauchi, K.; Mitsunaga, T.; Inagaki, M.; Suzuki, T. Synthesized quercetin derivatives stimulate melanogenesis in B16 melanoma cells by influencing the expression of melanin biosynthesis proteins MITF and p38 MAPK. Bioorg. Med. Chem. 2014, 22, 3331–3340. [Google Scholar] [CrossRef] [PubMed]
- Ye, Y.; Chou, G.X.; Wang, H.; Chu, J.H.; Yu, Z.L. Flavonoids, apigenin and icariin exert potent melanogenic activities in murine B16 melanoma cells. Phytomedicine 2010, 18, 32–35. [Google Scholar] [CrossRef] [PubMed]
- Yoon, H.S.; Lee, S.R.; Ko, H.C.; Choi, S.Y.; Park, J.G.; Kim, J.K.; Kim, S.J. Involvement of extracellular signal-regulated Kinase in nobiletin-induced melanogenesis in murine B16-F10 melanoma cells. Biosci. Biotechnol. Biochem. 2007, 71, 1781–1784. [Google Scholar] [CrossRef] [PubMed]
- Horibe, I.; Satoh, Y.; Shiota, Y.; Kumagai, A.; Horike, N.; Takemori, H.; Uesato, S.; Sugie, S.; Obata, K.; Kawahara, H.; Nagaoka, Y. Induction of melanogenesis by 4′-O-methylated flavonoids in B16-F10 melanoma cells. J. Nat. Med. 2013, 67, 705–710. [Google Scholar] [CrossRef] [PubMed]
- Park, W.S.; Kwon, O.; Yoon., T.J.; Jin, H.C. Anti-graying effect of the extract of Pueraria thunbergiana via upregulation of cAMP/MITF-M signaling pathway. J. Dermatol. Sci. 2014, 75, 154–156. [Google Scholar] [CrossRef] [PubMed]
- El Mofty, A.M. Observations on the use of Ammi Majus Linn. in vitiligo. Br. J. Dermatol. 1952, 64, 431–441. [Google Scholar] [CrossRef] [PubMed]
- Ortonne, J.P. Psoralen therapy in vitiligo. Clin. Dermatol. 1989, 7, 120–135. [Google Scholar] [CrossRef]
- Micali, G.; Nasca, M.R.; Musumeci, M.L. Severe phototoxic reaction secondary to the application of a fig leaves’ decoction used as a tanning agent. Contact Dermatitis 1995, 33, 212–213. [Google Scholar] [CrossRef] [PubMed]
- Späth, E.; Manjunath, B.L.; Pailer, M.; Jois, H.S. Synthese und konstitution des psoralens. Eur. J. Inorg. Chem. 1936, 69, 1087–1090. [Google Scholar] [CrossRef]
- Ekiert, H.; Gomółka, E. Coumarin compounds in Ammi majus L. callus cultures. Pharmazie 2000, 55, 684–687. [Google Scholar] [PubMed]
- Innocenti, G.; Bettero, A.; Caporale., G. Determination of the coumarinic constituents of Ficus carica leaves by HPLC. Farmaco Sci. 1982, 37, 475–485. [Google Scholar] [PubMed]
- Felsten, L.M.; Alikhan, A.; Petronic-Rosic, V. Vitiligo: A comprehensive overview: Part II: Treatment options and approach to treatment. J. Am. Acad. Dermatol. 2011, 6, 493–514. [Google Scholar] [CrossRef] [PubMed]
- Iannella, G.; Greco, A.; Didona, D.; Didona, B.; Granata, G.; Manno, A.; Pasquariello, B.; Magliulo, G. Vitiligo: Pathogenesis, clinical variants and treatment approaches. Autoimmun. Rev. 2016, 15, 335–343. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, H.; Hirata, N.; Kawaguchi, Y.; Yamazaki, M.; Naruto, S.; Shibano, M.; Taniguchi, M.; Baba, K.; Kubo, M. Melanogenesis stimulation in murine B16 melanoma cells by umberiferae plant extracts and their coumarin constituents. Biol. Pharm. Bull. 2005, 28, 1229–1233. [Google Scholar] [CrossRef] [PubMed]
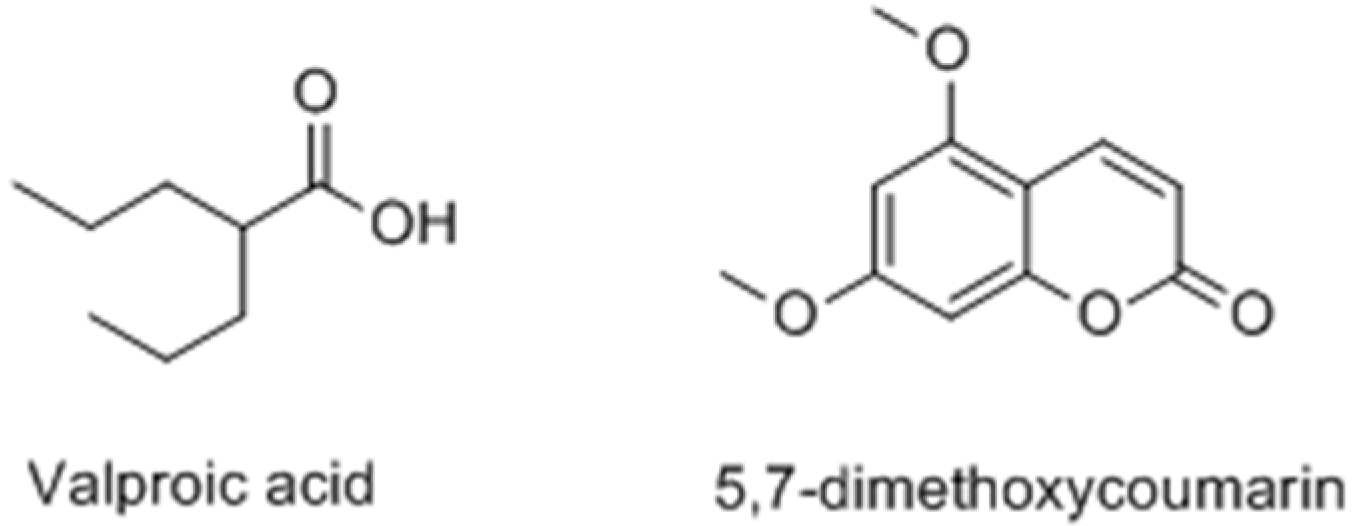
- Chodurek, E.; Orchel, A.; Orchel, J.; Kurkiewicz, S.; Gawlik, N.; Dzierżewicz, Z.; Stęppień, K. Evaluation of melanogenesis in A-375 melanoma cells treated with 5,7-dimethoxycoumarin and valproic acid. Cell. Mol. Biol. Lett. 2012, 17, 616–632. [Google Scholar]
- Pang, G.X.; Niu, C.; Mamat, N.; Aisa, H.A. Synthesis and in vitro biological evaluation of novel Coumarin derivatives containing isoxazole moieties on melanin synthesis in B16 cells and inhibition on bacteria. Bioorg. Med. Chem. Lett. 2017, 27, 2674–2677. [Google Scholar] [CrossRef] [PubMed]
- Niu, C.; Pang, G.X.; Li, G.; Dou, J.; Nie, L.F.; Himit, H.; Kabas, M.; Aisa, H.A. Synthesis and biological evaluation of furocoumarin derivatives on melanin synthesis in murine B16 cells for the treatment of vitiligo. Bioorg. Med. Chem. 2016, 24, 5960–5968. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Jung, E.; Park, J.; Jung, K.; Park, E.; Kim, J.; Hong, S.; Park, J.; Park, S.; Lee, S.; et al. Glycyrrhizin induces melanogenesis by elevating a cAMP level in B16 melanoma cells. J. Investig. Dermatol. 2005, 124, 405–411. [Google Scholar] [CrossRef] [PubMed]
- Lan, W.J.; Wan, H.Y.; Lan, W.; Wang, K.Y. Geniposide enhances melanogenesis by stem cell factor/c-kit signalling in norepinephrine-exposed normal human epidermal melanocyte. Basic Clin. Pharmacol. Toxicol. 2008, 103, 88–93. [Google Scholar] [CrossRef] [PubMed]
- Villareal, M.O.; Han, J.; Matsuyama, K.; Sekii, Y.; Smaoui, A.; Shigemori, H.; Isoda, H. Lupenone from Erica multiflora leaf extract stimulates melanogenesis in B16 murine melanoma cells through the inhibition of ERK1/2 activation. Planta Med. 2013, 79, 236–243. [Google Scholar] [CrossRef] [PubMed]
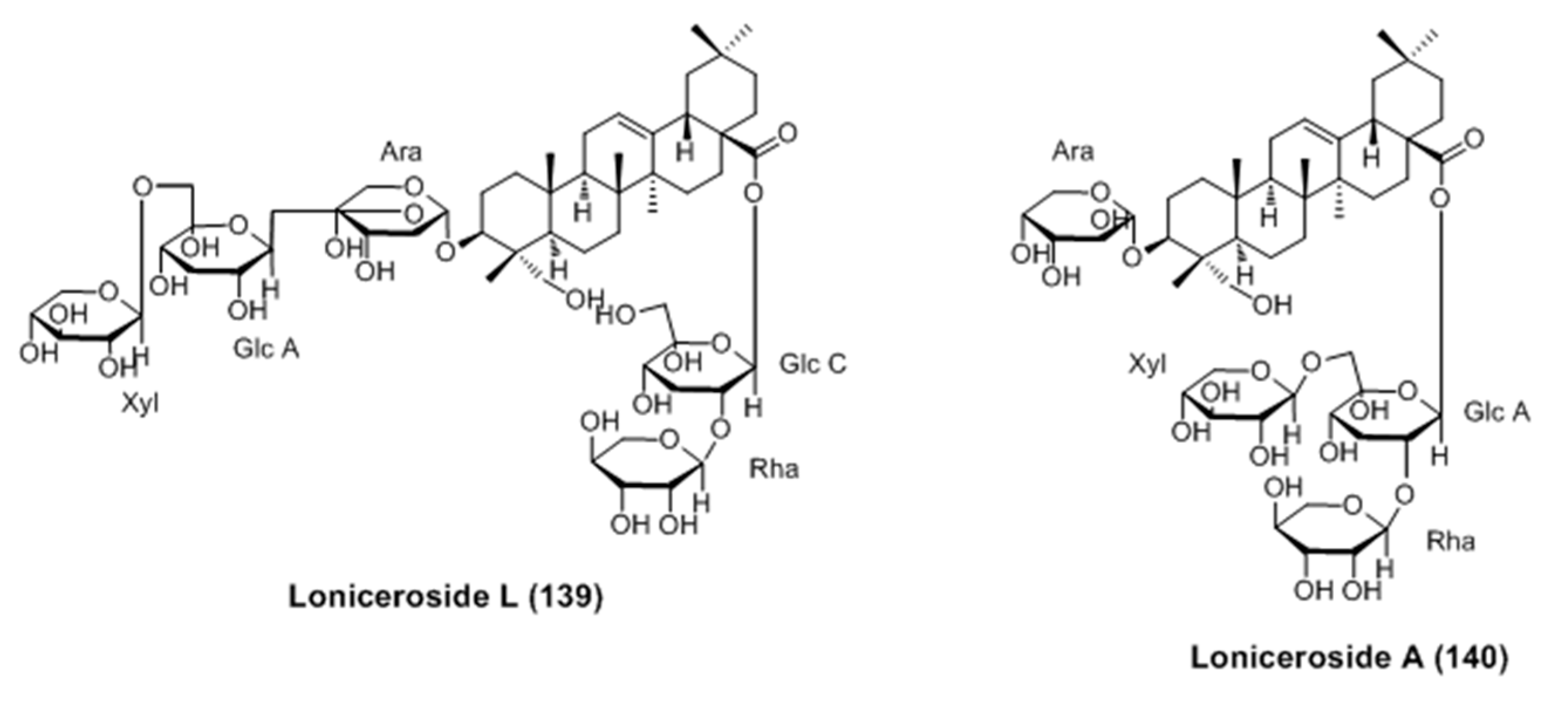
- Won, Y.M.; Seong, Z.K.; Kim, J.L.; Kim, H.S.; Song, H.H.; Kim, D.Y.; Kim, J.H.; Oh, S.R.; Cho, H.W.; Cho, J.H.; Lee, H.K. Triterpene glycosides with stimulatory activity on melanogenesis rom the aerial parts of Weigela subsessilis. Arch. Pharm. Res. 2015, 38, 1541–1551. [Google Scholar] [CrossRef] [PubMed]
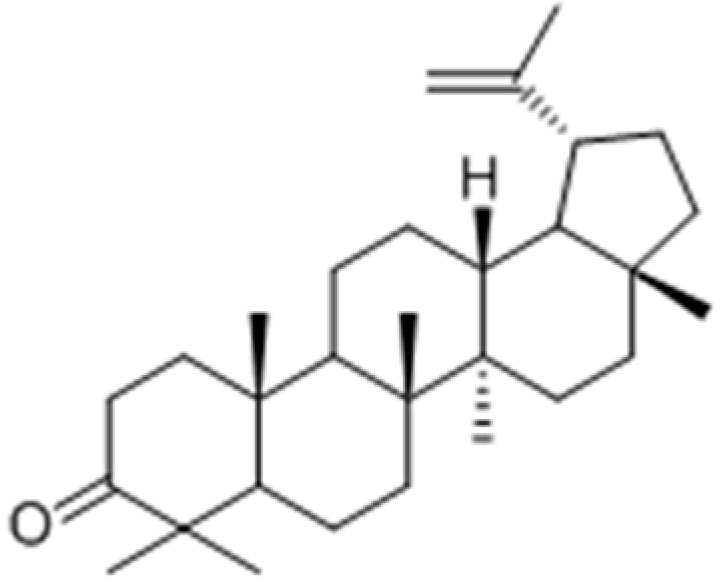
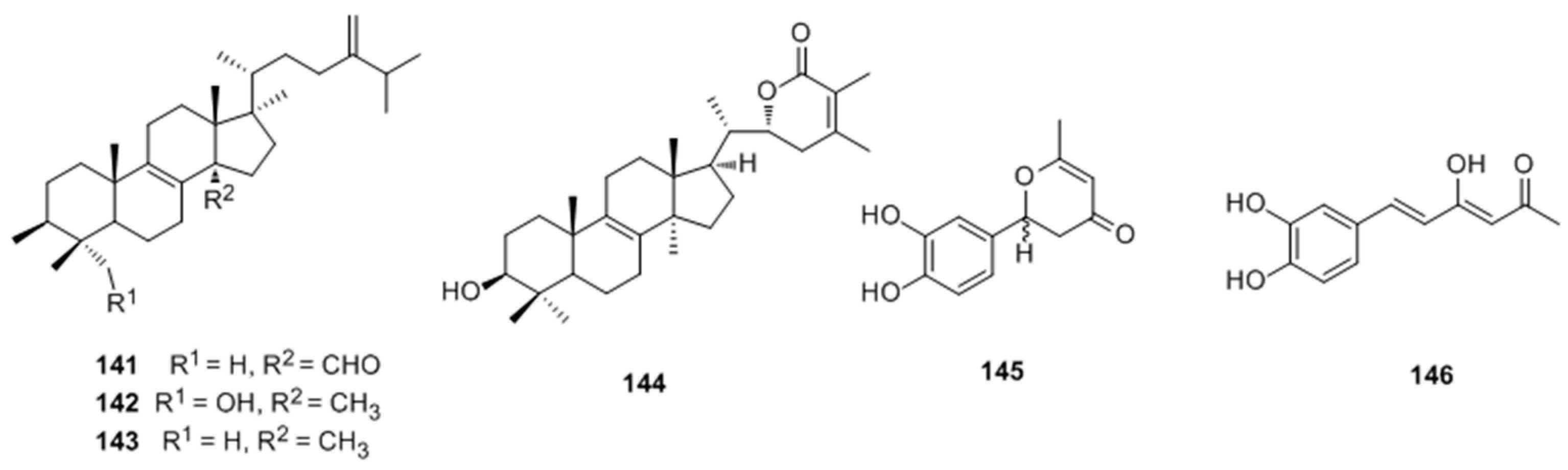
- Ren, Q.; Lu, X.Y.; Han, J.X.; Aisa, H.A.; Yuan, T. Triterpenoids and phenolics from the fruiting bodies of Inonotus hispidus and their activations of melanogenesis and tyrosinase. Chin. Chem. Lett. 2017, 28, 1052–1056. [Google Scholar] [CrossRef]
- Guan, S.; Su, W.; Wang, N.; Li, P.; Wang, Y. A potent tyrosinase activator from radix Polygoni multiflori and its melanogenesis stimulatory effect in B16 melanoma cells. Phytother. Res. 2008, 22, 660–663. [Google Scholar] [CrossRef] [PubMed]
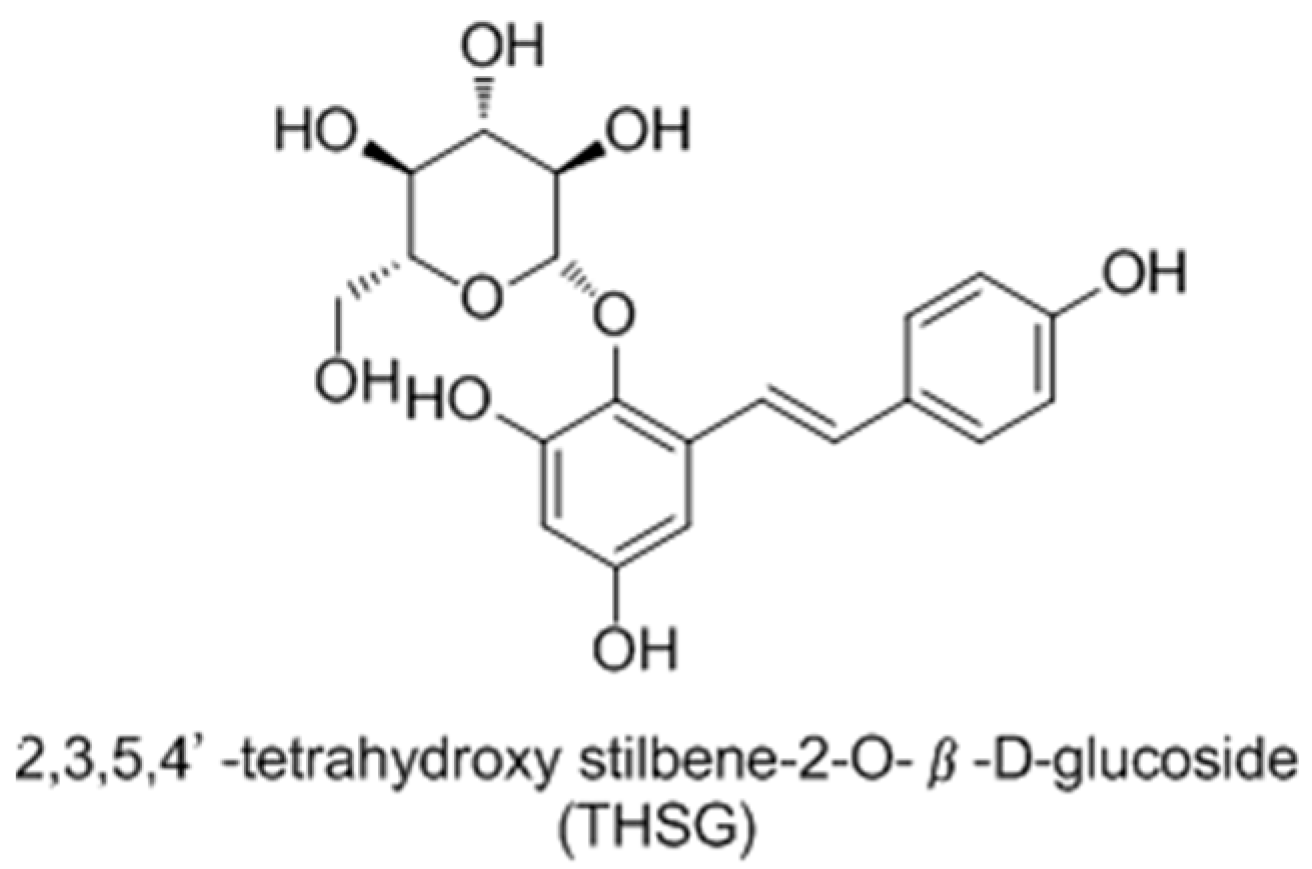
- Jiang, Z.Q.; Xu, J.M.; Long, M.H.; Tu, Z.M.; Yang, G.X.; He, G.Y. 2, 3, 5, 4′-tetrahydroxystilbene-2-O-β-d-glucoside (THSG) induces melanogenesis in B16 cells by MAP kinase activation and tyrosinase upregulation. Life Sci. 2009, 85, 345–350. [Google Scholar] [CrossRef] [PubMed]
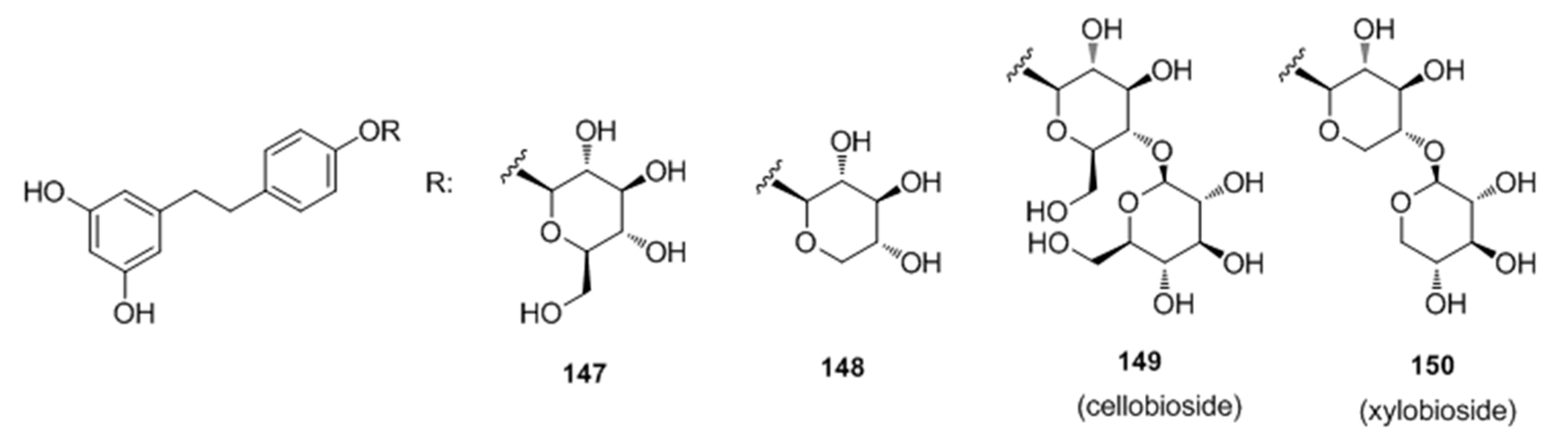
- Oode, C.; Shimada, W.; Yokota, M.; Yamada, Y.; Nihei, K. Dihydroresveratrol cellobioside and xylobioside as effective melanogenesis activators. Carbohyd. Res. 2016, 436, 45–49. [Google Scholar] [CrossRef] [PubMed]
- Dubois, C.; Haudecoeur, R.; Orio, M.; Belle, C.; Bochot, C.; Boumendjel, A.; Hardré, R.; Jamet, H.; Réglier, M. Versatile effects of aurone structure on mushroom tyrosinase activity. Chem. Bio. Chem. 2012, 13, 559–565. [Google Scholar] [CrossRef] [PubMed]
- Haudecoeur, R.; Gouron, A.; Dubois, C.; Jamet, H.; Lightbody, M.; Hardré, R.; Milet, A.; Bergantino, E.; Bubacco, L.; Belle, C.; et al. Investigation of binding-site homology between mushroom and bacterial tyrosinases by using aurones as effectors. Chem. Biol. Chem. 2014, 15, 1325–1333. [Google Scholar] [CrossRef] [PubMed]
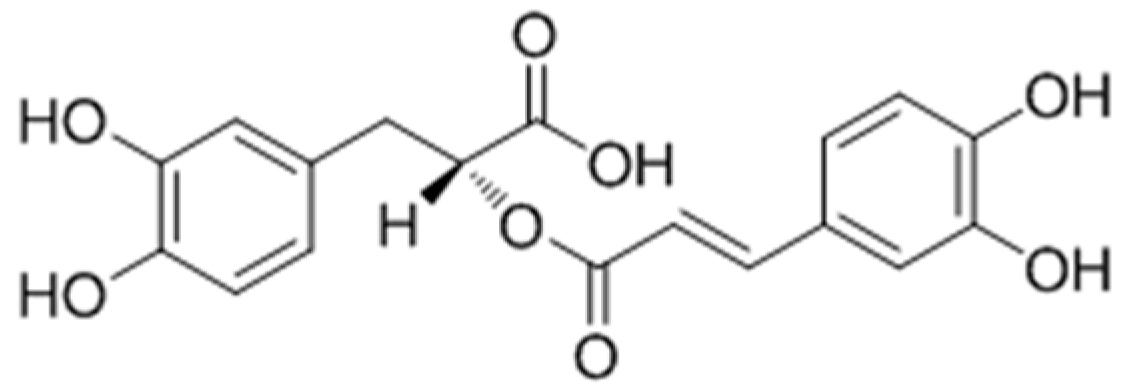
- Oliveira, K.B.; Palú, É.; Weffort-Santos, A.M.; Oliveira, B.H. Influence of rosmarinic acid and Salvia officinalis extracts on melanogenesis of B16-F10 cells. Rev. Bras. Farmacogn. 2013, 23, 249–258. [Google Scholar] [CrossRef]
- Lee, J.; Kim, Y.S.; Park, D. Rosmarinic acid induces melanogenesis through protein kinase A activation signaling. Biochem. Pharmacol. 2007, 74, 960–968. [Google Scholar] [CrossRef] [PubMed]
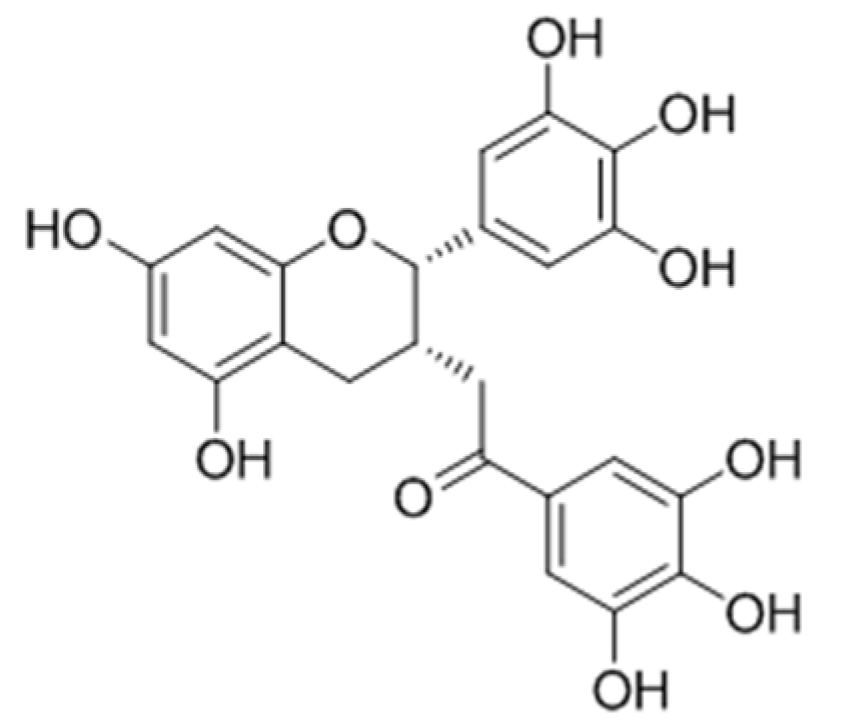
- Zhu, Y.P.; Wang, S.Q.; Lina, F.Q.; Li, Q.; Xua, A.E. The therapeutic effects of EGCG on vitiligo. Fitoterapia 2014, 99, 243–251. [Google Scholar] [CrossRef] [PubMed]
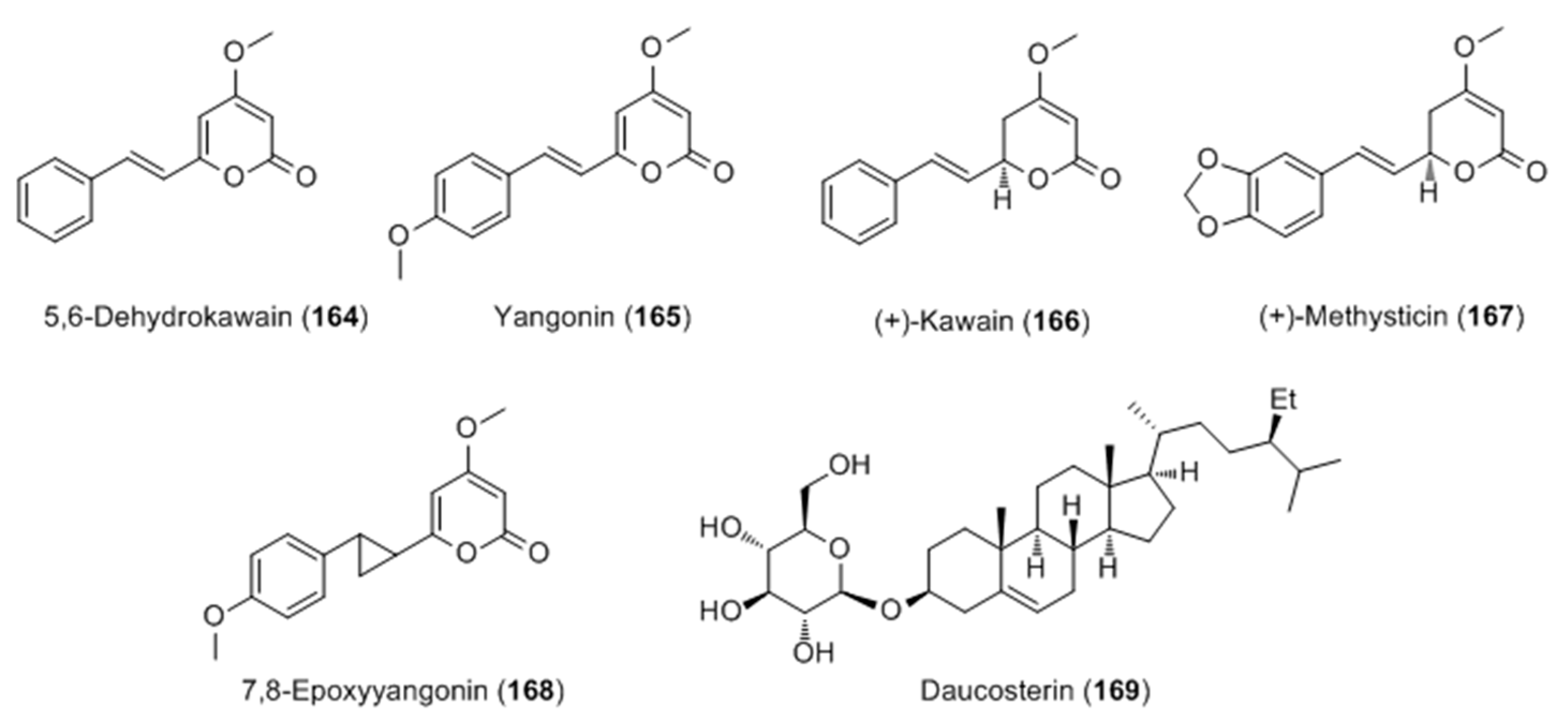
- Matsuda, H.; Hirata, N.; Kawaguchi, Y.; Naruto, S.; Takata, T.; Oyama, M.; Iinuma, M.; Kubo, M. Melanogenesis stimulation in murine B16 melanoma cells by Kava (Piper methysticum) rhizome extract and kavalactones. Biol. Pharm. Bull. 2006, 29, 834–837. [Google Scholar] [CrossRef] [PubMed]
- Hirata, N.; Naruto, S.; Ohguchi, K.; Akao, Y.; Nozawa, Y.; Iinumac, M.; Matsuda, H. Mechanism of the melanogenesis stimulation activity of (-)-cubebin in murine B16 melanoma cells. Bioorg. Med. Chem. 2007, 15, 4897–4902. [Google Scholar] [CrossRef] [PubMed]
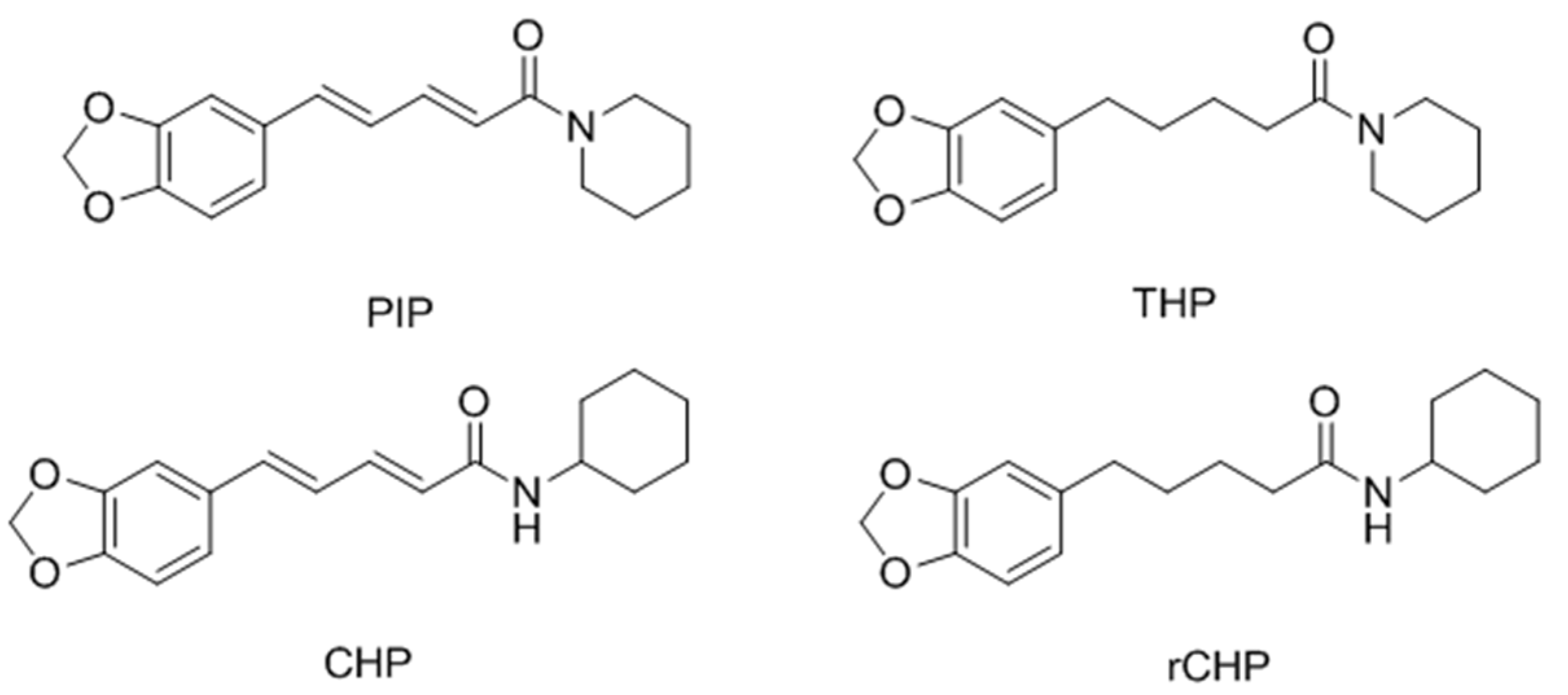
- Faas, L.; Venkatasamy, R.; Hider, R.C.; Young, A.R.; Soumyanath, A. In vivo evaluation of piperine and synthetic analogues as potential treatments for vitiligo using a sparsely pigmented mouse model. Br. J. Dermatol. 2008, 158, 941–950. [Google Scholar] [CrossRef] [PubMed]
- Dutta, D.; Mohanakumar, K.P. Tea and Parkinson’s disease: Constituents of tea synergize with antiparkinsonian drugs to provide better therapeutic benefits. Neurochem. Int. 2015, 89, 181–190. [Google Scholar] [CrossRef] [PubMed]
- Lubbe, S.J.; Escott-Price, V.; Brice, A.; Gasser, T.; Pittman, A.M.; Bras, J.; Hardy, J.; Heutink, P.; Wood, N.M.; Singleton, A.B.; et al. Rare variants analysis of cutaneous malignant melanoma genes in Parkinson’s disease. Neurobiol. Aging 2016, 48, 222.e1–222.e7. [Google Scholar] [CrossRef] [PubMed]
- Pei, T.L.; Zheng, C.L.; Huang, C.; Chen, X.T.; Guo, Z.H.; Fu, Y.X.; Liu, J.L.; Wang, Y.H. Systematic understanding the mechanisms of vitiligo pathogenesis and its treatment by Qubaibabuqi formula. J. Ethnopharmacol. 2016, 190, 272–287. [Google Scholar] [CrossRef] [PubMed]
- Cakilcioglu, U.; Khatun, S.; Turkoglu, I.; Hayta, S. Ethnopharmacological survey of medicinal plants in Maden (Elazig-Turkey). J. Ethnopharmacol. 2011, 137, 469–486. [Google Scholar] [CrossRef] [PubMed]
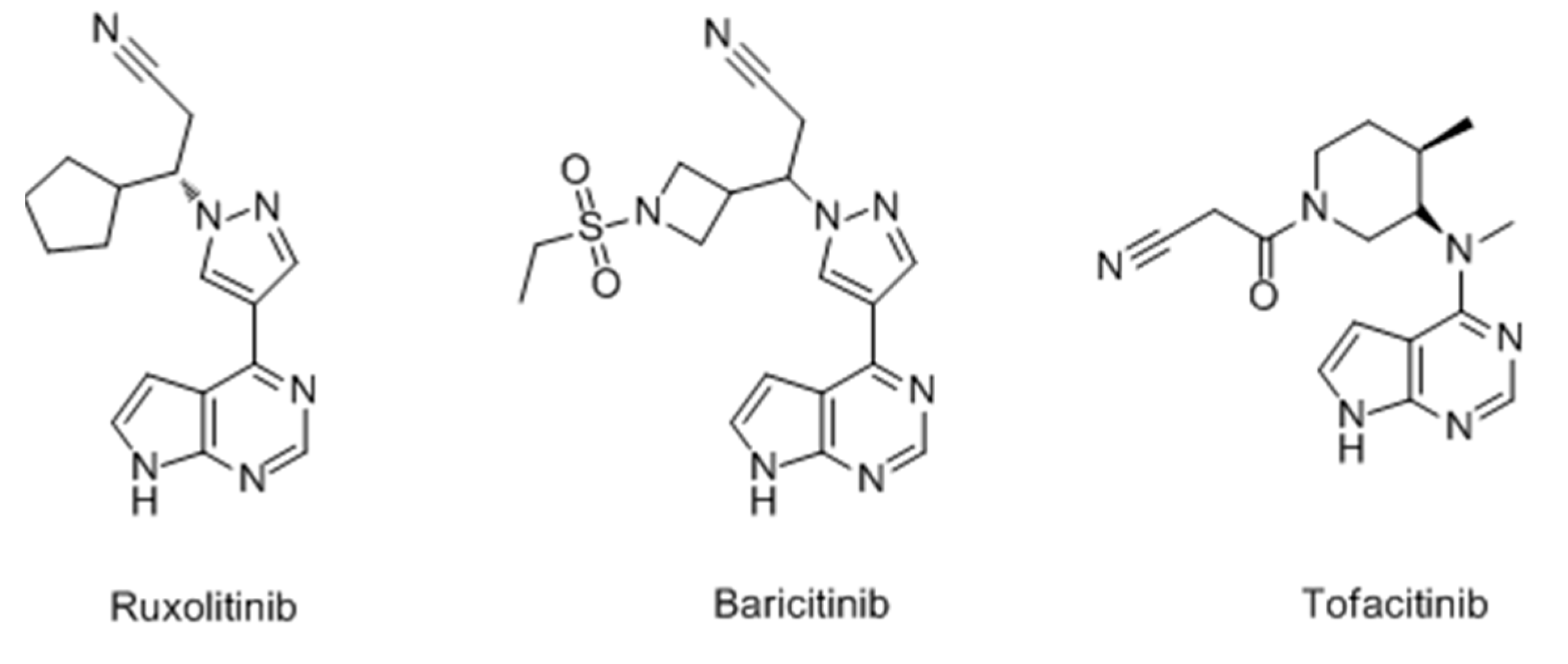
- Damsky, W.; King, B.A. JAK inhibitors in dermatology: The promise of a new drug class. J. Am. Acad. Dermatol. 2017, 76, 736–744. [Google Scholar] [CrossRef] [PubMed]
- Harris, J.E.; Rashighi, M.; Nguyen, N.; Jabbari, A.; Ulerio, G.; Clynes, R.; Christiano, A.M.; Mackay-Wiggan, J. Rapid skin repigmentation on oral ruxolitinib in a patient with coexistent vitiligo and alopecia areata (AA). J. Am. Acad. Dermatol. 2016, 74, 370–371. [Google Scholar] [CrossRef] [PubMed]










| Botanical Name | Extraction Parts | Solvents | Items Up-Regulated | Types of Cultured Cells | Related Signaling Pathway | Animal Experiments | Ref. |
|---|---|---|---|---|---|---|---|
| Daphne gnidium | Leaf | Ethyl acetate | Melanin, Tyrosinase activity | B16-F0 | Ns a | Ns | [37] |
| Daphne gnidium | Leaf | Chloroform | Melanin, Tyrosinase activity | B16-F0 | Ns | Ns | [38] |
| Moricandia arvensis | Leaf | Chloroform | Melanin, Tyrosinase activity | B16-F0 | Ns | Ns | [39] |
| Polygonum multiflorum | Root | Water | MITF, Melanocyte migration | Human melanocytes | Ns | Ns | [40] |
| Eclipta Prostrate | Whole herb | Water | Melanin, Tyrosinase activity, MITF, Melanocyte migration | Human melanocytes | Ns | Ns | [40] |
| Rehmannia Glutinosa | Root | Water | Melanin, MITF | Human melanocytes | Ns | Ns | [40] |
| Cassia alata | Leaf | Nm b | Melanin, Tyrosinase activity, Dendritogenesis, Migration | Melb-a melanoblast | Ns | Ns | [41] |
| Cassia occidentalis | Leaf | Methanol | Melanin, Tyrosinase activity, Dendritogenesis, Migration | Melb-a melanoblast | Ns | Ns | [42] |
| Pyrostegia venusta | Leaf, Flower | Ethanol:Water (70:30, v/v) | Melanin | B16-F10 | Ns | Ns | [43] |
| Pyrostegia venusta | Leaf | Ethanol:Water (70:30, v/v) | Epidermal melanin, Dermal pigmentation | - | Ns | C56BL/6 mice | [44] |
| Vernonia anthelmintica | Fruit | Ethanol:Water (60:40, v/v) | Melanin, Tyrosinase activity, Tyrosinase expression, p38 MAPK phosphorylation, MITF expression | B16-F10, Human melanocytes | p38 MAPK | Ns | [45] |
| Vernonia anthelmintica | Seed | Ethanol:Water (80:20, v/v) | TYR, TRP-1, TRP-2 and MITF expression | B16 murinemelanoma | Ns | Ns | [46] |
| Melissa officinalis | Whole herb | Nm | Melanin | Human keratinocytes | Ns | Ns | [49] |
| Melia azedarach | Nm | Ethanol:Water (70:30, v/v) | Melanin, TRP-1 expression | B16-F10 | Ns | NS | [50] |
| Capparis spinosa | Nm | Nm | Melanin, Tyrosinase expression | B16 murinemelanoma | Ns | Ns | [51] |
| Erica multiflora | Nm | Nm | Melanin, Tyrosinase expression | B16 murine melanoma | Ns | Ns | [51] |
| Citrus paradisi, Citrus grandis, Fructus aurantii immaturus, Fructus aurantii | Rind | Ethyl acetate | Melanin, Tyrosinase expression | B16 murine melanoma | Ns | Ns | [52] |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Niu, C.; Aisa, H.A. Upregulation of Melanogenesis and Tyrosinase Activity: Potential Agents for Vitiligo. Molecules 2017, 22, 1303. https://doi.org/10.3390/molecules22081303
Niu C, Aisa HA. Upregulation of Melanogenesis and Tyrosinase Activity: Potential Agents for Vitiligo. Molecules. 2017; 22(8):1303. https://doi.org/10.3390/molecules22081303
Chicago/Turabian StyleNiu, Chao, and Haji A. Aisa. 2017. "Upregulation of Melanogenesis and Tyrosinase Activity: Potential Agents for Vitiligo" Molecules 22, no. 8: 1303. https://doi.org/10.3390/molecules22081303
APA StyleNiu, C., & Aisa, H. A. (2017). Upregulation of Melanogenesis and Tyrosinase Activity: Potential Agents for Vitiligo. Molecules, 22(8), 1303. https://doi.org/10.3390/molecules22081303