Structures and Functions of Snake Venom Metalloproteinases (SVMP) from Protobothrops venom Collected in Japan
Abstract
:1. Introduction
2. Classification of the Domain Structures of SVMP
3. Structures and Characterization of SVMP
4. FL Activity
5. Hemorrhagic Activity
6. Inhibition of Platelet Aggregation
7. Conclusions
Acknowledgments
Conflicts of Interest
References
- Kini, R.M. Anticoagulant proteins from snake venoms: Structure, function, and mechanism. Biochem. J. 2006, 397, 377–387. [Google Scholar] [CrossRef] [PubMed]
- Jia, L.G.; Wang, X.M.; Shannon, J.D.; Biarnason, J.D.; Fox, J.W. Inhibition of platelet aggregation by the recombinant cysteine-rich domain of the hemorrhagic snake venom metalloproteinase, atrolysin A. Arch. Biochem. Biophys. 2000, 373, 281–286. [Google Scholar] [CrossRef] [PubMed]
- Takeda, S.; Takeya, H.; Iwanaga, S. Snake venom metalloproteinases: Structure, function and relevance to the mammalian ADAM/ADAMTS family proteins. Biochim. Biophys. Acta 2012, 1824, 164–176. [Google Scholar] [CrossRef] [PubMed]
- Hite, L.A.; Jia, L.-G.; Bjarnason, J.B.; Fox, J.W. cDNA sequences for four snake venom metalloproteinases: Structure, classification, and their relationship to mammalian reproductive proteins. Arch. Biochem. Biophys. 1994, 308, 6203–6211. [Google Scholar] [CrossRef] [PubMed]
- Iwanaga, S.; Suzuki, T. Enzymes in snake venom. In Handbook of Experimental Pharmacology; Born, G.V.R., Farah, A., Herken, H., Welch, A.D., Eds.; Springer: New York, NY, USA, 1979; Volume 52, pp. 61–158. [Google Scholar]
- Bjarnason, J.B.; Fox, J.W. Hemorrhagic metalloproteinases from snake venoms. Pharmacol. Ther. 1994, 62, 325–372. [Google Scholar] [CrossRef]
- Fox, J.W.; Serrano, S.M. Structural considerations of the snake venom metalloproteinases, key members of the M12 reprolysin family of metalloproteinases. Toxicon 2005, 45, 969–985. [Google Scholar] [CrossRef] [PubMed]
- Fox, J.W.; Serrano, S.M. Timeline of key events in snake venom metalloproteinase research. J. Proteom. 2009, 72, 200–209. [Google Scholar] [CrossRef] [PubMed]
- Takeya, H.; Iwanaga, S. Proteases that induce hemorrhagic. In Enzymes from Snake Venom; Bailey, G.S., Ed.; Alaken: Fort Collins, CO, USA, 1998; pp. 11–38. [Google Scholar]
- Araki, S.; Masuda, S.; Maeda, H.; Ying, M.J.; Hayashi, H. Involvement of specific integrins in apoptosis induced by vascular apoptosis-inducing protein 1. Toxicon 2002, 40, 535–542. [Google Scholar] [CrossRef]
- Fox, J.W.; Serrano, S.M. Snake venom metalloproteinase. In Handbook of Venoms and Toxins of Reptiles; Mackessy, S.P., Ed.; CRC Press: Boca Raton, FL, USA, 2010; pp. 95–113. [Google Scholar]
- Kang, T.S.; Georgieva, D.; Genov, N.; Murakami, M.T.; Sinha, M.; Kumar, R.P.; Kaur, P.; Kumar, S.; Dey, S.; Sharma, S.; et al. Enzymatic toxins from snake venom: Structural characterization and mechanism of catalysis. FEBS 2011, 278, 4544–4576. [Google Scholar] [CrossRef] [PubMed]
- Bjarnason, J.B.; Fox, J.W. Snake venom metalloproteinases: Reprolysins. Methods Enzymol. 1995, 248, 345–368. [Google Scholar] [PubMed]
- Kishimoto, M.; Takahashi, T. Molecular cloning of HR1a and HR1b, high molecular hemorrhagic factors, from Trimeresurus flavoviridis venom. Toxicon 2002, 40, 1369–1375. [Google Scholar] [CrossRef]
- Takeya, H.; Oda, K.; Miyata, T.; Omori-Satoh, T.; Iwanaga, S. The complete amino acid sequence of the high molecular mass hemorrhagic protein HR1B isolated from the venom of Trimeresurus flavoviridis. J. Biol. Chem. 1990, 265, 16068–16073. [Google Scholar] [PubMed]
- Takeya, H.; Nishida, S.; Miyata, T.; Kawada, S.; Saisaka, Y.; Morita, T.; Iwanaga, S. A novel metalloproteinase with disintegrin (platelet aggregation inhibitor)-like and C-type lectin-like domains. J. Biol. Chem. 1992, 267, 14109–14117. [Google Scholar] [PubMed]
- Gowda, D.C.; Jackson, C.M.; Hensley, P.; Davidson, E.A. Factor X-activating glycoprotein of Russell’s viper venom. Polypeptide composition and characterization of the carbohydrate moieties. J. Biol. Chem. 1994, 269, 10644–10650. [Google Scholar] [PubMed]
- Gomis-Ruth, F.X. Structural aspects of the metzincin clan of metalloendopeptidases. J. Mol. Biotechnol. 2003, 24, 157–202. [Google Scholar] [CrossRef]
- Kumasaka, T.; Yamamoto, M.; Moriyama, H.; Tanaka, N.; Sato, M.; Katsube, Y.; Yamakawa, Y.; Omori-Satoh, T.; Iwanaga, S.; Ueki, T. Crystal structure of H2-proteinase from the venom of Trimeresurus flavoviridis. J. Biochem. 1996, 119, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Oyama, E.; Kitagawa, Y.; Takahashi, H. Primary structure and characterization of a non hemorrhagic metalloproteinase with fibrinolytic activity, from the snake venom of Protobothrops tokarensis (Tokara-habu). Toxicon 2013, 70, 153–161. [Google Scholar] [CrossRef] [PubMed]
- Takeya, H.; Arakawa, H.; Miyata, T.; Iwanaga, S.; Omori-Satoh, T. Primary structure of H2-proteinase, a non-hemorrhagic proteinase, isolated from the venom of the habu snake, Trimeresurus flavoviridis. J. Biochem. 1989, 106, 843–853. [Google Scholar] [CrossRef]
- Miyata, T.; Takeya, H.; Ozeki, Y.; Arakawa, M.; Tokunaga, F.; Iwanaga, S.; Omori-Satoh, T. Primary structure of hemorrhagic protein, HR2a, isolated from the venom of Trimeresurus flavoviridis. J. Biochem. 1989, 105, 847–853. [Google Scholar] [CrossRef] [PubMed]
- Iha, M.; Qi, Z.Q.; Kannki, T.; Tomihara, Y.; Yonaha, K. The primary structure of a hemorrhagic factor, HR2b, from the venom of Okinawa habu (Trimeresurus flavoviridis). Toxicon 1995, 33, 229–239. [Google Scholar] [CrossRef]
- Kishimoto, M.; Takahashi, T. Molecular cloning and sequence analysis of cDNA encoding flavoviridin, a disintegrin from the venom of Trimeresurus flavoviridis. Toxicon 2002, 40, 1033–1040. [Google Scholar] [CrossRef]
- Scaloni, A.; Di Martino, E.; Miraglia, N.; Pelagalli, A.; Della Marte, R.; Staiano, N.; Pucci, P. Amino acid sequence and molecular modelling of glycoprotein IIb-IIIa and fibronectin receptor iso-antagonists from Trimeresurus elegans venom. Biochem. J. 1996, 313, 775–782. [Google Scholar] [CrossRef]
- Masuda, S.; Hayashi, H.; Atoda, H.; Morita, T.; Araki, S. Purification, cDNA cloning and characterization of the vascular apoptosis-inducing protein, HV1, from Trimeresurus flavoviridis. Eur. J. Biochem. 2001, 268, 3339–3345. [Google Scholar] [CrossRef] [PubMed]
- Takeda, S.; Igarashi, T.; Mori, H.; Araki, S. Crystal structure of VAP1 reveal ADAMs MDC domain architecture and its unique C-shaped scaffold. EMBO J. 2006, 25, 2388–2396. [Google Scholar] [CrossRef] [PubMed]
- Takeda, S. Three-dimensional domain architecture of the ADAM family proteinases. Semin. Cell Dev. Biol. 2009, 20, 146–152. [Google Scholar] [CrossRef] [PubMed]
- Takeya, H.; Nishida, S.; Nishino, S.; Makinose, Y.; Omori-Satoh, T.; Nikai, T.; Sugihara, H.; Iwanaga, S. Primary structures of platelet aggregation inhibitors (disintegrin) autoproteolytically released from snake venom hemorrhagic metalloproteinases and new fluorogenic peptide substrates for these enzyme. J. Biochem. 1993, 113, 473–483. [Google Scholar] [CrossRef] [PubMed]
- Hite, L.A.; Shannon, J.D.; Biarnason, J.B.; Fox, J.W. Sequence of a cDNA clone encoding the zinc metalloproteinase hemorrhagic toxin e from Crotalus atrox: Evidence for signal, zymogen, and disintegrin-like structures. Biochemistry 1992, 31, 6203–6211. [Google Scholar] [CrossRef] [PubMed]
- Kini, R.M.; Evans, H.J. Structural domains in venom proteins: Evidence that metalloproteinases and nonenzymatic platelet aggregation inhibitors (disintegrins) from snake venom are derived by proteolysis from a common precursor. Toxicon 1992, 30, 265–293. [Google Scholar] [CrossRef]
- Okuda, D.; Koike, H.; Morita, T. A new gene structure of the disintegrin family: A subunit of dimeric disintegrin has a short coding region. Biochemistry 2002, 41, 14248–14254. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.F.; Holt, J.C.; Lukasiewicz, H.; Niewiarowski, S. Trigramin. A low molecular weight peptide inhibiting fibrinogen interaction with platelet receptors expressed on glycoprotein IIb-IIIa complex. J. Biol. Chem. 1987, 262, 16157–16163. [Google Scholar] [PubMed]
- Calvete, J.J.; Marcinkiewicz, C.; Monleon, D.; Esteve, V.; Celba, B.; Juarez, P.; Sanz, L. Snake venom disintegrins: Evolution of structure and function. Toxicon 2005, 45, 1063–1074. [Google Scholar] [CrossRef] [PubMed]
- Siigur, E.; Siigur, J. Purification and characterization of lebetase, a fibrinolytic enzyme from Vipera lebetina (snake) venom. Biochim. Biophys. Acta 1991, 1074, 223–229. [Google Scholar] [CrossRef]
- Esnouf, M.P.; Tunnah, G.W. The isolation and properties of the thrombin-like activity from Angistrodon rhodostoma venom. Br. J. Haematol. 1967, 13, 582–590. [Google Scholar] [CrossRef]
- Markland, F.S.; Damus, P.S. Purification and properties of a thrombin-like enzyme from the venom of Crotalus adamanteus (Eastern diamondback rattlesnake). J. Biol. Chem. 1971, 246, 6460–6473. [Google Scholar] [PubMed]
- Oyama, E.; Takahashi, H. Substrate specificity of two thrombin like enzymes (elegaxobin, elegaxobinII) from the venom of Trimeresurus elegans (Sakishima-habu), using neutralizing antibody. Toxicon 2006, 48, 601–610. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wisner, A.; Maroun, R.C.; Choumet, V.; Xiong, Y.; Bon, C. Trimeresurus stejnegeri snake venom plasminogen activator. J. Biol. Chem. 1997, 272, 20531–20537. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, F.F.; Santos, C.I.; Magalhaes, A.; Diniz, C.R.; Figueiredo, S.; Gilroy, J.; Richardson, M. Isolation of a proteinase with plasminogen-activating activity from Lachesis muta muta (Bushmaster) snake venom. Arch. Biochem. Biophys. 2000, 378, 131–141. [Google Scholar] [CrossRef] [PubMed]
- Robbins, K.C.; Summaria, L.; Hsieh, B.; Shah, R.J. The peptide chains of human plasmin. Mechanism of activation of human plasminogen to plasmin. J. Biol. Chem. 1967, 242, 2333–2342. [Google Scholar] [PubMed]
- Forsgren, M.; Raden, B.; Israelsson, M.; Larsson, K.; Heden, L.O. Molecular cloning and characterization of a full-lengh cDNA clone for human plasminogen. FEBS Lett. 1987, 213, 254–260. [Google Scholar] [CrossRef]
- Nikai, T.; Mori, N.; Kishida, M.; Yuko, K.; Takenaka, C.; Murakami, T.; Shigezane, S.; Sugihara, H. Isolation and characterization of hemorrhagic factors and b from the venom of the Chinese habu snake (Trimeresurus mucrosquamatus). Biochim. Biophys. Acta 1985, 838, 122–131. [Google Scholar] [CrossRef]
- Nikai, T.; Mori, N.; Kishida, M.; Sugihara, H.; Tu, A.T. Isolation and biochemical characterization of hemorrhagic toxin f from the venom of Crotalus atrox (western diamondback rattlesnake). Arch. Biochem. Biophys. 1984, 231, 309–319. [Google Scholar] [CrossRef]
- Kim, J.-Y.; Lee, S.-B.; Kwon, K.R.; Choi, S.-H. Isolation and Characterization of a 32-kDa fibrinolytic enzyme (FE-32 kDa) from Gloydius blomhoffii siniticus venom. Pharmacopuncture 2014, 17, 44–50. [Google Scholar] [CrossRef] [PubMed]
- Ohsaka, A. Handbook of Experimental Pharmacology, Snake Venoms; Lee, C.-Y., Ed.; Springer-Verlag: New York, NY, USA, 1979; Volume 52, pp. 480–546. [Google Scholar]
- Takahashi, T.; Ohsaka, A. Purification and characterization of a proteinase in the venom of Trimeresurus flavoviridis. Complete separation of the enzyme from hemorrhagic activity. Biochim. Biophys. Acts 1970, 198, 293–307. [Google Scholar] [CrossRef]
- Kini, R.M.; Evans, H.J. Effects of snake venom proteins on blood platelets. Toxicon 1990, 28, 1387–1422. [Google Scholar] [CrossRef]
- Teng, C.M.; Huang, T.F. Snake venom constituents that affect platelet function. Platelets 1991, 2, 77–87. [Google Scholar] [CrossRef] [PubMed]
- Yamakawa, Y.; Omori-Satoh, T.; Maeyama, J. Primary structures of cytotoxic factors isolated from Habu (Trimeresurus flavoviridis) venom. J. Biochem. 1991, 109, 667–669. [Google Scholar] [CrossRef] [PubMed]
- Musial, J.; Niewiarowski, S.; Rucinski, B.; Stewart, G.J.; Cook, J.J.; Williams, J.A.; Edmunds, L.H., Jr. Inhibition of platelet adhesion to surfaces of extracorporeal circuits by disintegrins. RGD-containing peptides from viper venoms. Circulation 1990, 82, 261–273. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.F.; Sheu, J.R.; Teng, C.M.; Chen, S.W.; Liu, C.S. Triflavin, an antiplatelet Arg-Gly-Asp-containing peptide, is a specific antagonist of platelet membrane glycoprotein IIb-IIIa complex. J. Biochem. 1991, 109, 328–334. [Google Scholar] [PubMed]
- Williams, J.; Rucinski, B.; Holt, J.; Niewiarow, S. Elegantin and albolabrin purified peptides from viper venoms: Homologies with the RGDS domain of fibrinogen and von Willebrand factor. Biochim. Biophys. Acta 1990, 31, 81–89. [Google Scholar] [CrossRef]
- Oyama, E.; Takahashi, H. Purification and characterization of two high molecular mass snake venom metalloproteinase (P-III SVMPs), named SV-PAD-2 and HR-Ele-1, from the venom of Protobothrops elegans (Sakishima-habu). Toxicon 2015, 103, 30–38. [Google Scholar] [CrossRef] [PubMed]
- Queiroz, M.R.; Mamede, C.C.; Fonseca, K.C.; De Morais, N.C.G.; De Sousa, B.B.; Santos-Filbo, N.A.; Beletti, M.E.; Arantes, E.C.; Stanziola, L.; De Oliveira, F. Rapid purification of a new P-I Crass metalloproteinase from Bothrops moojeni venom with antiplatelet activity. BioMed Res. Int. 2014, 2014, 352420. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, E.F.; Richardson, M.; Gremski, L.H.; Veiga, S.S.; Yarleque, A.; Niland, E.S.; Lima, A.M.; Estevao-Costa, M.I.; Eble, J.A. A novel fibrinolytic metalloproteinase, barnettlysin-1 from Bothrops barnetti (barnett’s pitviper) snake venom with anti-platelet properties. Biochim. Biophys. Acta 2016, 1860, 542–556. [Google Scholar] [CrossRef] [PubMed]
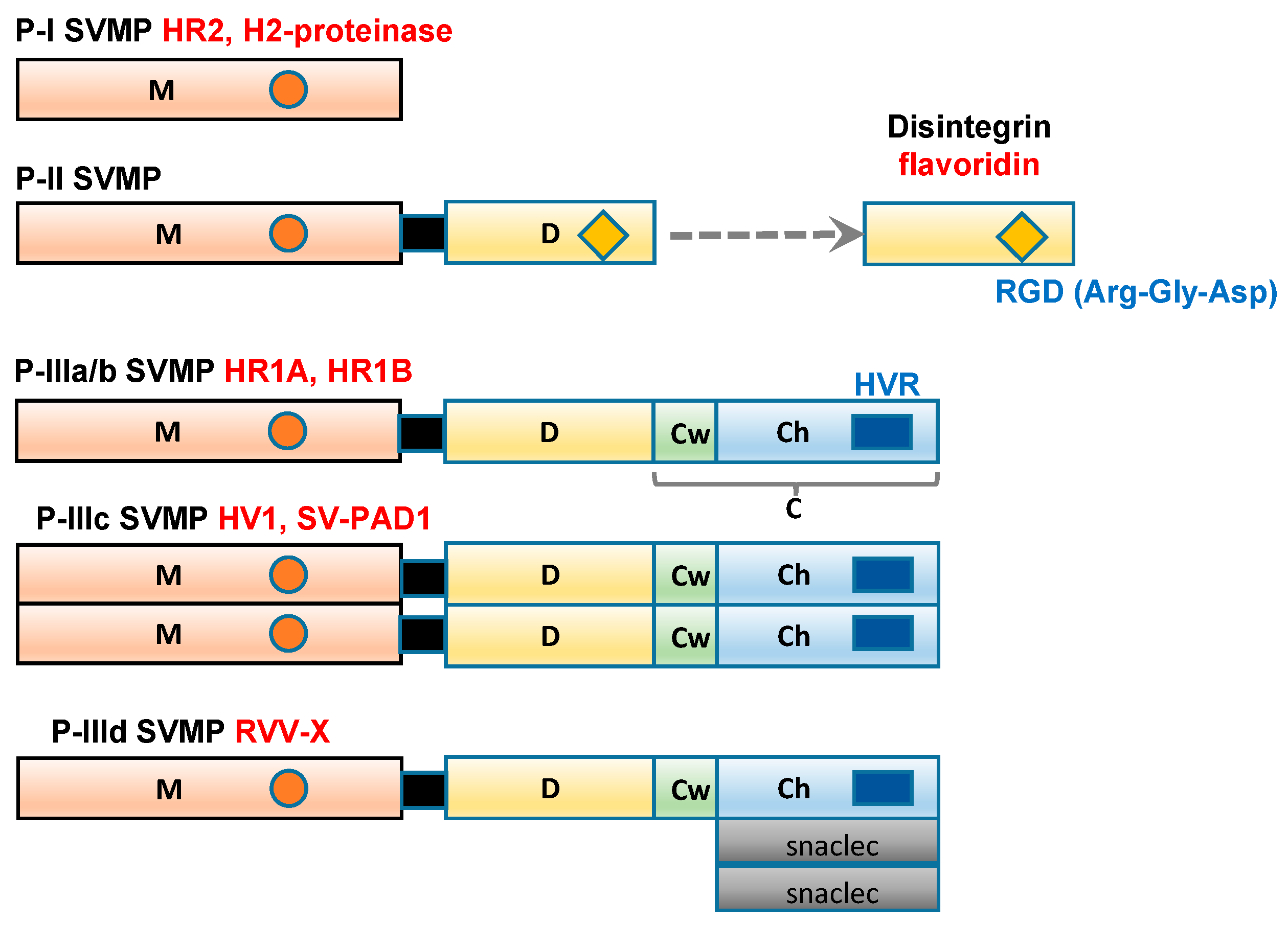

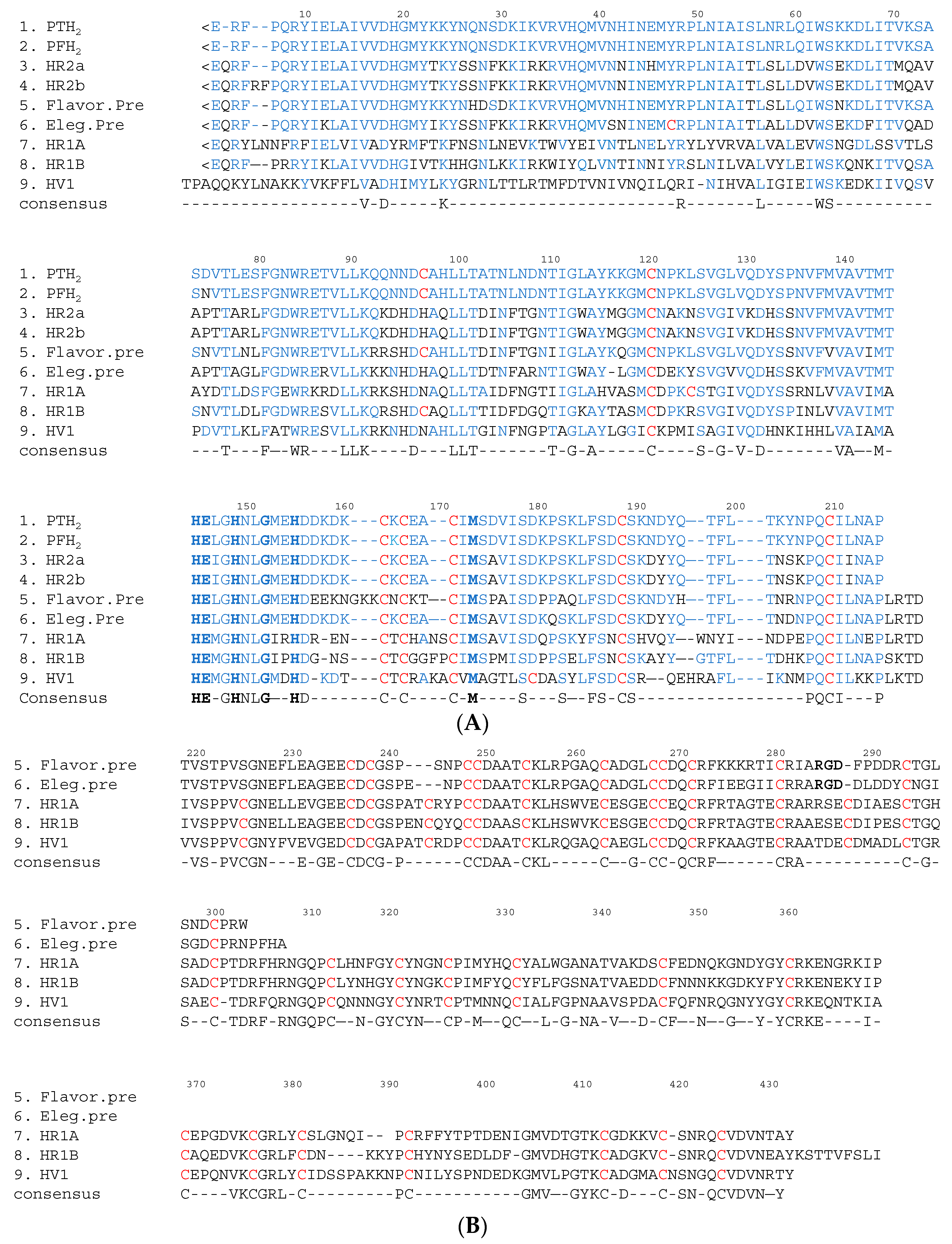
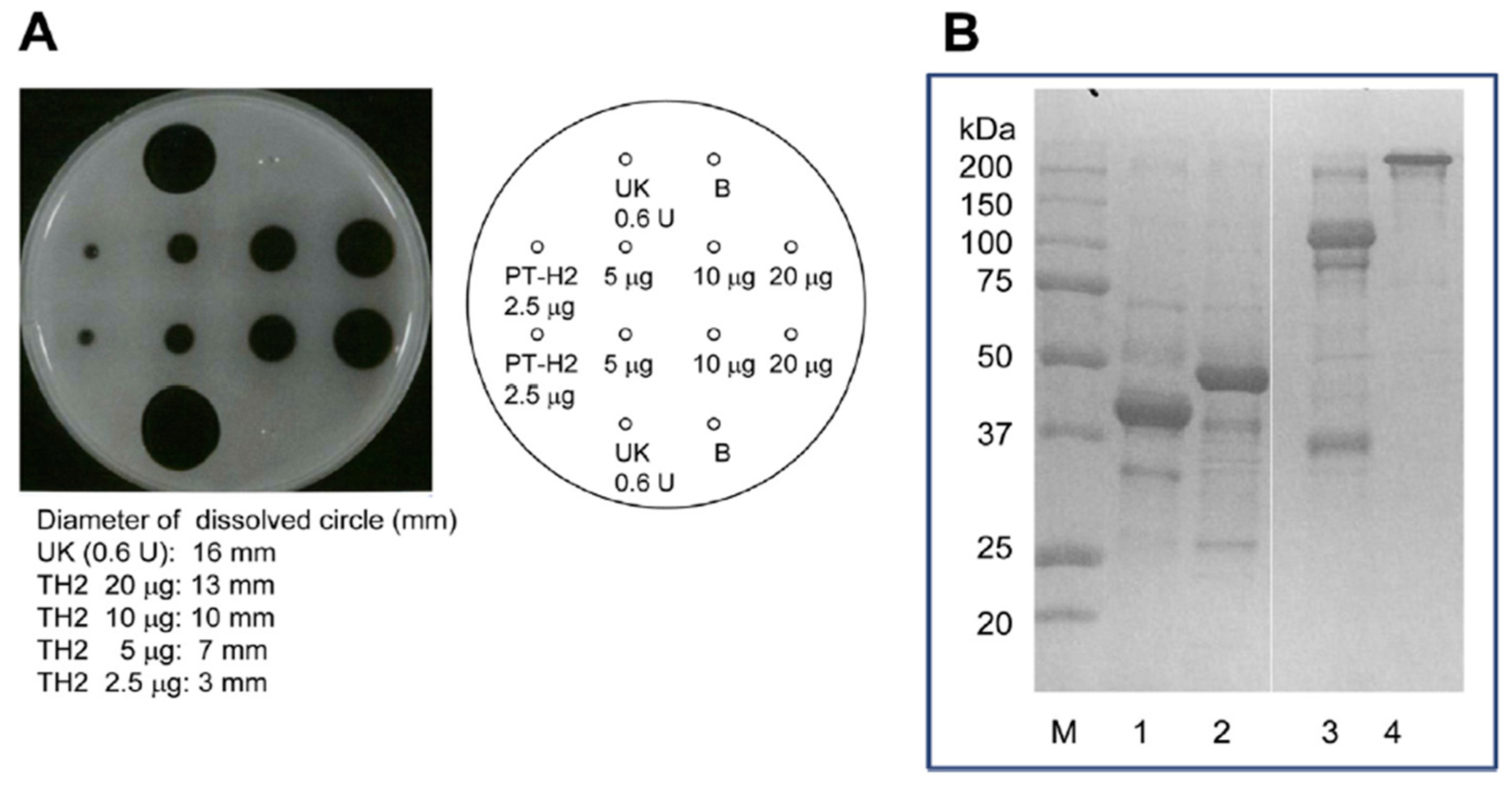
| Class | Domains | Sorce | Biological Activities | ||
|---|---|---|---|---|---|
| HR1B | P-IIIa/b | MDC | P. flavoviridis | Hemorrhagic | |
| HR1A | P-IIIa/b | MDC | P. flavoviridis | Hemorrhagic | |
| HR-Ele-1 | P-IIIa/b | MDC | P. elegans | Hemorrhagic | |
| HV1 | P-IIIc | MDC (dimer) | P. flavoviridis | N.D. | Appoptotic |
| SV-PAD-2 | P-IIIc | MDC (dimer) | P. elegans | Non-hemorrhagic | Inhibition of platelet aggregation |
| Flavoridin Pre. | P-II | MD | P. flavoviridis | N.D. | Precursor of disintegrin |
| Elegantin Pre. | P-II | MD | P. elegans | N.D. | Precursor of disintegrin |
| HR2a | P-I | M | P. flavoviridis | Hemorrhagic | |
| HR2b | P-I | M | P. flavoviridis | Hemorrhagic | |
| H2 proteinase | P-I | M | P. flavoviridis | Non-hemorrhagic | Proteolytic |
| PT-H2 proteinase | P-I | M | P. tokarensis | Non-hemorrhagic | Fibrinolytic, inhibition of platelet aggregation |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oyama, E.; Takahashi, H. Structures and Functions of Snake Venom Metalloproteinases (SVMP) from Protobothrops venom Collected in Japan. Molecules 2017, 22, 1305. https://doi.org/10.3390/molecules22081305
Oyama E, Takahashi H. Structures and Functions of Snake Venom Metalloproteinases (SVMP) from Protobothrops venom Collected in Japan. Molecules. 2017; 22(8):1305. https://doi.org/10.3390/molecules22081305
Chicago/Turabian StyleOyama, Etsuko, and Hidenobu Takahashi. 2017. "Structures and Functions of Snake Venom Metalloproteinases (SVMP) from Protobothrops venom Collected in Japan" Molecules 22, no. 8: 1305. https://doi.org/10.3390/molecules22081305




