Pharmacokinetic Study of Biotransformation Products from an Anxiolytic Fraction of Tilia americana
Abstract
:1. Introduction
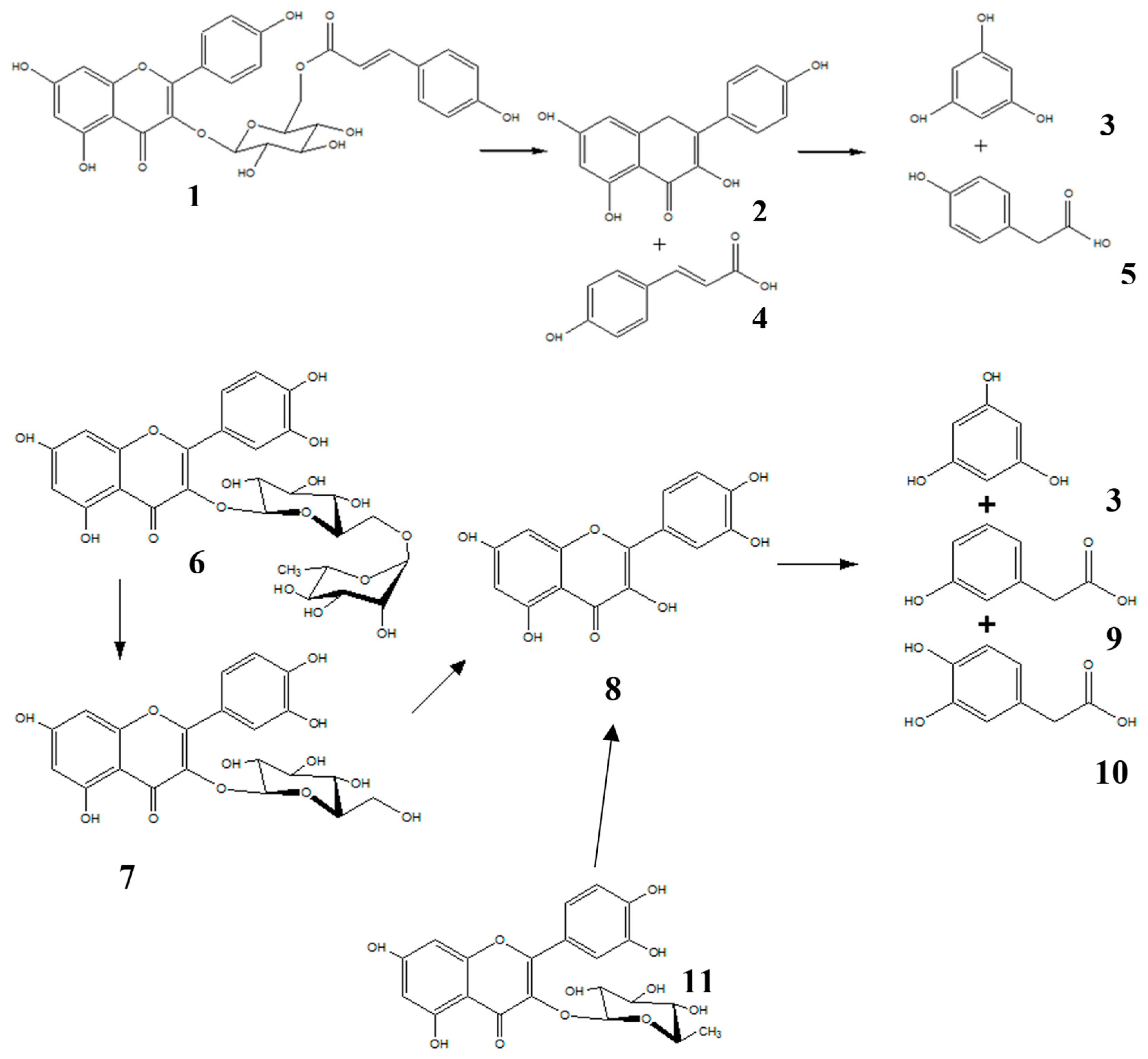
2. Results and Discussion
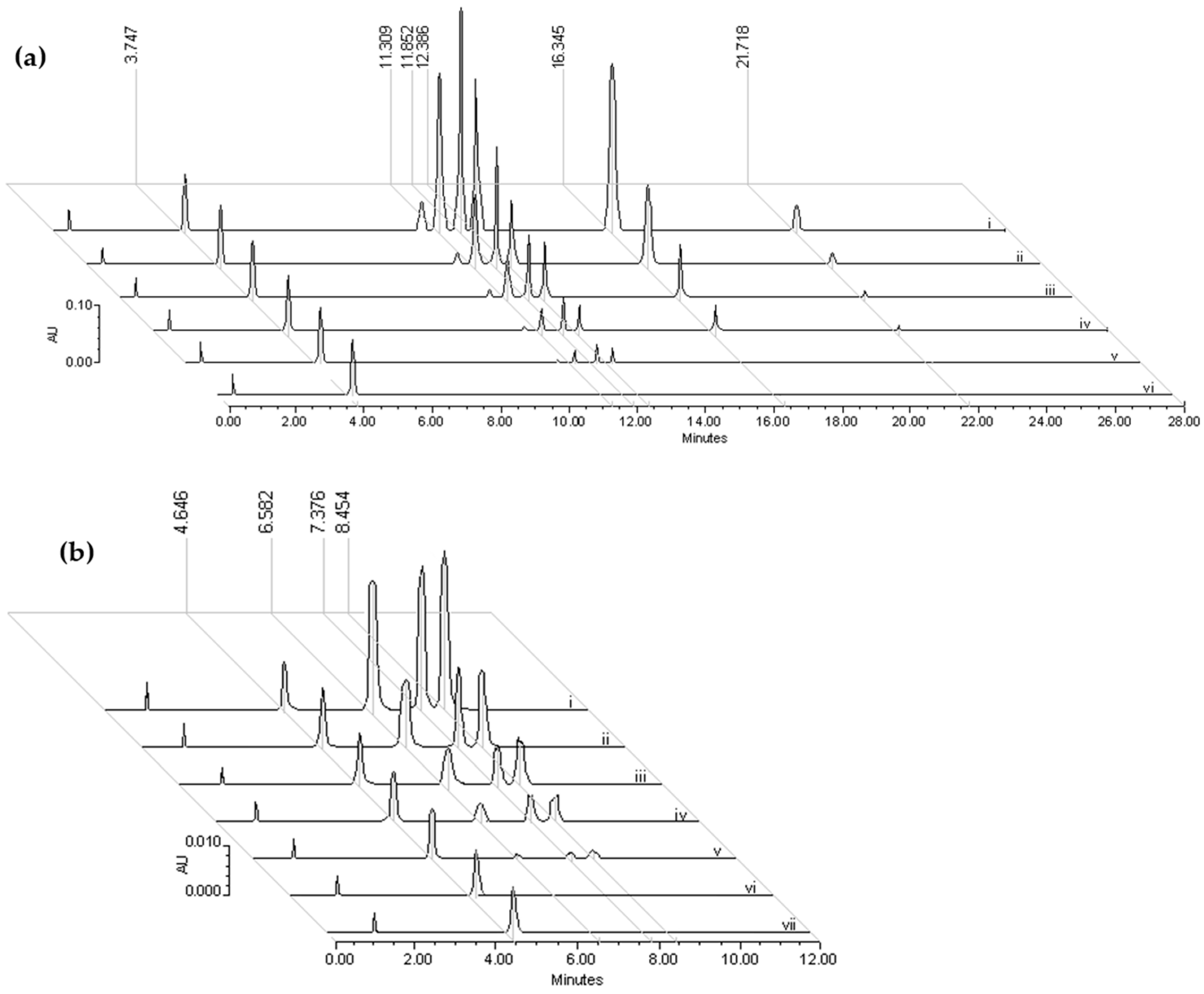
2.1. Chromatographic Profiling of Flavonoids, m-HPAA, p-HPAA and DOPAC Standards
2.2. Validation Methods
2.2.1. Standardization of the Chromatographic Process
2.2.2. Standardization of the Extraction Process
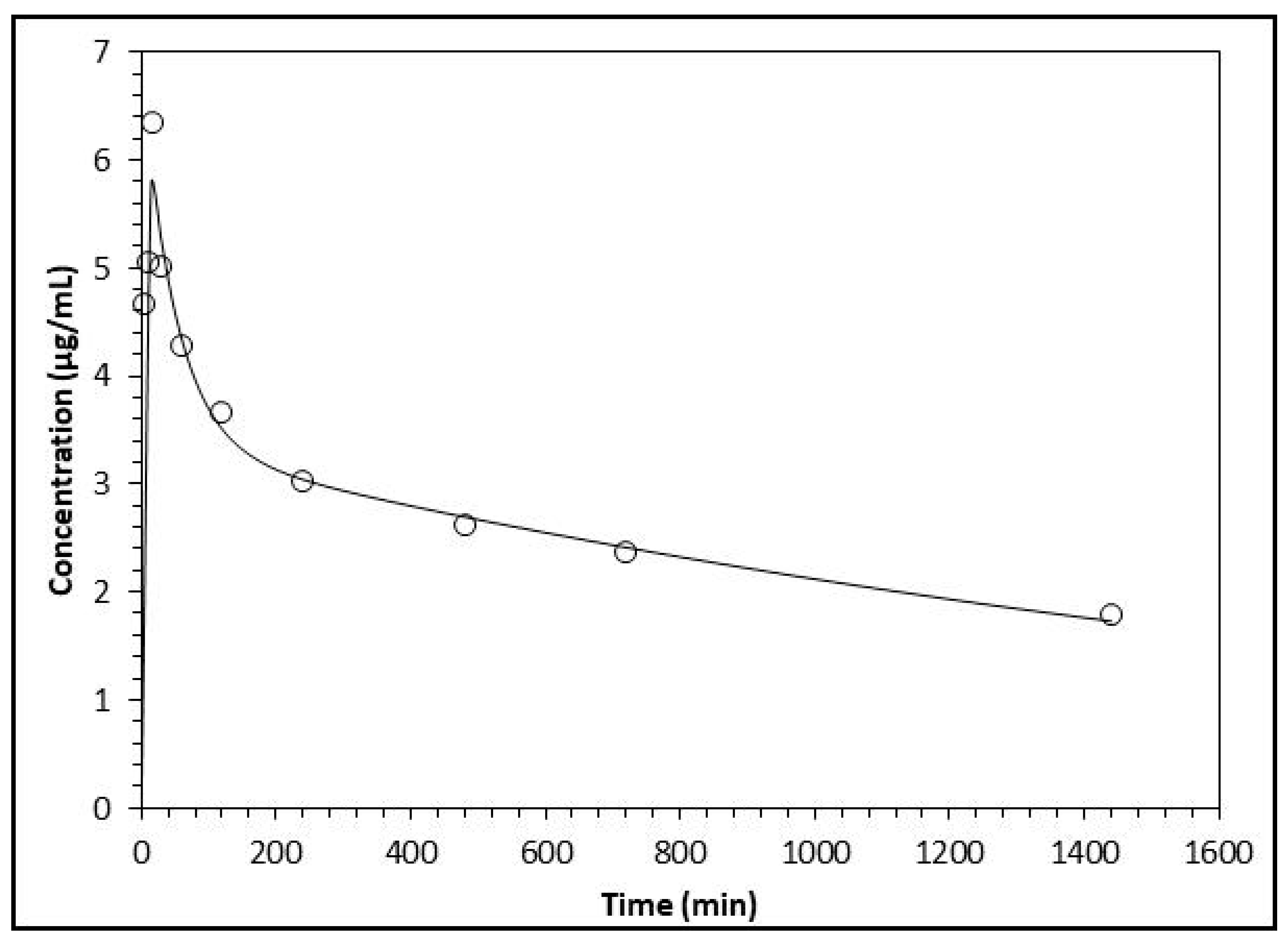
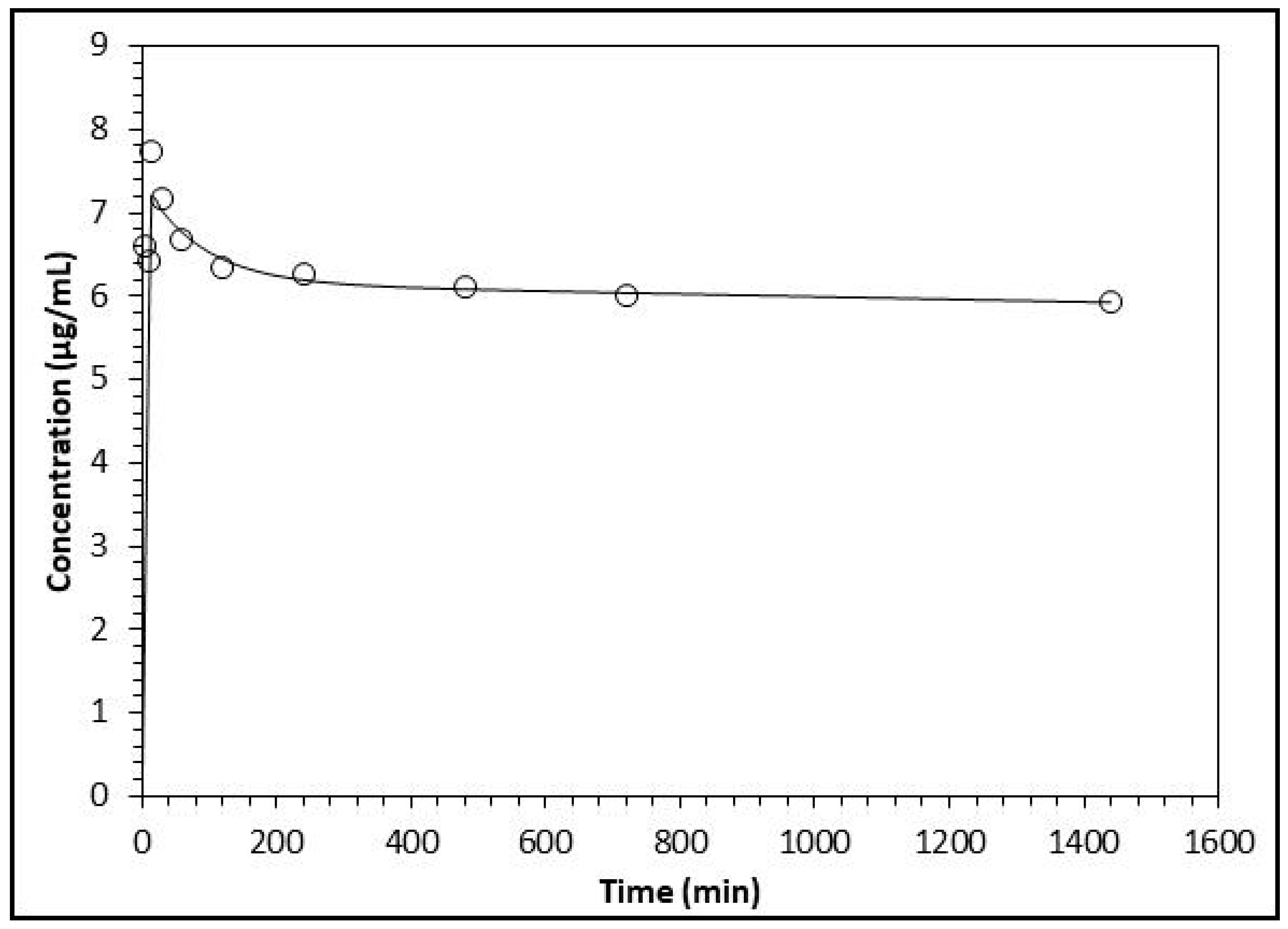
2.3. Plasma Level of Flavonoids and Relative Products
3. Materials and Methods
3.1. Chemicals
3.2. Plant Material
3.3. Extraction Process of Tilia americana
Active Fraction Preparation
3.4. Animals
3.5. Standards and Internal Standard Stock Solutions for Chromatographic Profiling
3.6. HPLC Calibration Curve of Flavonoids, p-HPAA, m-HPAA and DOPAC in Plasma
3.7. Experimental Design
3.8. Quantification of Flavonoids and Biotransformation Products in Plasma
3.9. HPLC Calibration Curve for Flavonoids and Biotransformation Products
3.10. Validation Method
3.11. Linearity and Sensitivity Test
3.12. Specificity Test
3.13. Accuracy and Precision Test
3.14. Recovery (Extraction Efficiency)
3.15. Stability Study
3.16. Pharmacokinetic Analysis
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Baxter, A.J.; Scott, K.M.; Vos, T.; Whiteford, H.A. Global prevalence of anxiety disorders: A systematic review and meta-regression. Psychol. Med. 2013, 43, 897–910. [Google Scholar] [CrossRef] [PubMed]
- Canadian Agency for Drugs and Technologies in Health. Short- and Long-Term Use of Benzodiazepines in Patients with Generalized Anxiety Disorder: A Review of Guidelines. Available online: https://www.cadth.ca/sites/default/files/pdf/htis/july-2015/RA0782%20Long-Term%20Benzodiazepine%20Safety%20Final.pdf (accessed on 11 July 2015).
- Argueta Villamar, A.; Cano Asseleih, L.M.; Rodarte, M.L. Atlas de las Plantas de las Medicina Tradicional Mexicana; Instituto Nacional Indigenista: Mexico, Mexico, 1994; p. 1786. ISBN 1093031427, 9682973244-9682973252. [Google Scholar]
- Martinez, M.; Matuda, E. 1979 Flora del Estado de Mexico; Edicion Facsimilar de los Fasciculos Publicados en los Anos de 1953 a 1972; Biblioteca Enciclopedica del Estado de Mexico: Toluca Edo de Mex, Mexico, 1979; Volume 3. [Google Scholar]
- Aguirre-Hernández, E.; Martínez, A.L.; González-Trujano, M.E.; Moreno, J.; Vibrans, H.; Soto-Hernández, M. Pharmacological evaluation of the anxiolytic and sedative effects of Tilia americana L. var. mexicana in mice. J. Ethnopharmacol. 2007, 109, 140–145. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Ortega, G.; Guevara-Fefer, P.; Chávez, M.; Herrera, J.; Martínez, A.; Martínez, A.L.; González-Trujano, M.E. Sedative and anxiolytic efficacy of Tilia americana var. Mexicana inflorescences used traditionally by communities of State of Michoacan, Mexico. J. Ethnopharmacol. 2008, 116, 461–468. [Google Scholar] [CrossRef] [PubMed]
- Herrera-Ruiz, M.; Román-Ramos, R.; Zamilpa, A.; Tortoriello, J.; Jiménez-Ferrer, J.E. Flavonoids from Tilia americana with anxiolytic activity in plus-maze test. J. Ethnopharmacol. 2008, 118, 312–317. [Google Scholar] [CrossRef] [PubMed]
- Noguerón-Merino, M.C.; Jiménez-Ferrer, E.; Román-Ramos, R.; Zamilpa, A.; Tortoriello, J.; Herrera-Ruiz, M. Interactions of a standardized flavonoid fraction from Tilia americana with serotoninergic drugs in elevated plus maze. J. Ethnopharmacol. 2015, 164, 319–327. [Google Scholar] [CrossRef] [PubMed]
- Vissiennon, C.; Nieber, K.; Kelber, O.; Butterweck, V. Route of administration determines the anxiolytic activity of the flavonols kaempferol, quercetin and myricetin—Are they prodrugs? J. Nutr. Biochem. 2012, 23, 733–740. [Google Scholar] [CrossRef] [PubMed]
- Analytical Procedures and Methods Validation for Drugs and Biologics. Guidance for Industry. Available online: https://www.fda.gov/downloads/drugs/guidances/ucm386366.pdf (accessed on 11 July 2015).
- Viola, H.; Wolfman, C.; Levi de Stein, M.; Wasowski, C.; Peña, C.; Medina, J.H.; Paladini, A.C. Isolation of pharmacologically active benzodiacepines receptor ligands from Tilia tomentosa (Tiliaceae). J. Ethnopharmacol. 1994, 44, 47–53. [Google Scholar] [CrossRef]
- Morganb, A.; Dayb, A.J. Transport of trans-tiliroside (kaempferol-3-β-d-(6″-p-coumaroyl-glucopyranoside) and related flavonoids across Caco-2 cells, as a model of absorption and metabolism in the small intestine. Xenobiotica 2015, 45, 722–730. [Google Scholar]
- Erlund, I.; Kosonen, T.; Alfthan, G.; Mäenpää, J.; Perttunen, K.; Kenraali, J.; Parantainen, J.; Aro, A. Pharmacokinetics of quercetin from quercetin aglycone and rutin in healthy volunteers. Eur. J. Clin. Pharmacol. 2000, 56, 545–553. [Google Scholar] [CrossRef] [PubMed]
- Labib, S.; Erb, A.; Kraus, M.; Wickert, T.; Richling, E. The pig caecum model: A suitable tool to study the intestinal metabolism of flavonoids. Mol. Nutr. Food Res. 2004, 48, 326–332. [Google Scholar] [CrossRef] [PubMed]
- Crespy, V.; Morand, C.; Besson, C.; Cotelle, N.; Vézin, H.; Demigné, C.; Rémésy, C. The splanchnic metabolism of flavonoids highly differed according to the nature of the compound. Am. J. Physiol. Gastrointest. Liver Physiol. 2003, 284, G980–G988. [Google Scholar] [CrossRef] [PubMed]
- Grundmann, O.; Nakajima, J.; Kamata, K.; Seo, S.; Butterweck, V. Kaempferol from the leaves of Apocynum venetum possesses anxiolytic activities in the elevated plus maze test in mice. Phytomedicine 2009, 16, 295–302. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Huo, M.; Zhou, J.; Xie, S. PKSolver: An add-in program for pharmacokinetic and pharmacodynamic data analysis in Microsoft Excel. Comput. Methods Programs Biomed. 2010, 99, 306–314. [Google Scholar] [CrossRef] [PubMed]
- Olofsen, E.; Dahan, A. Using Akaike’s information theoretic criterion in mixed-effects modeling of pharmacokinetic data: A simulation study. F1000 Res. 2014, 2, 71. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds of tiliroside are available from the authors. |

| Compound | Nominal Conc. (µg/mL) | Observed Conc. (µg/mL) | Accuracy Bias (%) | RSD (%) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 h | 8 h | 24 h | 0 h | 8 h | 24 h | 0 h | 8 h | 24 h | |||||
| Room temperature (25 °C) | Rutin | 1.75 | 1.74 ± 0.11 | 1.65 ± 0.10 | 1.64 ± 0.10 | 5.76 | 5.46 | 0.33 | 6.74 | 6.58 | 6.57 | ||
| 3.50 | 3.39 ± 0.16 | 3.67 ± 0.30 | 3.38 ± 0.27 | 3.17 | −4.97 | 2.93 | 4.76 | 8.25 | 8.14 | ||||
| 7.00 | 7.27 ± 0.41 | 7.17 ± 0.21 | 7.13 ± 0.24 | −1.99 | −2.45 | −3.93 | 5.72 | 2.95 | 3.39 | ||||
| Quercetin glucoside | 11.02 | 11.16 ± 0.27 | 10.84 ± 0.67 | 11.35 ± 0.61 | −1.24 | 1.62 | −2.98 | 2.42 | 6.18 | 5.38 | |||
| 22.18 | 20.86 ± 1.35 | 20.84 ± 1.24 | 20.44 ± 1.65 | 5.94 | 6.02 | 7.83 | 6.46 | 5.99 | 8.08 | ||||
| 44.37 | 43.10 ± 2.20 | 42.48 ± 1.06 | 41.37 ± 1.17 | 2.86 | 4.28 | 6.76 | 5.10 | 2.49 | 2.84 | ||||
| Quercitrin | 11.02 | 11.27 ± 0.75 | 11.56 ± 0.78 | 10.99 ± 0.72 | −2.26 | −4.83 | 0.30 | 6.67 | 6.74 | 6.59 | |||
| 22.18 | 22.94 ± 1.87 | 22.65 ± 1.85 | 23.11 ± 1.89 | −3.39 | −2.11 | −4.15 | 8.17 | 8.16 | 8.18 | ||||
| 44.37 | 44.12 ± 2.82 | 44.96 ± 1.84 | 43.90 ± 1.61 | 0.56 | −1.33 | 1.05 | 6.40 | 4.10 | 3.66 | ||||
| Tiliroside | 11.02 | 10.54 ± 0.74 | 10.33 ± 0.61 | 10.53 ± 0.79 | 4.59 | 6.26 | 4.48 | 7.09 | 5.89 | 7.50 | |||
| 22.18 | 21.44 ± 2.13 | 22.84 ± 2.57 | 20.60 ± 2.05 | 3.36 | −2.95 | 7.14 | 9.98 | 9.95 | 9.99 | ||||
| 44.37 | 43.77 ± 4.28 | 44.65 ± 4.37 | 41.25 ± 2.04 | 1.35 | −0.64 | 7.03 | 9.80 | 9.79 | 9.80 | ||||
| Kaempferol | 1.12 | 1.16 ± 0.099 | 1.21 ± 0.83 | 1.15 ± 0.098 | −3.29 | −7.28 | −2.28 | 8.53 | 6.92 | 8.52 | |||
| 2.24 | 2.24 ± 0.20 | 2.15 ± 0.19 | 2.42 ± 0.22 | 0.53 | 4.60 | −8.12 | 9.06 | 9.03 | 9.10 | ||||
| 4.50 | 4.45 ± 0.41 | 4.32 ± 0.21 | 4,.17 ± 0.34 | −4.64 | −1.74 | 1.77 | 9.34 | 5.05 | 8.26 | ||||
| m-HPAA | 1.95 | 1.91 ± 0.2 | 1.91 ± 0.03 | 1.90 ± 0.04 | 0.47 | 2.23 | 2.73 | 0.33 | 1.61 | 1.98 | |||
| 7.81 | 7.80 ± 0.01 | 7.98 ± 0.12 | 7.82 ± 0.01 | 0.13 | 2.18 | 0.09 | 0.09 | 1.51 | 0.06 | ||||
| 31.25 | 31.40 ± 0.10 | 31.53 ± 0.19 | 31.34 ± 0.07 | 1.88 | 0.90 | 0.30 | 1.35 | 0.63 | 0.22 | ||||
| p-HPAA | 1.95 | 1.92 ± 0.02 | 1.92 ± 0.02 | 1.93 ± 0.01 | 1.44 | 1.40 | 0.85 | 1.03 | 1.01 | 0.60 | |||
| 7.81 | 7.86 ± 0.11 | 7.94 ± 0.11 | 7.89 ± 0.05 | 0.65 | 2.05 | 1.01 | 0.46 | 1.42 | 0.71 | ||||
| 31.25 | 31.76 ± 0.28 | 31.64 ± 0.28 | 31.64 ± 0.31 | 1.64 | 1.26 | 1.26 | 1.14 | 0.88 | 0.88 | ||||
| DOPAC | 1.95 | 1.98 ± 0.020 | 1.92 ± 0.02 | 1.91 ± 0.03 | 0.02 | 0.03 | 0.03 | 1.26 | 1.46 | 1.61 | |||
| 7.81 | 7.85 ± 0.032 | 7.86 ± 0.03 | 7.78 ± 0.02 | 0.03 | 0.03 | 0.02 | 0.40 | 0.41 | 0.27 | ||||
| 31.25 | 31.61 ± 0.26 | 31.64 ± 0.24 | 31.53 ± 0.19 | 0.26 | 0.25 | 0.19 | 0.82 | 0.80 | 0.62 | ||||
| Nominal Conc. (µg/mL) | Observed Conc. (µg/mL) | Accuracy Bias (%) | RSD (%) | ||||||||||
| Autosampler (4 °C; 8 h) | Long-term (−70 °C; 1 month) | Autosampler (4 °C; 8 h) | Long-term (−70°C; 1 month) | Autosampler (4°C; 8 h) | Long-term (−70°C; 1 month) | ||||||||
| Storage stability | Rutin | 1.75 | 1.86 ± 0.19 | 1.69 ± 0.12 | −6.41 | 2.92 | 10.58 | 7.24 | |||||
| 3.50 | 3.44 ± 0.15 | 3.51 ± 0.28 | 1.58 | −0.45 | 4.39 | 7.97 | |||||||
| 7.00 | 7.08 ± 0.34 | 7.10 ± 0.38 | −1.17 | −1.56 | 4.81 | 5.43 | |||||||
| Quercetin glucoside | 11.02 | 11.22 ± 0.59 | 11.41 ± 0.71 | 2.40 | 6.22 | 5.29 | 6.25 | ||||||
| 22.18 | 20.96 ± 1.31 | 20.57 ± 2.06 | 5.48 | 7.26 | 6.28 | 10.02 | |||||||
| 44.37 | 43.30 ± 2.02 | 41.60 ± 2.26 | −1.82 | −3.52 | 4.66 | 5.45 | |||||||
| Quercitrin | 11.02 | 11.62 ± 1.02 | 11.64 ± 0.99 | −5.47 | −2.89 | 8.82 | 8.76 | ||||||
| 22.18 | 22.79 ± 2.26 | 22.49 ± 1.66 | −2.73 | −1.41 | 9.92 | 7.40 | |||||||
| 44.37 | 44.44 ± 4.52 | 44.17 ± 2.95 | −0.17 | 0.44 | 10.17 | 6.69 | |||||||
| Tiliroside | 11.02 | 10.58 ± 0.81 | 10.40 ± 0.99 | 3.97 | 5.54 | 7.69 | 9.54 | ||||||
| 22.18 | 22.94 ± 2.25 | 21.41 ± 1.82 | −3.45 | 3.42 | 9.81 | 8.51 | |||||||
| 44.37 | 42.74 ± 3.40 | 44.26 ± 2.49 | 3.66 | 0.24 | 7.97 | 5.64 | |||||||
| Kaempferol | 1.12 | 1.21 ± 0.07 | 1.15 ± 0.10 | −7.77 | −2.77 | 6.56 | 8.67 | ||||||
| 2.24 | 2.24 ± 0.20 | 2.15 ± 0.19 | 0.50 | 4.13 | 9.09 | 9.07 | |||||||
| 4.50 | 4.19 ± 0.37 | 4.35 ± 0.39 | 1.24 | −2.47 | 8.95 | 9.03 | |||||||
| m-HPAA | 1.95 | 1.93 ± 0.04 | 1.92 ± 0.03 | 0.09 | 0.90 | 0.22 | 0.63 | ||||||
| 7.81 | 7.82 ± 0.01 | 7.80 ± 0.12 | 0.30 | 2.18 | 0.06 | 1.51 | |||||||
| 31.25 | 31.34 ± 0.07 | 31.20 ± 0.19 | 2.73 | 2.23 | 1.98 | 1.61 | |||||||
| p-HPAA | 1.95 | 1.93 ± 0.02 | 1.92 ± 0.01 | 1.26 | 1.26 | 0.88 | 0.88 | ||||||
| 7.81 | 7.89 ± 0.11 | 7.97 ± 0.05 | 2.05 | 1.01 | 1.42 | 0.71 | |||||||
| 31.25 | 31.64 ± 0.28 | 31.64 ± 0.28 | 1.40 | 0.85 | 1.01 | 0.60 | |||||||
| DOPAC | 1.95 | 1.91 ± 0.03 | 1.99 ± 0.03 | 0.88 | 1.15 | 0.80 | 0.62 | ||||||
| 7.81 | 7.78 ± 0.02 | 7.85 ± 0.03 | 0.38 | 0.58 | 0.41 | 0.27 | |||||||
| 31.25 | 31.61 ± 0.19 | 31.53 ± 0.25 | 2.23 | 2.11 | 1.46 | 1.61 | |||||||
| Plasma Concentration (μg/mL) | ||||||||
|---|---|---|---|---|---|---|---|---|
| Time (min) | Rutin | Quercetin Glucoside | Quercitrin | Tiliroside | Kaempferol | m-HPAA | p-HPAA | DOPAC |
| 5 | ND | ND | ND | ND | ND | 4.67 ± 0.27 | 5.40 ± 0.36 | 6.60 ± 0.43 |
| 10 | 5.06 ± 0.22 | 6.71 ± 0.44 | 6.43 ± 0.35 | |||||
| 15 | 4.75 ± 0.29 | 6.01 ± 0.33 | 7.73 ± 0.61 | |||||
| 30 | 4.52 ± 0.28 | 5.38 ± 0.51 | 7.17 ± 0.52 | |||||
| 60 | 4.29 ± 0.13 | 5.31 ± 0.54 | 6.88 ± 0.43 | |||||
| 120 | 4.17 ± 0.33 | 5.29 ± 0.32 | 6.34 ± 0.41 | |||||
| 240 | 4.11 ± 0.23 | 5.15 ± 0.23 | 6.26 ± 0.32 | |||||
| 480 | 4.04 ± 0.25 | 5.08 ± 0.24 | 6.10 ± 0.34 | |||||
| 720 | 3.94 ± 0.19 | 4.99 ± 0.35 | 6.01 ± 0.54 | |||||
| 1440 | 3.79 ± 0.28 | 4.91 ± 0.21 | 5.93 ± 0.49 | |||||
| m-HPAA | p-HPAA | DOPAC | Units. | |
|---|---|---|---|---|
| A | 3.49 | 3.19 | 1.23 | µg/mL |
| B | 3.36 | 3.75 | 6.16 | µg/mL |
| ka | 0.24 | 0.36 | 0.43 | l/min |
| k10 | 0.0009 | 0.0006 | 3.18 × 10−5 | 1/min |
| k12 | 0.009 | 0.007 | 0.002 | 1/min |
| k21 | 0.01 | 0.009 | 0.0096 | 1/min |
| t1/2α | 35.08 | 42.25 | 59.95 | min |
| t1/2β | 1501.88 | 1993.85 | 26,012.93 | min |
| t1/2ka | 2.84 | 1.92 | 1.61 | min |
| α | 0.02 | 0.02 | 0.01 | 1/min |
| β | 0.0005 | 0.0003 | 2.66 × 10−5 | 1/min |
| V/F | 30.45 | 29.46 | 27.21 | (mg/Kg)/(µg/mL) |
| CL/F | 0.026 | 0.018 | 0.0008 | (mg/Kg)/(μg/mL)/min |
| Tmax | 14.10 | 11.16 | 12.90 | min |
| Cmax | 5.76 | 6.26 | 7.18 | µg/mL |
| AUC0→∞ | 7434.23 | 10,962.77 | 231,124.48 | µg/mL min |
| AUC0→1440 | 3685.97 | 4423.64 | 8786.10 | µg/mL min |
| MRT | 2124.58 | 2831.66 | 37,514.31 | min |
| Diagnostics | ||||
| SS | 0.78 | 0.39 | 0.89 | |
| R2 | 0.99 | 0.99 | 0.998 | |
| AIC | 7.49 | 0.77 | 8.84 | |
| SC | 9.00 | 2.27 | 10.36 | |
| Compound | Concentration (µg/mL) | Level |
|---|---|---|
| Rutin | 1.75 | Low |
| 3.50 | Medium | |
| 7.00 | High | |
| Quercetin glucoside | 11.02 | Low |
| Quercitrin | 22.18 | Medium |
| Tiliroside | 34.37 | High |
| Kaempferol | 1.125 | Low |
| 2.25 | Medium | |
| 4.50 | High | |
| m-HPAA | 1.95 | Low |
| p-HPAA | 7.81 | Medium |
| DOPAC | 31.25 | High |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Juárez Ramírez, V.A.; Jiménez-Beltrán, M.I.; Zamilpa, A.; Herrera-Ruiz, M.; Abarca-Vargas, R.; Lombardo-Earl, G.; Tortoriello, J.; Jiménez-Ferrer, E. Pharmacokinetic Study of Biotransformation Products from an Anxiolytic Fraction of Tilia americana. Molecules 2017, 22, 1260. https://doi.org/10.3390/molecules22081260
Juárez Ramírez VA, Jiménez-Beltrán MI, Zamilpa A, Herrera-Ruiz M, Abarca-Vargas R, Lombardo-Earl G, Tortoriello J, Jiménez-Ferrer E. Pharmacokinetic Study of Biotransformation Products from an Anxiolytic Fraction of Tilia americana. Molecules. 2017; 22(8):1260. https://doi.org/10.3390/molecules22081260
Chicago/Turabian StyleJuárez Ramírez, Virgilio Alfonso, María Isabel Jiménez-Beltrán, Alejandro Zamilpa, Maribel Herrera-Ruiz, Rodolfo Abarca-Vargas, Galia Lombardo-Earl, Jaime Tortoriello, and Enrique Jiménez-Ferrer. 2017. "Pharmacokinetic Study of Biotransformation Products from an Anxiolytic Fraction of Tilia americana" Molecules 22, no. 8: 1260. https://doi.org/10.3390/molecules22081260






