Evaluation of Novel Dual Acetyl- and Butyrylcholinesterase Inhibitors as Potential Anti-Alzheimer’s Disease Agents Using Pharmacophore, 3D-QSAR, and Molecular Docking Approaches
Abstract
:1. Introduction
2. Results and Discussion
2.1. Inhibiting Activity of DL0410 Derivatives on AChE and BuChE
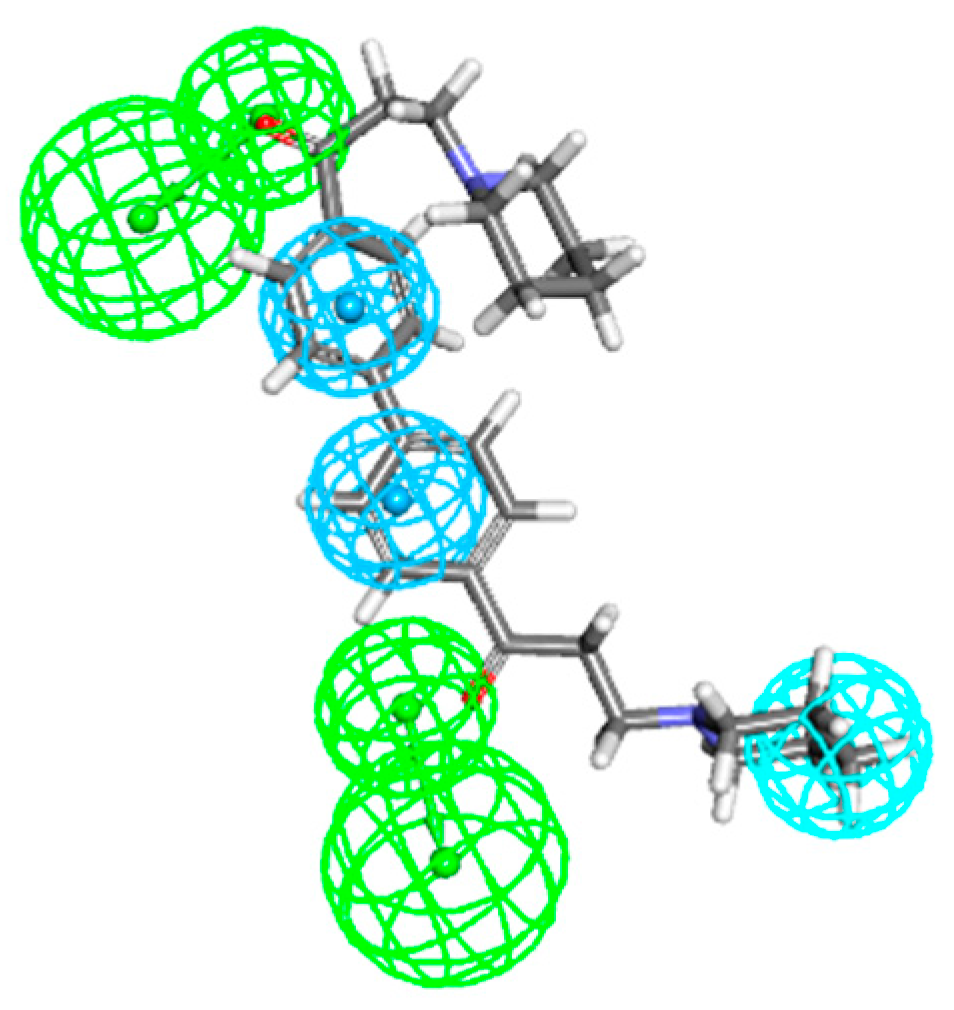
2.2. Common Feature Pharmacophore Models
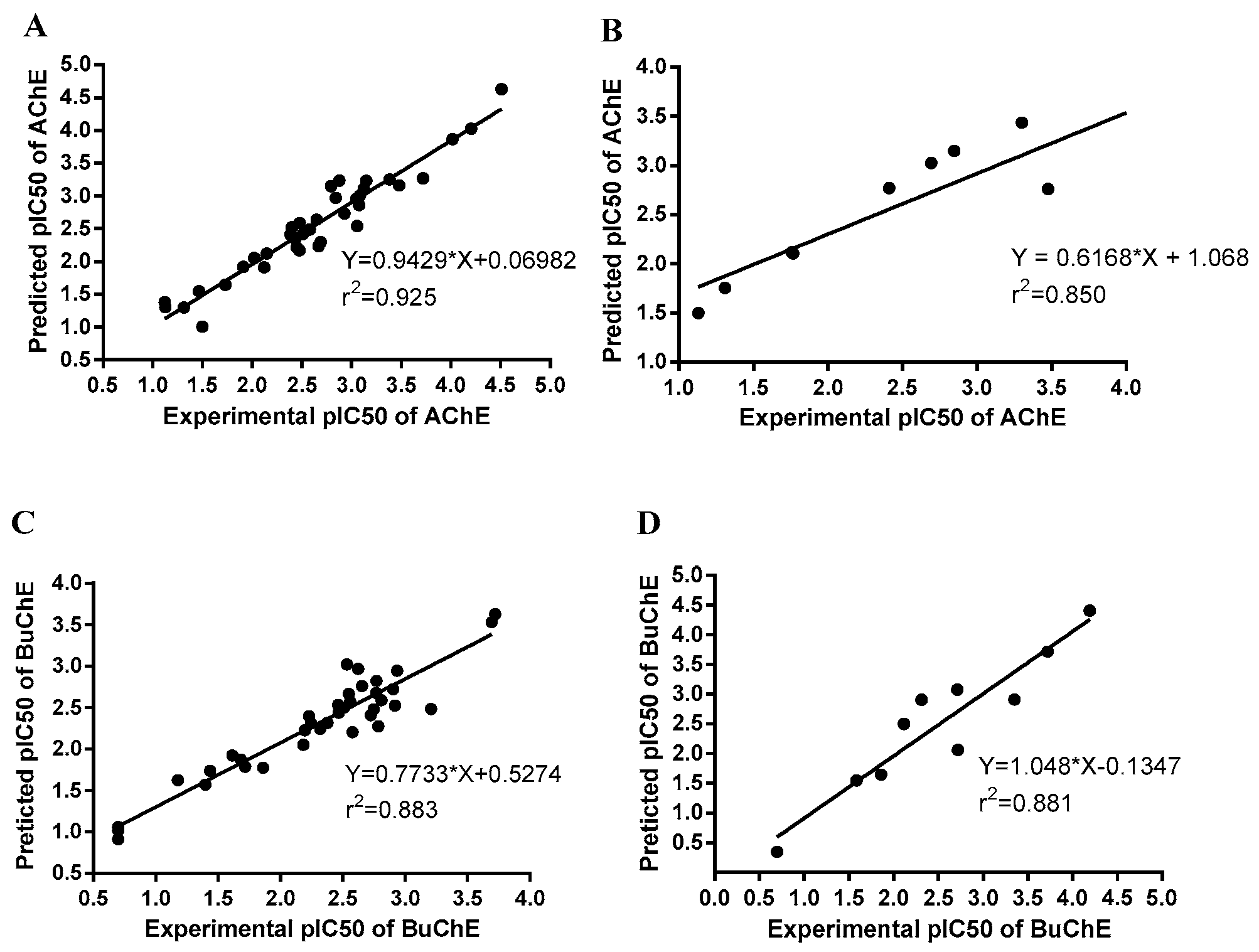
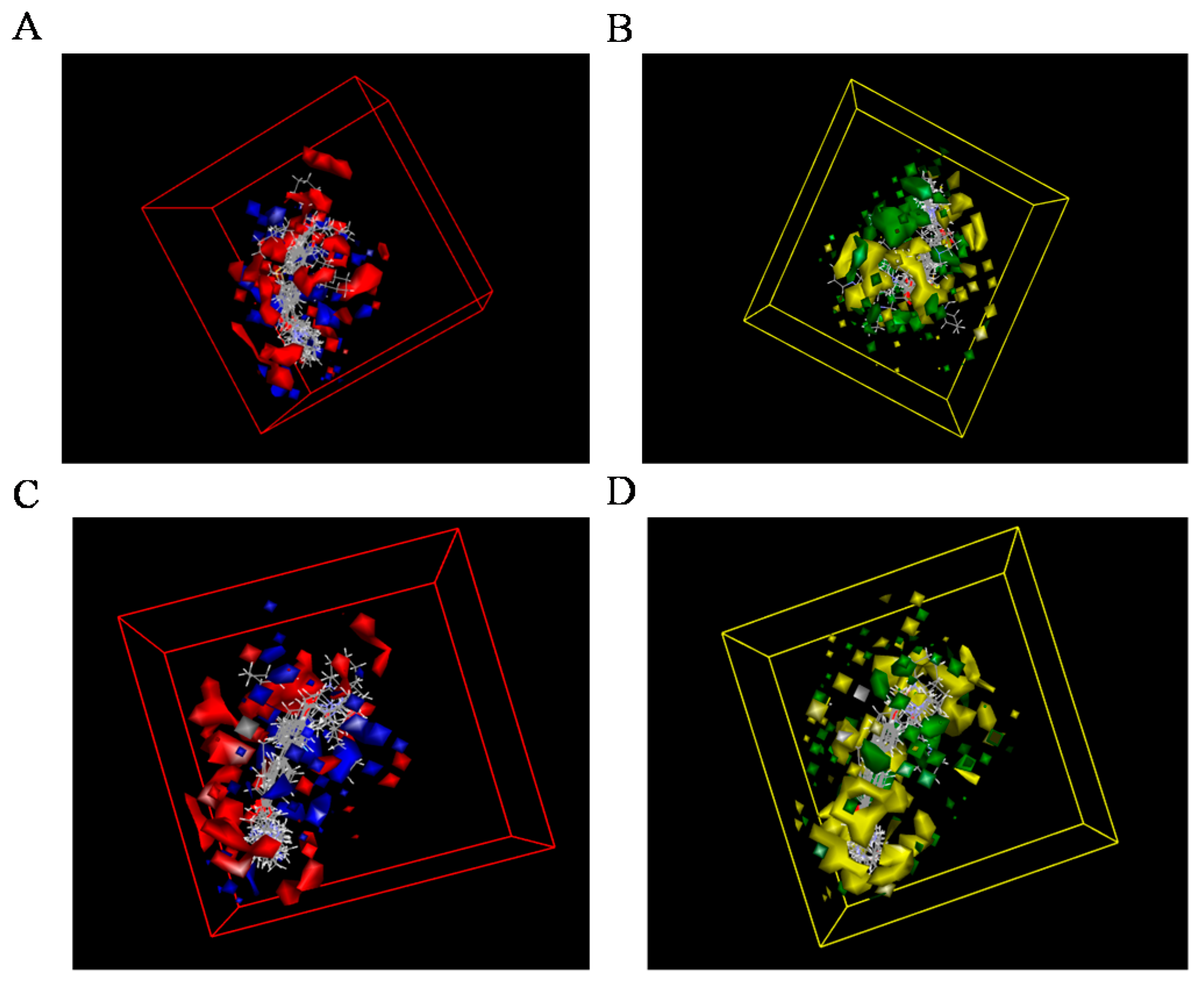
2.3. 3D-QSAR Models
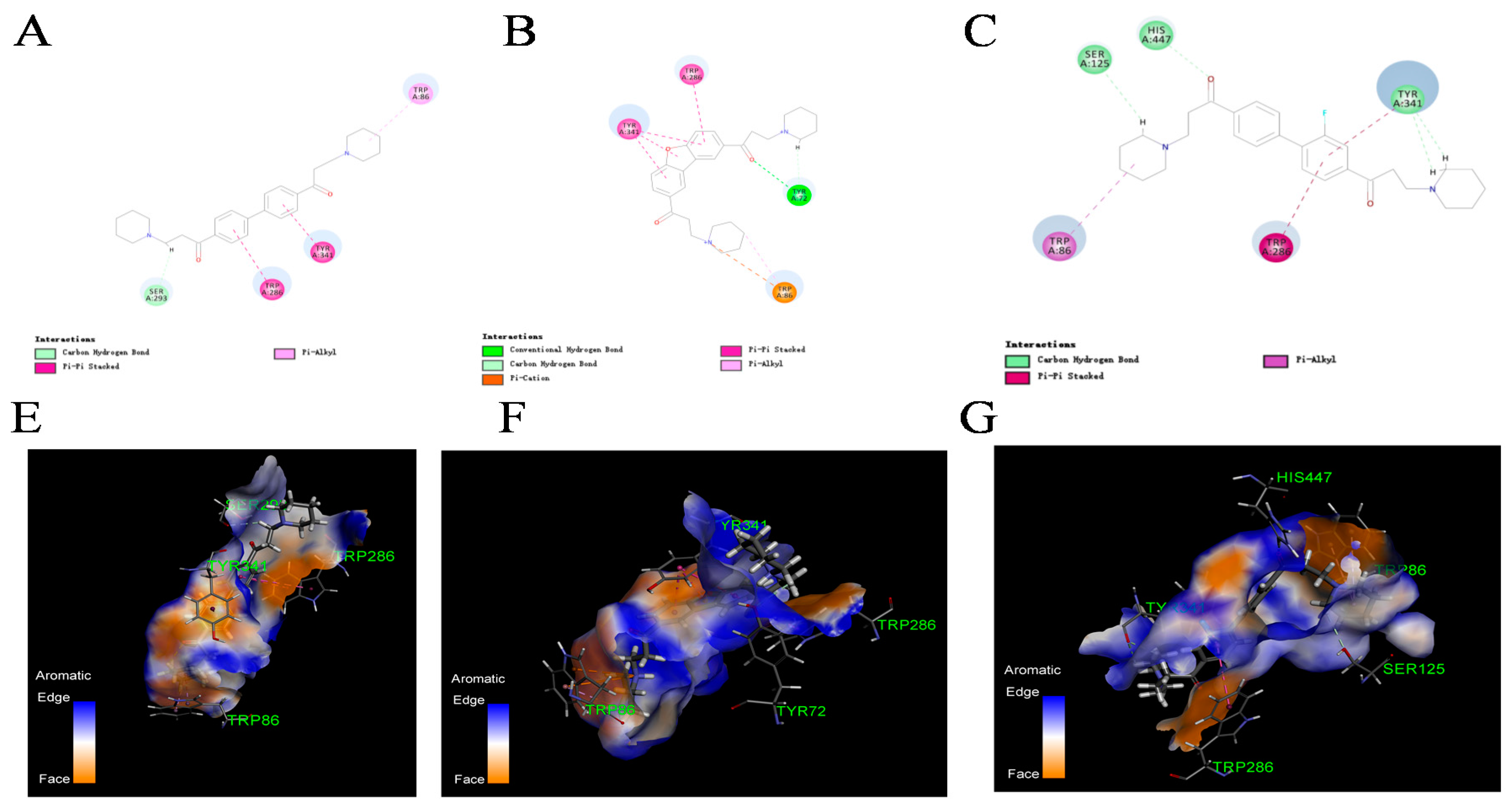
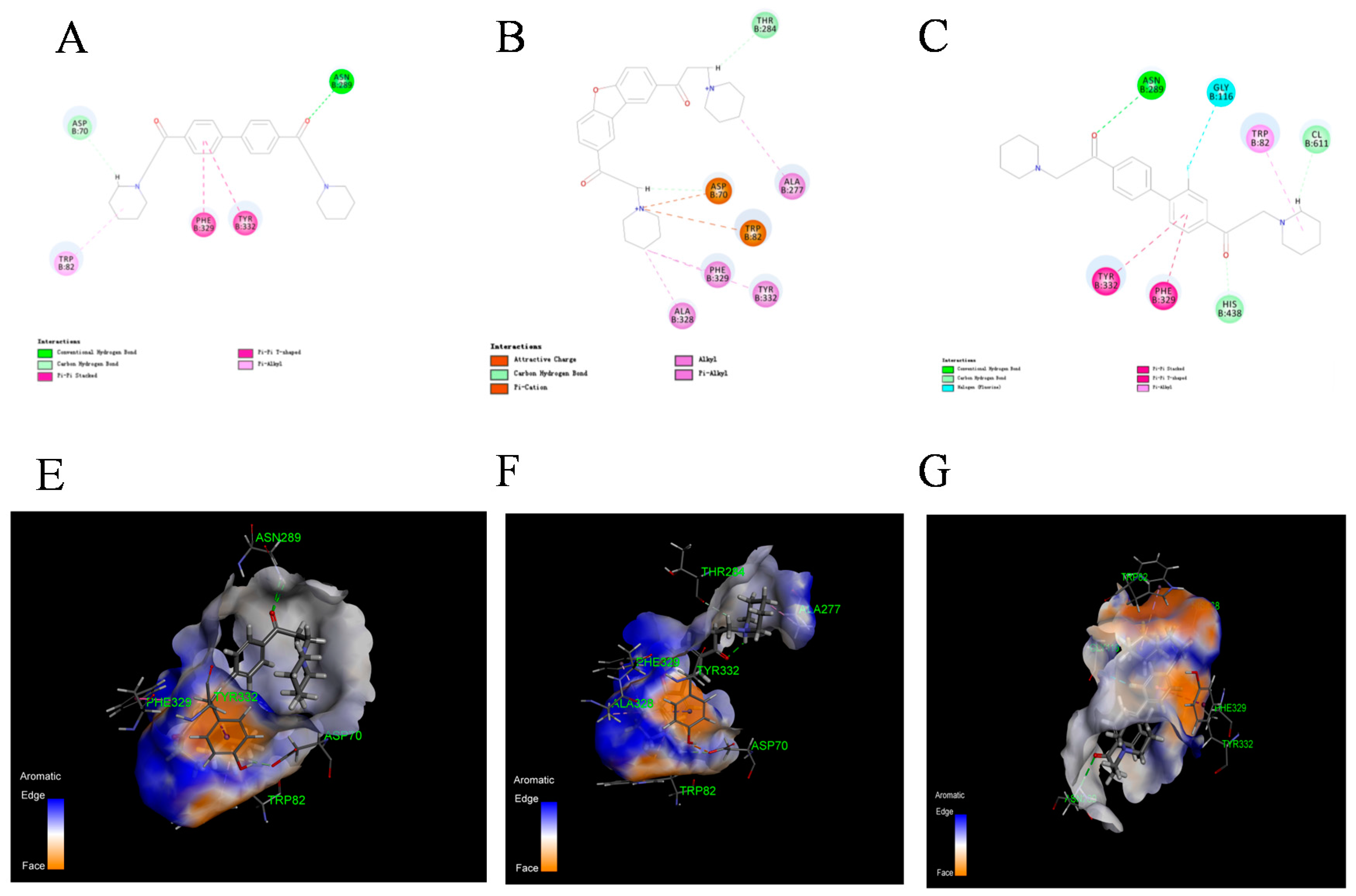
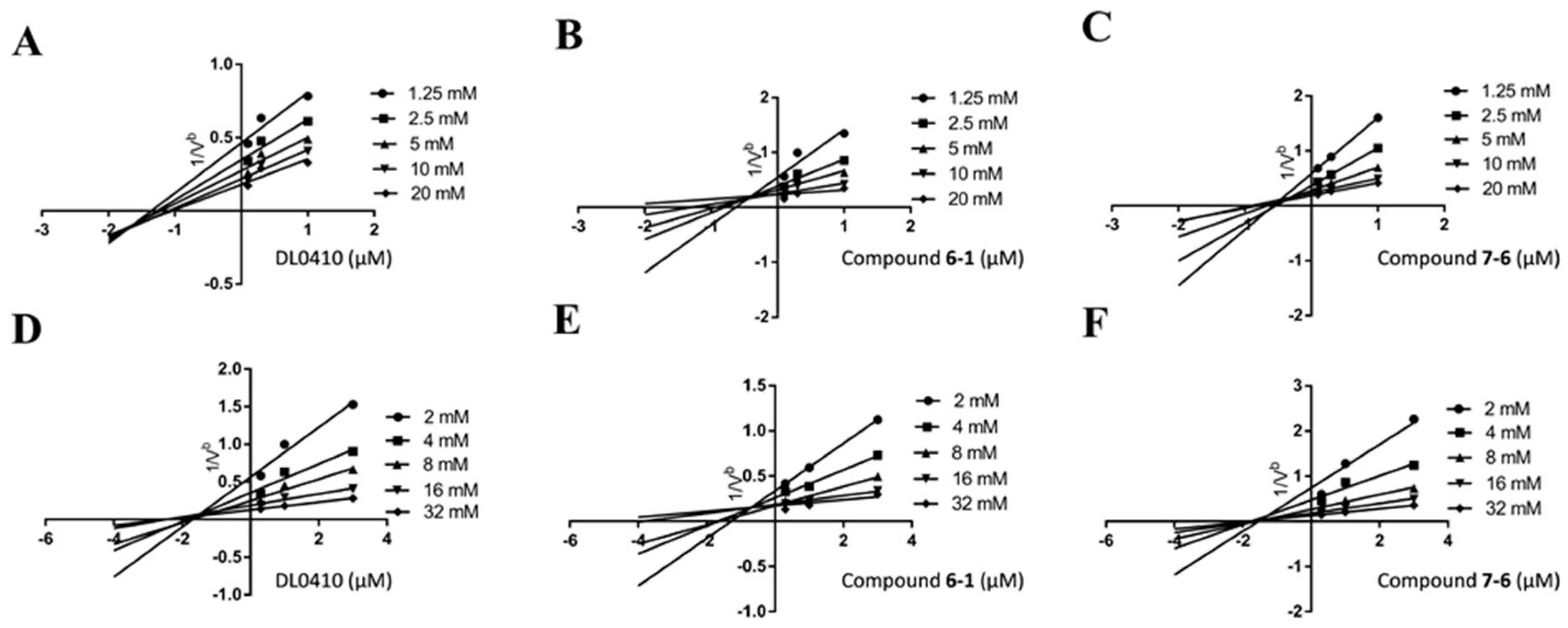
2.4. Kinetics Study and Molecular Docking
2.5. The Evaluation of Absorption and BBB Permeability
3. Methods
3.1. In Vitro AChE Inhibitory Assay
3.2. In Vitro BuChE Inhibitory Assay
3.3. AChE and BuChE Kinetic Studies
3.4. Common Feature Pharmacophore Models
3.5. 3D-QSAR Models
3.6. Molecular Docking
3.7. Absorption, Distribution, Metabolism, and Excretion (ADME)
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Oboudiyat, C.; Glazer, H.; Seifan, A.; Greer, C.; Isaacson, R.S. Alzheimer’s disease. Semin. Neurol. 2013, 33, 313–329. [Google Scholar] [CrossRef] [PubMed]
- Mucke, L. Neuroscience: Alzheimer’s disease. Nature 2009, 461, 895–897. [Google Scholar] [CrossRef] [PubMed]
- Wisniewski, T.; Konietzko, U. Amyloid-beta immunization for Alzheimer’s disease. Lancet Neurol. 2008, 7, 805–811. [Google Scholar] [CrossRef]
- Querfurth, H.W.; LaFerla, F.M. Alzheimer’s disease. N. Engl. J. Med. 2010, 362, 329–344. [Google Scholar] [CrossRef] [PubMed]
- Citron, M. Alzheimer’s disease: Strategies for disease modification. Nat. Rev. Drug Discov. 2010, 9, 387–398. [Google Scholar] [CrossRef]
- Maruszak, A.; Żekanowski, C. Mitochondrial dysfunction and Alzheimer’s disease. Prog. Neuropsychopharmacol. Biol. Psychiatry 2011, 35, 320–330. [Google Scholar] [CrossRef] [PubMed]
- Winkler, J.; Thal, L.J.; Gage, F.H.; Fisher, L.J. Cholinergic strategies for Alzheimer’s disease. J. Mol. Med. 1998, 76, 555–567. [Google Scholar] [CrossRef] [PubMed]
- Ferreira-Vieira, T.H.; Guimaraes, I.M.; Silva, F.R.; Ribeiro, F.M. Alzheimer’s disease: Targeting the cholinergic system. Curr. Neuropharmacol. 2016, 14, 101–115. [Google Scholar] [CrossRef] [PubMed]
- Akhondzadeh, S.; Abbasi, S.H. Herbal medicine in the treatment of Alzheimer’s disease. Am. J. Alzheimers Dis. Other Demen. 2006, 21, 113–118. [Google Scholar] [CrossRef] [PubMed]
- Mushtaq, G.; Greig, N.H.; Khan, J.A.; Kamal, M.A. Status of acetylcholinesterase and butyrylcholinesterase in Alzheimer’s disease and type 2 diabetes mellitus. CNS Neurol. Disord. Drug Targets 2014, 13, 1432–1439. [Google Scholar] [CrossRef] [PubMed]
- Cummings, J.L.; Morstorf, T.; Zhong, K. Alzheimer’s disease drug development pipeline: Few candidates, frequent failures. Alzheimers Res. Ther. 2014, 6, 37. [Google Scholar] [CrossRef] [PubMed]
- Deb, P.K.; Sharma, A.; Piplani, P.; Akkinepally, R.R. Molecular docking and receptor-specific 3D-QSAR studies of acetylcholinesterase inhibitors. Mol. Divers. 2012, 16, 803–823. [Google Scholar] [CrossRef] [PubMed]
- Silman, I.; Sussman, J. L. Acetylcholinesterase: How is structure related to function. Chem. Biol. Interact. 2008, 175, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Kryger, G.; Silman, I.; Sussman, J.L. Structure of acetylcholinesterase complexed with E2020 (Aricept): Implications for the design of new anti-Alzheimer drugs. Structure 1999, 7, 297–307. [Google Scholar] [CrossRef]
- Ahmad, S.; Iftikhar, F.; Ullah, F.; Sadiq, A.; Rashid, U. Rational design and synthesis of dihydropyrimidine based dual binding site acetylcholinesterase inhibitors. Bioorg. Med. Chem. 2016, 69, 91–101. [Google Scholar] [CrossRef] [PubMed]
- Nicolet, Y.; Lockridge, O.; Masson, P.; Fontecilla-Camps, J.C.; Nachon, F. Crystal structure of human butyrylcholinesterase and of its complexes with substrate and products. J. Biol. Chem. 2003, 278, 41141–41147. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.M.; Feng, B.; Fu, H.; Liu, A.L.; Wang, L.; Du, G.H.; Wu, S. Design, synthesis, and biological evaluation of a new series of biphenyl/bibenzyl derivatives functioning as dual inhibitors of acetylcholinesterase and butyrylcholinesterase. Molecules 2017, 22, 172. [Google Scholar] [CrossRef] [PubMed]
- Zhou, D.; Zhou, W.; Song, J.K.; Feng, Z.Y.; Yang, R.Y.; Wu, S.; Wang, L.; Liu, A.L.; Du, G.H. DL0410, a novel dual cholinesterase inhibitor, protects mouse brains against Aβ-induced neuronal damage via the Akt/JNK signaling pathway. Acta Pharmacol. Sin. 2016, 37, 1401–1412. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.Y.; Zhao, G.; Wang, D.M.; Pang, X.C.; Wang, S.B.; Fang, J.S.; Li, C.; Liu, A.L.; Wu, S.; Du, G.H. DL0410 can reverse cognitive impairment, synaptic loss and reduce plaque load in APP/PS1 transgenic mice. Pharmacol. Biochem. Behav. 2015, 139, 15–26. [Google Scholar] [CrossRef] [PubMed]
- Lian, W.W.; Fang, J.S.; Xu, L.J.; Zhou, W.; Kang, D.; Xiong, W.; Jia, H.; Liu, A.L.; Du, G.H. DL0410 ameliorates memory and cognitive impairments induced by scopolamine via increasing cholinergic neurotransmission in Mice. Molecules 2017, 22, 410. [Google Scholar] [CrossRef] [PubMed]
- Fang, J.S.; Yang, R.Y.; Gao, L.; Zhou, D.; Yang, S.Q.; Liu, A.L.; Du, G.H. Predictions of BuChE inhibitors using support vector machine and naive bayesian classification techniques in drug discovery. J. Chem. Inf. Model. 2015, 53, 3009–3020. [Google Scholar] [CrossRef] [PubMed]
- Fang, J.S.; Li, Y.J.; Liu, R.; Pang, X.C.; Li, C.; Yang, R.Y.; He, Y.Y.; Lian, W.W.; Liu, A.L.; Du, G.H. Discovery of multitarget-directed ligands against Alzheimer’s disease through systematic prediction of chemical-protein interactions. J. Chem. Inf. Model. 2015, 55, 149–164. [Google Scholar] [CrossRef] [PubMed]
- Chai, H.H.; Lim, D.; Chai, H.Y.; Jung, K. Molecular modeling of small molecules as BVDV RNA-Dependent RNA polymerase allosteric inhibitors. Bull. Korean Chem. Soc. 2013, 34, 837–850. [Google Scholar] [CrossRef]
- Li, C.Y.; Li, Q.S.; Yan, L.; Sun, X.G.; Wei, R.; Gong, H.B.; Zhu, H.L. Synthesis, biological evaluation and 3D-QSAR studies of novel 4,5-dihydro-1H-pyrazole niacinamide derivatives as BRAF inhibitors. Bioorg. Med. Chem. 2012, 20, 3746–3755. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Jeong, S.K.; Kim, B.C.; Park, K.W.; Dash, A. Donepezil across the spectrum of Alzheimer’s disease: Dose optimization and clinical relevance. Acta Neurol. Scand. 2015, 131, 259–267. [Google Scholar] [CrossRef] [PubMed]
- Kosak, U.; Brus, B.; Knez, D.; Sink, R.; Zakelj, S.; Trontelj, J.; Pislar, A.; Slenc, J.; Gobec, M.; Zivin, M.; et al. Development of an in-vivo active reversible butyrylcholinesterase inhibitor. Sci. Rep. 2016, 6, 39495. [Google Scholar] [CrossRef] [PubMed]
- Schlamowitz, M.; Shaw, A.; Jackson, W.T. Limitations of the Dixon plot for ascertaining nature of enzyme inhibition. Tex. Rep. Biol. Med. 1969, 27, 483–488. [Google Scholar] [PubMed]
- Dixon, M. The determination of enzyme inhibitor constants. Biochem. J. 1953, 55, 170–171. [Google Scholar] [CrossRef] [PubMed]
- Cheng, F.; Li, W.; Zhou, Y.; Shen, J.; Wu, Z.; Liu, G.; Lee, P.W.; Tang, Y. AdmetSAR: A comprehensive source and free tool for assessment of chemical ADMET properties. J. Chem. Inf. Model. 2012, 52, 3099–3105. [Google Scholar] [CrossRef] [PubMed]
- Nisha, C.M.; Kumar, A.; Vimal, A.; Vimal, A.; Bai, B.M.; Pal, D.; Kumar, A. Docking and ADMET prediction of few GSK-3 inhibitors divulges 6-bromoindirubin-3-oxime as a potential inhibitor. J. Chem. Inf. Model. 2016, 65, 100–107. [Google Scholar] [CrossRef] [PubMed]
- Ellman, G.L.; Courtney, K.D.; Andres, V.; Feather-Stone, R.M. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol. 1961, 7, 88–95. [Google Scholar] [CrossRef]
- Bharate, S.B.; Manda, S.; Joshi, P.; Singh, B.; Vishwakarma, R.A. Total synthesis and anti-cholinesterase activity of marine-derived bis-indole alkaloid fascaplysin. Med. Chem. Commun. 2012, 3, 1098–1103. [Google Scholar] [CrossRef]
- Sathishkumar, N.; Karpagam, V.; Sathiyamoorthy, S.; Woo, M.J.; Kim, Y.J.; Yang, D.K. Computer-aided identification of EGFR tyrosine kinase inhibitors using ginsenosides from Panax ginseng. Comput. Biol. Med. 2013, 43, 786–797. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.C.; Qin, Y.J.; Wang, P.F.; Yang, Y.A.; Wen, Q.; Zhang, X.; Qiu, H.Y.; Duan, Y.T.; Wang, Y.T.; Sang, Y.L.; et al. Sulfonamides containing coumarin moieties selectively and potently inhibit carbonic anhydrases II and IX: Design, synthesis, inhibitory activity and 3D-QSAR analysis. Eur. J. Med. Chem. 2013, 66, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Mavel, S.; Mincheva, Z.; Méheux, N.; Carcenac, Y.; Guilloteau, D.; Abarbri, M.; Emond, P. QSAR study and synthesis of new phenyltropanes as ligands of the dopamine transporter (DAT). Bioorg. Med. Chem. 2012, 20, 1388–1395. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Wang, X.Y.; Lv, P.C.; Zhu, H.L. Discovery of a series of novel phenylpiperazine derivatives as EGFR TK inhibitors. Sci. Rep. 2015, 5, 13934. [Google Scholar] [CrossRef] [PubMed]
- Roncaglioni, A.; Toropov, A.A.; Toropova, A.P.; Benfenati, E. In silico methods to predict drug toxicity. Curr. Opin. Pharmacol. 2013, 13, 802–806. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Sample of the compound DL0410 is available from the authors. |

 | ||||
| Compound No. | R1 | R2 | IC50(μM) | |
| AChE | BuChE | |||
| 1-1 |  |  | 0.096 | 1.25 |
| 1-2 |  |  | 0.84 | 2.22 |
| 1-3 |  |  | 1.32 | 5.9 |
| 1-4 |  |  | 0.82 | 2.65 |
| 1-5 |  |  | 0.41 | 1.88 |
| 1-6 |  |  | 3.32 | 4.87 |
| 1-7 |  |  | 49.05 | 36.58 |
| 1-8 |  |  | 0.75 | 0.19 |
| 1-9 |  |  | 1.18 | 0.74 |
| 1-10 |  |  | 0.88 | 2.83 |
| 1-11 |  |  | 0.71 | 2.39 |
| 1-12 |  |  | 0.33 | 1.16 |
| 1-13 |  |  | 1.43 | 1.94 |
| 1-14 |  |  | 1.61 | 13.77 |
| 1-15 |  |  | 0.97 | 0.72 |
| 1-16 |  |  | 2.15 | 1.91 |
| 1-17 |  |  | 3.33 | 6.56 |
| 1-18 |  |  | 9.52 | >200 |
| 1-19 |  |  | 0.25 | >200 |
 | ||||
| Compound No. | R1 | R2 | IC50(μM) | |
| AChE | BuChE | |||
| 2-1 |  |  | 75.54 | 20.72 |
| 2-2 |  |  | 74.13 | 66.51 |
| 2-3 |  |  | 0.50 | 3.41 |
| 2-4 |  |  | 3.67 | 19.18 |
| 2-5 |  |  | 0.19 | 1.64 |
| 2-6 |  |  | 12.24 | >200 |
| 2-7 |  |  | 3.53 | 6.38 |
| 2-8 |  |  | 62.27 | 9.11 |
| 2-9 |  |  | 2.62 | 1.21 |
| 2-10 |  |  | 3.96 | 2.77 |
 | ||||
| Compound No. | R1 | R2 | IC50(μM) | |
| AChE | BuChE | |||
| 3-1 |  |  | 4.11 | 4.2 |
| 3-2 |  |  | 7.09 | 7.65 |
| 3-3 |  |  | 48.53 | 40.00 |
| 3-4 |  |  | 7.55 | 24.35 |
| 3-5 |  |  | 3.32 | 1.72 |
 | ||||
| Compound No. | R1 | R2 | IC50(μM) | |
| AChE | BuChE | |||
| 4-1 |  |  | 4.89 | 6.00 |
| 4-2 |  |  | 54.44 | 5.95 |
| 4-3 |  |  | 3.07 | 3.09 |
| 4-4 |  |  | 17.40 | 2.93 |
| 4-5 |  |  | 15.67 | 16.32 |
 | ||||
| Compound No. | R | IC50(μM) | ||
| AChE | BuChE | |||
| 5-1 | O | 0.89 | 5.68 | |
| 5-2 | S | 3.89 | 3.44 | |
| 5-3 |  | 14.08 | >200 | |
 | ||||
| Compound No. | R | IC50(μM) | ||
| AChE | BuChE | |||
| 6-1 | O | 0.06 | 0.61 | |
| 6-2 | S | 2.03 | 1.71 | |
| Compound No. | Structure | IC50(μM) | ||
| AChE | BuChE | |||
| 7-1 |  | 31.74 | 13.78 | |
| 7-2 |  | 17.13 | 1.55 | |
| 7-3 |  | 34.09 | 0.06 | |
| 7-4 |  | 18.53 | >200 | |
| 7-5 |  | 3.35 | 0.20 | |
| 7-6 |  | 0.03 | 0.44 | |
| 7-7 |  | 1.42 | 4.79 | |
| ADMET Prediction Tool | ID | DL0410 | Compound 6-1 | Compound 7-6 |
|---|---|---|---|---|
| PreADMET | Caco-2 permeability (nm/s) | 55.75 | 49.58 | 57.90 |
| MDCK permeability (nm/s) | 0.18 | 4.88 | 0.91 | |
| ADMET module in DS | ADMET_AlogP98 | 5.17 | 5.38 | 2.03 |
| ADMET_BBB | 0.79 | 0.85 | −0.37 | |
| admetSAR | Caco-2 permeability (LogPapp, cm/s) | 1.11 | 1.10 | 0.68 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pang, X.; Fu, H.; Yang, S.; Wang, L.; Liu, A.-L.; Wu, S.; Du, G.-H. Evaluation of Novel Dual Acetyl- and Butyrylcholinesterase Inhibitors as Potential Anti-Alzheimer’s Disease Agents Using Pharmacophore, 3D-QSAR, and Molecular Docking Approaches. Molecules 2017, 22, 1254. https://doi.org/10.3390/molecules22081254
Pang X, Fu H, Yang S, Wang L, Liu A-L, Wu S, Du G-H. Evaluation of Novel Dual Acetyl- and Butyrylcholinesterase Inhibitors as Potential Anti-Alzheimer’s Disease Agents Using Pharmacophore, 3D-QSAR, and Molecular Docking Approaches. Molecules. 2017; 22(8):1254. https://doi.org/10.3390/molecules22081254
Chicago/Turabian StylePang, Xiaocong, Hui Fu, Shilun Yang, Lin Wang, Ai-Lin Liu, Song Wu, and Guan-Hua Du. 2017. "Evaluation of Novel Dual Acetyl- and Butyrylcholinesterase Inhibitors as Potential Anti-Alzheimer’s Disease Agents Using Pharmacophore, 3D-QSAR, and Molecular Docking Approaches" Molecules 22, no. 8: 1254. https://doi.org/10.3390/molecules22081254
APA StylePang, X., Fu, H., Yang, S., Wang, L., Liu, A.-L., Wu, S., & Du, G.-H. (2017). Evaluation of Novel Dual Acetyl- and Butyrylcholinesterase Inhibitors as Potential Anti-Alzheimer’s Disease Agents Using Pharmacophore, 3D-QSAR, and Molecular Docking Approaches. Molecules, 22(8), 1254. https://doi.org/10.3390/molecules22081254




