Molecular Dynamics Simulations of the Host Defense Peptide Temporin L and Its Q3K Derivative: An Atomic Level View from Aggregation in Water to Bilayer Perturbation
Abstract
:1. Introduction
2. Results
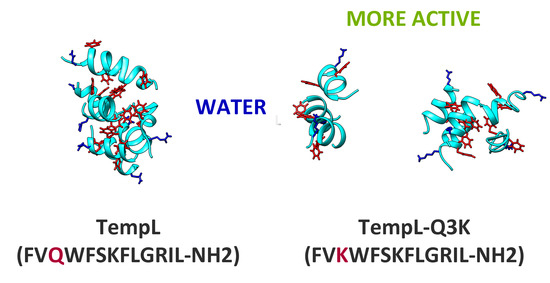
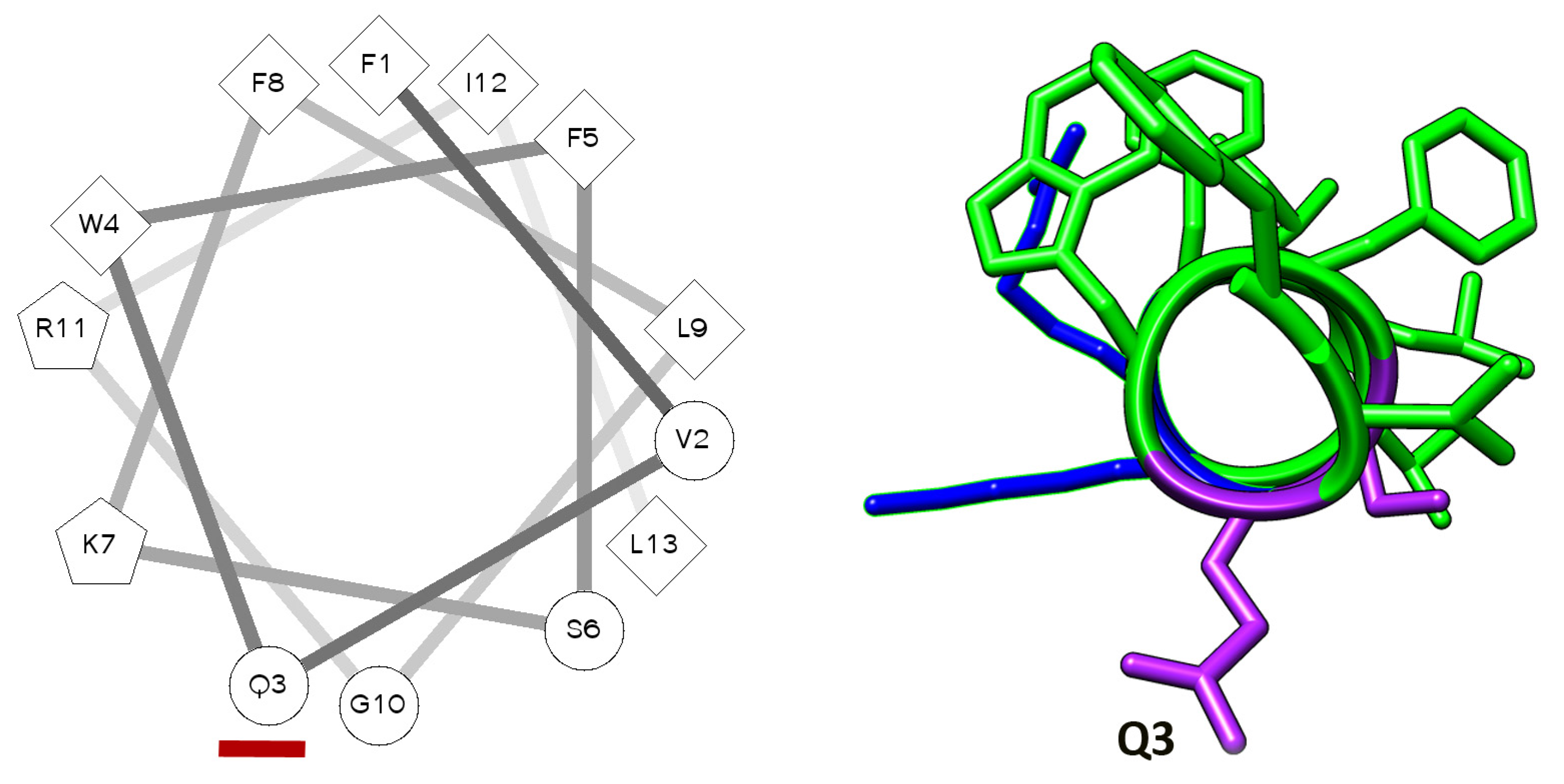
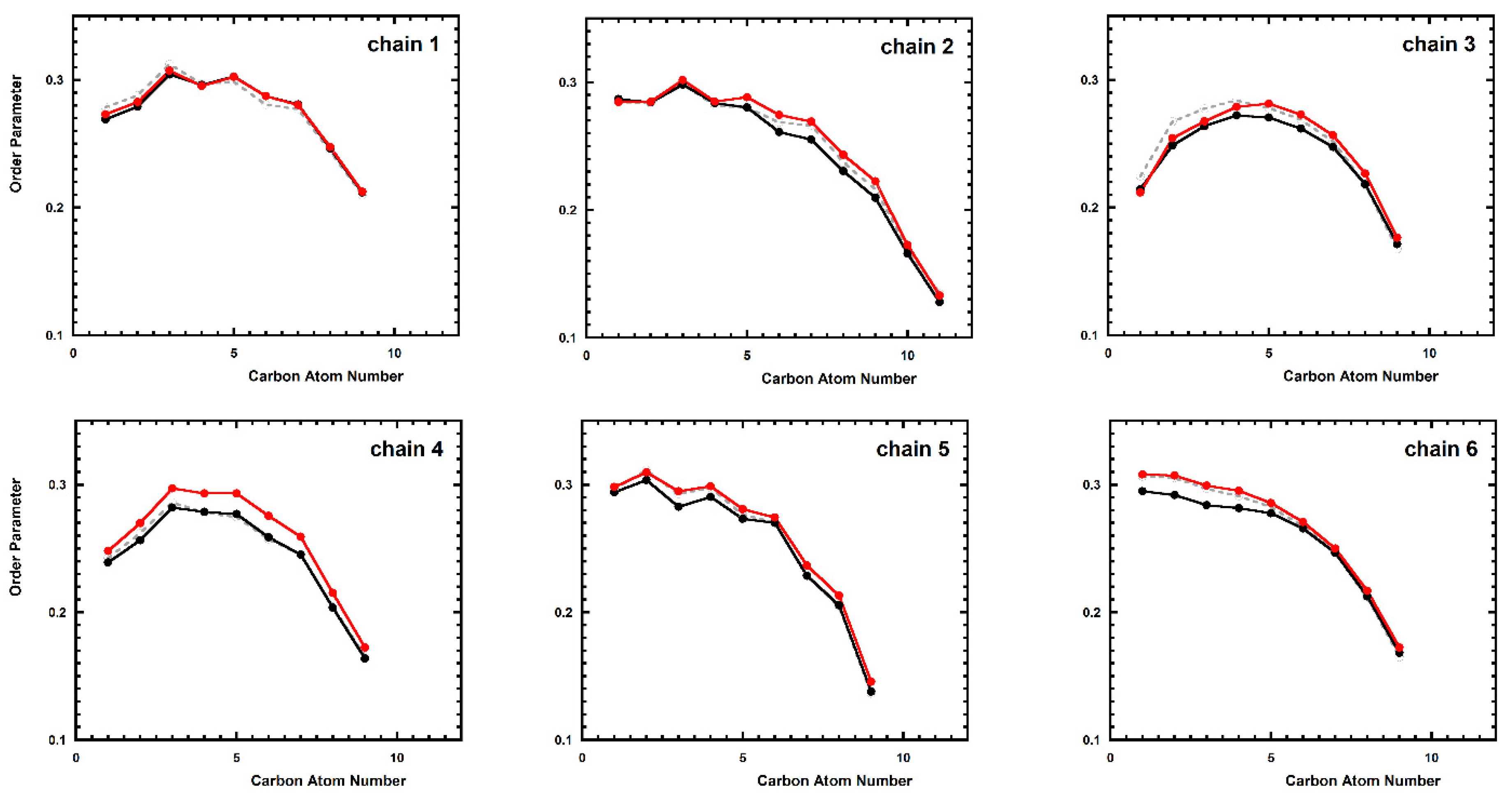
2.1. Aggregation of the Peptides in Water Solution
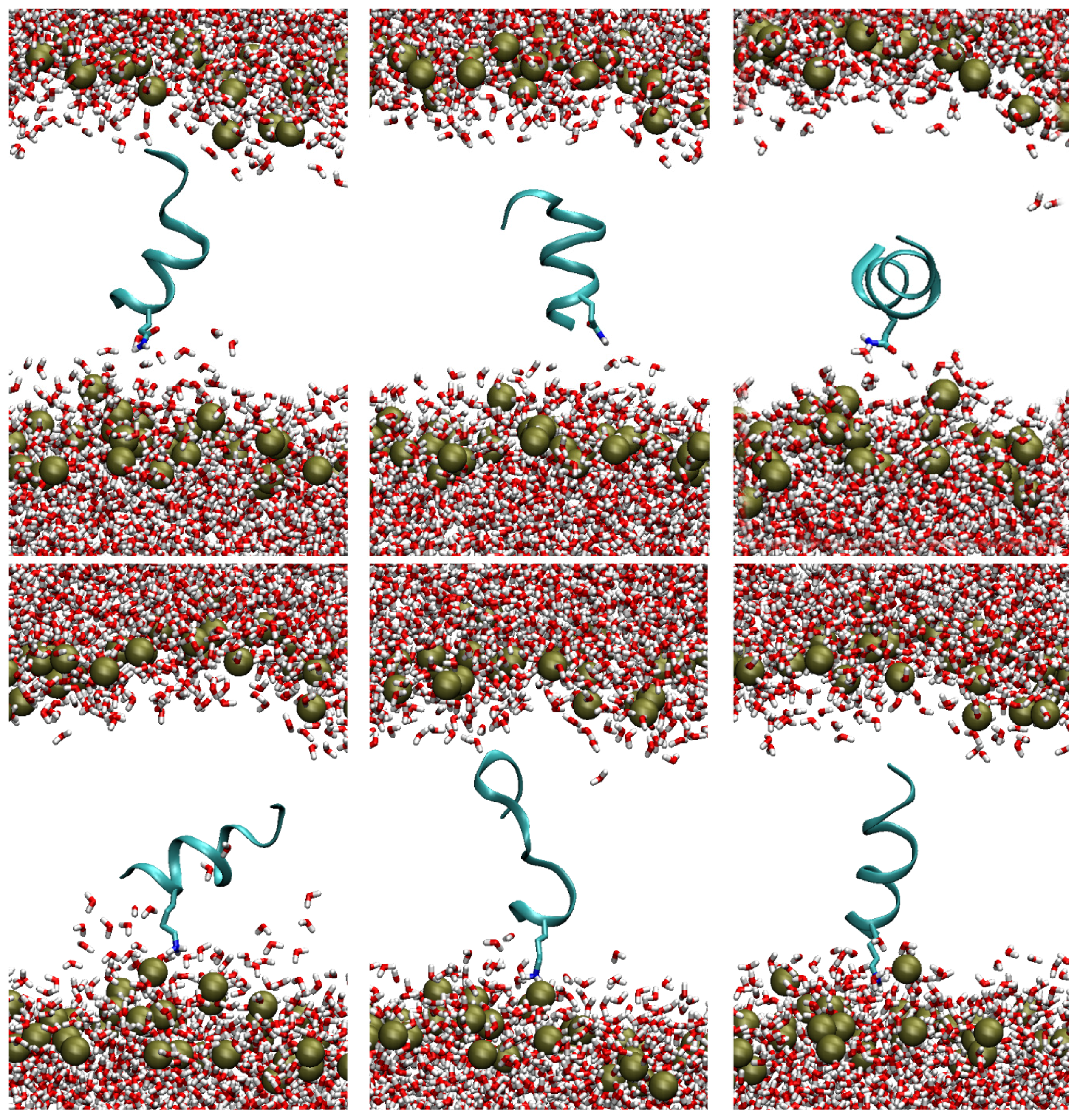
2.2. Binding Energy of TempL and Q3K-TempL to Bilayers Mimicking the Outer Membrane of Gram-negative Bacteria
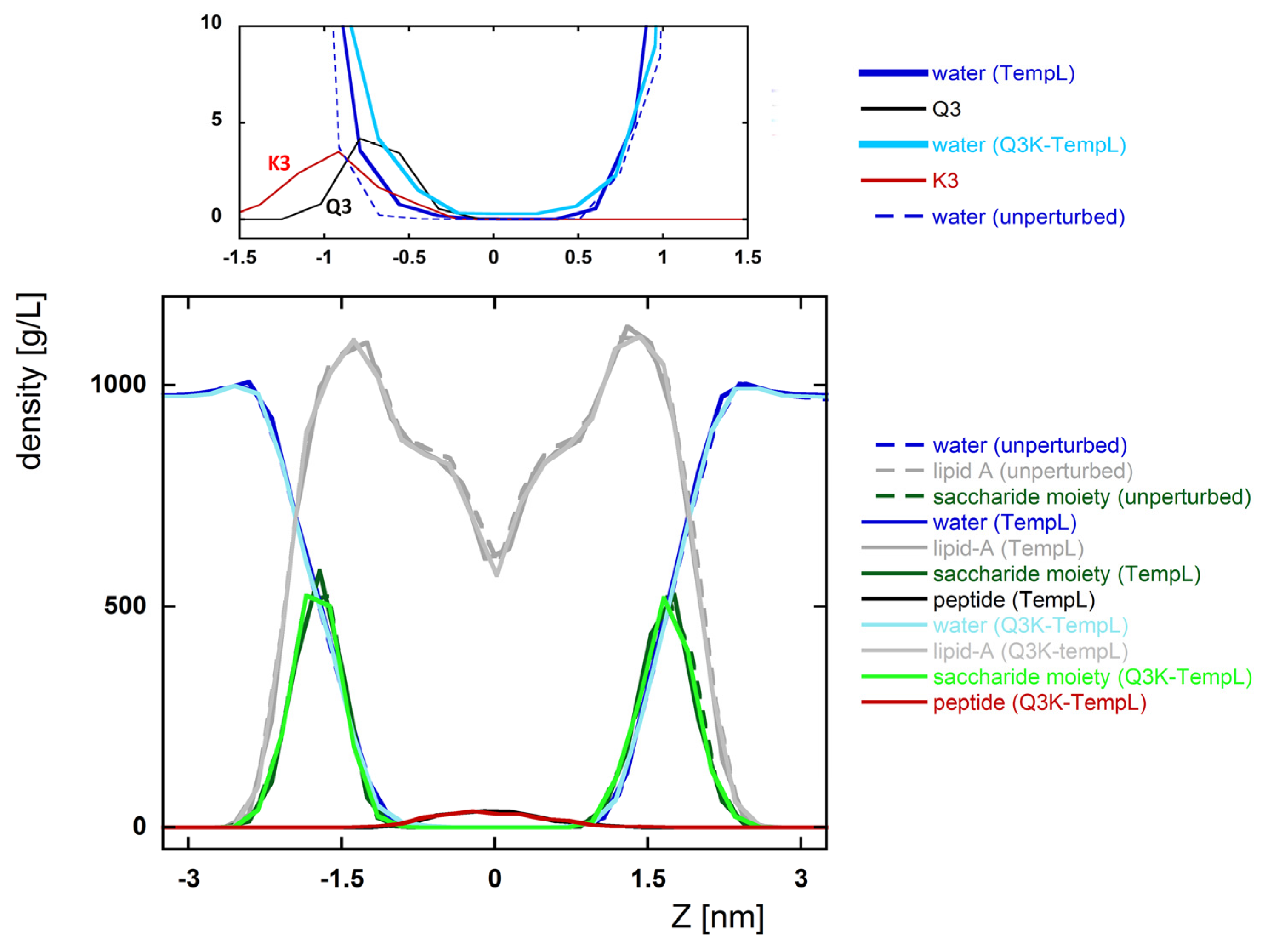
2.3. Effects of the TempL and Q3K-TempL Insertion on the lipid-A Bilayer
3. Discussion
4. Methods
4.1. Simulations in Water
4.2. PMF Calculations
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Zhang, L.; Falla, T.J. Potential therapeutic application of host defence peptides. Methods Mol. Biol. 2010, 38, 8102–8111. [Google Scholar] [CrossRef]
- De la Fuente-Núñez, C.; Reffuveille, F.; Fernández, L.; Hancock, R.E.W. Bacterial biofilm development as a multicellular adaptation: Antibiotic resistance and new therapeutic strategies. Curr. Opin. Microbiol. 2013, 16, 580–589. [Google Scholar] [CrossRef] [PubMed]
- Filipa, H.S.; Victor, C.; Craig, S.; Peter, G.B. Antiviral Host Defence Peptide in Host Defense Peptides and Their Potential as Therapeutic Agents; Epand, R.M., Ed.; Springer International Publishing: Basel, Switzerland, 2016; ISBN 978-3-319-32949-9. [Google Scholar]
- Fjell, C.D.; Hiss, J.A.; Hancock, R.E.; Schneider, G. Designing antimicrobial peptides: Form follows function. Nat. Rev. Drug. Discov. 2012, 11, 37–51. [Google Scholar] [CrossRef]
- Mahalka, A.K.; Kinnunen, P.K. Binding of amphipathic alpha-helical antimicrobial peptides to lipid membranes: Lessons from temporins B and L. BBA-Biomembrances 2009, 1788, 1600–1609. [Google Scholar] [CrossRef] [PubMed]
- Slaninová, J.; Mlsová, V.; Kroupová, H.; Alán, L.; Tumová, T.; Monincová, L.; Borovicková, L.; Fucík, V.; Cevrovsky, V. Toxicity study of antimicrobial peptides from wild bee venom and their analogs toward mammalian normal and cancer cells. Peptides 2012, 33, 18–26. [Google Scholar] [CrossRef] [PubMed]
- Schweizer, F. Cationic amphiphilic peptides with cancer-selective toxicity. Eur. J. Pharmacol. 2009, 625, 190–194. [Google Scholar] [CrossRef] [PubMed]
- Reddy, K.V.; Yedery, R.D.; Aranha, C. Antimicrobial peptides: Premises and promises. Int. J. Antimicrob. Agents 2004, 24, 536–547. [Google Scholar] [CrossRef] [PubMed]
- Hoskin, D.W.; Ramamoorthy, A. Studies on anticancer activity of antimicrobial peptides. BBA-Biomembrances 2008, 1778, 357–375. [Google Scholar] [CrossRef] [PubMed]
- Dubos, R.J. Studies on bactericidal agent extracted from a soil Bacillus: I. Preparation of the agent. Its activity in vitro. J. Exp. Med. 1939, 70, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Dubos, R.J. Studies on bactericidal agent extracted from a soil Bacillus: II. Protective effect of the vactericidal agent against experimental Pneumococcus infections in mice. J. Exp. Med. 1939, 70, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Li, X.; Wang, Z. APD3: The antimicrobial peptide database as a tool for research and education. Nucleic Acids Res. 2016, 44, D1087–D1093. [Google Scholar] [CrossRef] [PubMed]
- Wimley, W.C. Describing the mechanism of antimicrobial peptide action with the interfacial activity model. ACS Chem. Biol. 2010, 5, 905–917. [Google Scholar] [CrossRef] [PubMed]
- Brown, K.L.; Hancock, R.E.W. Cationic host defense (antimicrobial) peptides. Curr. Opin. Immunol. 2006, 18, 24–30. [Google Scholar] [CrossRef] [PubMed]
- Steiner, H.; Hultmark, D.; Engstrom, A.; Bennich, H.; Boman, H.G. Sequence and specificity of two antibacterial proteins involved in insect immunity. Nature 1981, 292, 246–248. [Google Scholar] [CrossRef] [PubMed]
- Zasloff, M. Antimicrobial peptides of multicellular organisms. Nature 2002, 415, 389–395. [Google Scholar] [CrossRef] [PubMed]
- Wimley, W.C.; Hristova, K. Antimicrobial peptides: Successes, challenges and unanswered questions. J. Membr. Biol. 2011, 239, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Bocchinfuso, G.; Bobone, S.; Mazzuca, C.; Palleschi, A.; Stella, L. Fluorescence spectroscopy and molecular dynamics simulations in studies on the mechanism of membrane destabilization by antimicrobial peptides. Cell. Mol. Life Sci. 2011, 68, 2281–2301. [Google Scholar] [CrossRef] [PubMed]
- Mueller, P. Membrane excitation through voltage-induced aggregation of channel precursors. Ann. N. Y. Acad. Sci. 1975, 264, 247–264. [Google Scholar] [CrossRef] [PubMed]
- Qian, S.; Wang, W.; Yang, L.; Huang, H.W. Structure of the alamethicin pore reconstructed by X-ray diffraction analysis. Biophys. J. 2008, 94, 3512–3522. [Google Scholar] [CrossRef] [PubMed]
- Matsuzaki, K.; Murase, O.; Fuiji, N.; Miyajima, K. An antimicrobial peptide, magainin-2, induced rapid-flip flop of phospholipids coupled with pore formation and peptide translocation. Biochemistry 1996, 35, 11361–11368. [Google Scholar] [CrossRef] [PubMed]
- Shai, Y. Mode of action of membrane active antimicrobial peptides. Biopolymers 2002, 66, 236–248. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Harroun, T.A.; Weiss, T.M.; Ding, L.; Huang, H.W. Barrel-stave or toroidal model? A case study on melittin pores. Biophys. J. 1991, 81, 1475–1485. [Google Scholar] [CrossRef]
- Sevcsik, E.; Pabst, G.; Jilek, A.; Lohner, K. How lipids influence the mode of action of membrane-active peptides. BBA-Biomembrances 2007, 1768, 2586–2595. [Google Scholar] [CrossRef] [PubMed]
- Lacapere, J.J.; Pebay-Peyroula, E.; Neumann, J.M.; Etchebest, C. Determining membrane protein structures: Still a challenge! Trends Biochem. Sci. 2007, 32, 259–270. [Google Scholar] [CrossRef] [PubMed]
- La Rocca, P.; Biggin, P.C.; Tieleman, D.P.; Sansom, M.S.P. Simulation studies of the interaction of antimicrobial peptides and lipid bilayers. BBA-Biomembrances 1999, 1462, 185–200. [Google Scholar] [CrossRef]
- Sansom, M.S.; Shrivastava, I.H.; Bright, J.N.; Tate, J.; Capener, C.E.; Biggin, P.C. Potassium channels: Structures, models, simulations. BBA-Biomembrances 2002, 1565, 294–307. [Google Scholar] [CrossRef]
- Nielsen, S.O.; Lopez, C.F.; Srinivas, G.; Klein, M.L. Coarse grain models and the computer simulation of soft materials. J. Phys. Condens. Matter 2004, 16, R481–R512. [Google Scholar] [CrossRef]
- Matyus, E.; Kandt, C.; Tieleman, D.P. Computer simulation of antimicrobial peptides. Curr. Med. Chem. 2007, 14, 2789–2798. [Google Scholar] [CrossRef] [PubMed]
- Sapay, N.; Tieleman, D.P. Molecular dynamics simulation of lipid–protein interactions. Curr. Top. Membr. 2008, 60, 111–130. [Google Scholar] [CrossRef]
- Bennuna, S.V.; Hoopesb, M.I.; Xingc, C.; Faller, R. Coarse grained modeling of lipids. Chem. Phys. Lipids 2009, 159, 59–66. [Google Scholar] [CrossRef] [PubMed]
- Marrink, S.J.; de Vries, A.H.; Tieleman, D.P. Lipids on the move: Simulations of membrane pores, domains, stalks and curves. BBA-Biomembrances 2009, 1788, 149–168. [Google Scholar] [CrossRef] [PubMed]
- Gurtovenko, A.A.; Anwar, J.; Vattulainen, I. Defect-mediated trafficking across cell membranes: Insights from in silico modeling. Chem. Rev. 2010, 110, 6077–6103. [Google Scholar] [CrossRef] [PubMed]
- Venanzi, M.; Gatto, E.; Bocchinfuso, G.; Palleschi, A.; Stella, L.; Formaggio, F.; Toniolo, C. Dynamics of formation of a helix-turn-helix structure in a membrane active peptide: A time-resolved spectroscopic study. Eur. J. Chem. Biol. 2006, 7, 43–45. [Google Scholar] [CrossRef] [PubMed]
- Venanzi, M.; Gatto, E.; Bocchinfuso, G.; Palleschi, A.; Stella, L.; Baldini, C.; Formaggio, F.; Toniolo, C. Peptide folding dynamics: A time-resolved study from the nanosecond to the microsecond time regime. J. Phys. Chem. B 2006, 110, 22834–22841. [Google Scholar] [CrossRef] [PubMed]
- Venanzi, M.; Bocchinfuso, G.; Gatto, E.; Palleschi, A.; Stella, L.; Formaggio, F.; Toniolo, C. Metal binding properties of fluorescent analogues of Trichogin GA IV: A conformational study by time-resolved spectroscopy and molecular mechanics investigations. Eur. J. Chem. Biol. 2009, 10, 91–97. [Google Scholar] [CrossRef] [PubMed]
- Caruso, M.; Placidi, E.; Gatto, E.; Mazzuca, C.; Stella, L.; Bocchinfuso, G.; Palleschi, A.; Formaggio, F.; Toniolo, C.; Venanzi, M. Fibrils or globules? Tuning the morphology of peptide aggregates from helical building blocks. J. Phys. Chem. B 2013, 117, 5448–5459. [Google Scholar] [CrossRef] [PubMed]
- Gatto, E.; Bocchinfuso, G.; Palleschi, A.; Stella, L.; Oncea, S.; De Zotti, M.; Formaggio, F.; Toniolo, C.; Venanzi, M. 3D-structure, dynamics, and activity of synthetic analogues of the peptaibiotic Trichodecenin I. Chem. Biodivers. 2013, 13, 887–903. [Google Scholar] [CrossRef] [PubMed]
- Bocchinfuso, G.; Conflitti, P.; Raniolo, S.; Caruso, M.; Mazzuca, C.; Gatto, E.; Placidi, E.; Formaggio, F.; Toniolo, C.; Venanzi, M.; et al. Aggregation propensity of Aib homo-peptides of different length: An insight from molecular dynamics simulations. J. Pept. Sci. 2014, 20, 494–507. [Google Scholar] [CrossRef] [PubMed]
- Bobone, S.; Bocchinfuso, G.; Park, Y.; Palleschi, A.; Hahm, K.S.; Stella, L. The importance of being kinked: Role of Pro residues in the selectivity of the helical antimicrobial peptide P5. J. Pept. Sci. 2013, 19, 758–769. [Google Scholar] [CrossRef] [PubMed]
- Farrotti, A.; Bocchinfuso, G.; Palleschi, A.; Rosato, N.; Salnikov, E.S.; Voievoda, N.; Bechinger, B.; Stella, L. Molecular dynamics methods to predict peptide locations in membranes: LAH4 as a stringent test case. BBA-Biomembrances 2015, 1848, 581–592. [Google Scholar] [CrossRef] [PubMed]
- Bobone, S.; Gerelli, Y.; De Zotti, M.; Bocchinfuso, G.; Farrotti, A.; Orioni, B.; Sebastiani, F.; Latter, E.; Penfold, J.; Senesi, R.; et al. Membrane thickness and the mechanism of action of the short peptaibol trichogin GA IV. BBA-Biomembrances 2013, 1828, 1013–1024. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bocchinfuso, G.; Palleschi, A.; Orioni, B.; Grande, G.; Formaggio, F.; Toniolo, C.; Park, Y.; Hahm, K.S.; Stella, L. Different mechanisms of action of antimicrobial peptides: Insights from fluorescence spectroscopy experiments and molecular dynamics simulations. J. Pept. Sci. 2009, 15, 550–558. [Google Scholar] [CrossRef] [PubMed]
- Orioni, B.; Bocchinfuso, G.; Kim, J.Y.; Palleschi, A.; Grande, G.; Bobone, S.; Venanzi, M.; Park, Y.; Kim, J.I.; Hahm, K.; et al. Membrane perturbation by the antimicrobial peptide PMAP-23: A fluorescence and molecular dynamics study. BBA-Biomembrances 2009, 1788, 1523–1533. [Google Scholar] [CrossRef] [PubMed]
- Mangoni, M.L.; Papo, N.; Barra, D.; Simmaco, M.; Bozzi, A.; Di Giulio, A.; Rinaldi, A.C. Effects of the antimicrobial peptide temporin L on cell morphology, membrane permeability and viability of Escherichia coli. Biochem. J. 2004, 380, 859–865. [Google Scholar] [CrossRef] [PubMed]
- Simmaco, M.; Mignogna, G.; Canofeni, S.; Miele, R.; Mangoni, M.; Barra, D. Temporins, antimicrobial peptides from the European Red Frog Rana temporaria. Eur. J. Biochem. 1996, 242, 788–792. [Google Scholar] [CrossRef] [PubMed]
- Grieco, P.; Carotenuto, A.; Auriemma, L.; Saviello, M.R.; Campiglia, P.; GomezMonterrey, I.M.; Marcellini, L.; Luca, V.; Barra, D.; Novellino, E.; et al. The effect of d-amino acid substitution on the selectivity of temporin L towards target cells: Identification of a potent anti-Candida peptide. BBA-Biomembrances 2013, 1828, 652–660. [Google Scholar] [CrossRef] [PubMed]
- Rosenfeld, Y.; Barra, D.; Simmaco, M.; Shai, Y.; Mangoni, M.L. A synergism between temporins toward Gram-negative bacteria overcomes resistance imposed by the lipopolysaccharide protective layer. J. Biol. Chem. 2006, 281, 28565–28574. [Google Scholar] [CrossRef] [PubMed]
- Mangoni, M.L.; Di Grazia, A.; Cappiello, F.; Casciaro, B.; Luca, V. Naturally occurring peptides from Rana temporaria: Antimicrobial properties and more. Curr. Top. Med. Chem. 2016, 16, 54–64. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, S.; Ghosh, J.K. Introduction of a lysine residue promotes aggregation of temporin L in lipopolysaccharides and augmentation of its antiendotoxin property. Antimicrob. Agents Chemother. 2013, 57, 2457–2466. [Google Scholar] [CrossRef] [PubMed]
- Carotenuto, A.; Malfi, S.; Saviello, M.R.; Campiglia, R.; Gomez-Monterrey, I.; Mangoni, M.L.; Marcellini, L.; Gaddi, H.; Novellino, E.; Grieco, P. A different molecular mechanism underlying antimicrobial and hemolytic actions of Temporins A and L. J. Med. Chem. 2008, 51, 2354–2362. [Google Scholar] [CrossRef] [PubMed]
- Mangoni, M.L.; Carotenuto, A.; Auriemma, L.; Saviello, M.R.; Campiglia, P.; Gomez-Monterrey, I.; Malfi, S.; Marcellini, L.; Barra, D.; Novellino, E.; et al. Structure-activity relationship, conformational and biological studies of Temporin L analogues. J. Med. Chem. 2011, 54, 1298–1307. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, S.; Kumar, A.; Tripathi, A.K.; Tandon, A.; Ghosh, J.K. Modulation of anti-endotoxin property of Temporin L by minor amino-acidsubstitution in identified phenylalanine zipper sequence. Biochem. J. 2016, 473, 4045–4062. [Google Scholar] [CrossRef] [PubMed]
- Bhunia, A.; Mohanram, H.; Domadia, P.; Torres, J.; Bhattacharjya, S. Designed β-boomerang antiendotoxic and antimicrobial peptides. J. Biol. Chem. 2009, 284, 21991–22004. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, R.; Srivastava, S.; Singh, M.; Bajpai, V.; Ghosh, J. Consequences of alteration in leucine zipper sequence of Melittin in its neutralization of lipopolysaccharide-induced proinflammatory response in Macrophage cells and interaction with lipopolysaccharide. J. Biol. Chem. 2012, 287, 1980–1995. [Google Scholar] [CrossRef] [PubMed]
- Bocchinfuso, G.; Mazzuca, C.; Conflitti, P.; Cori, D.; Coviello, T.; Palleschi, A. Relative stability of the Scleroglucan triple-helix and single strand: An insight from computational and experimental Techniques. Z. Phys. Chem. 2016, 230, 1395–1410. [Google Scholar] [CrossRef]
- Giacometti, A.; Cirioni, O.; Ghiselli, R.; Mocchegiani, F.; Orlando, F.; Silvestri, C.; Bozzi, A.; Di Giulio, A.; Luzi, C.; Mangoni, M.L.; et al. Interaction of antimicrobial peptide Temporin L with lipopolysaccharide in vitro and in experimental rat models of septic shock caused by Gram-negative bacteria. Antimicrob. Agents Chemother. 2006, 50, 2478–2486. [Google Scholar] [CrossRef] [PubMed]
- Bhunia, A.; Saravanan, R.; Mohanram, H.; Mangoni, M.L.; Bhattacharjya, S. NMR structures and interactions of Temporin-1Tl and Temporin-1Tb with lipopolysaccharide micelles. J. Biol. Chem. 2011, 286, 24394–24406. [Google Scholar] [CrossRef] [PubMed]
- Stella, L.; Mazzuca, C.; Venanzi, M.; Palleschi, A.; Didonè, M.; Formaggio, F.; Toniolo, C.; Pispisa, B. Aggregation and water-membrane partition as major determinants of the activity of the antibiotic peptide Trichogin GA IV. Biophys. J. 2004, 86, 936–945. [Google Scholar] [CrossRef]
- Mazzuca, C.; Stella, L.; Venanzi, M.; Formaggio, F.; Toniolo, C.; Pispisa, B. Mechanism of membrane activity of the antibiotic Trichogin GA IV: A two-state transition controlled by peptide concentration. Biophys. J. 2005, 88, 3411–3421. [Google Scholar] [CrossRef] [PubMed]
- Gatto, E.; Mazzuca, C.; Stella, L.; Venanzi, M.; Toniolo, C.; Pispisa, B. Effect of peptide lipidation on membrane perturbing activity: A comparative study on two Trichogin analogues. J. Phys. Chem. B 2006, 110, 22813–22818. [Google Scholar] [CrossRef] [PubMed]
- Roversi, D.; Luca, V.; Aureli, S.; Park, Y.; Mangoni, M.L.; Stella, L. How many antimicrobial peptide molecules kill a bacterium? The case of PMAP-23. ACS Chem. Biol. 2014, 9, 2003–2007. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Savini, F.; Luca, V.; Bocedi, A.; Massoud, R.; Park, Y.; Mangoni, M.L.; Stella, L. Cell-density dependence of host-defense peptide activity and selectivity in the presence of host cells. ACS Chem. Biol. 2017, 12, 52–56. [Google Scholar] [CrossRef] [PubMed]
- Daidone, I.; D’Abramo, M.; Di Nola, A.; Amadei, A. Theoretical characterization of r-Helix and â-Hairpin folding kinetics. J. Am. Chem. Soc. 2005, 127, 14825–14832. [Google Scholar] [CrossRef] [PubMed]
- Sal-Man, N.; Oren, Z.; Shai, Y. Preassembly of membrane-active peptides is an important factor in their selectivity toward target cells. Biochemistry 2002, 41, 11921–11930. [Google Scholar] [CrossRef] [PubMed]
- Papo, N.; Shai, Y. A molecular mechanism for lipopolysaccharide protection of Gram-negative bacteria from antimicrobial peptides. J. Biol. Chem. 2005, 280, 10378–10387. [Google Scholar] [CrossRef] [PubMed]
- Pronk, S.; Pall, S.; Schulz, R.; Larsson, P.; Bjelkmar, P.; Apostolov, R.; Shirts, M.R. GROMACS 4.5: A high-throughput and highly parallel open source molecular simulation toolkit. Bioinformatics 2013, 29, 845–854. [Google Scholar] [CrossRef] [PubMed]
- Oostenbrinck, C.; Soares, T.A.; van der Veght, N.F.; van Gusteren, W.F. Validation of the 53A6 GROMOS force field. Eur. Biophys. J. 2005, 34, 273–284. [Google Scholar] [CrossRef] [PubMed]
- Berendsen, H.J.C.; Postma, J.M.; van Gunsteren, W.F.; Hermans, J. Interaction models for water in relation to protein hydration. In Intermolecular Forces; Reidel Publishing Company: Dordrecht, The Netherlands, 1981; pp. 331–342. [Google Scholar]
- Berendsen, H.J.C.; Postma, J.M.; van Gunsteren, W.F.; Di Nola, A.; Haak, J.R. Molecular dynamics with coupling to an external bath. J. Chem. Phys. 1984, 81, 3684–3690. [Google Scholar] [CrossRef]
- Hess, B.; Bekker, H.; Berendsen, H.J.C.; Fraaije, J.G.E.M. LINCS: A linear constraint solver for molecular simulations. J. Comput. Chem. 1997, 18, 1463–1472. [Google Scholar] [CrossRef]
- Essmann, U.; Perera, L.; Berkowitz, M.L.; Darden, T.; Lee, H.; Pedersen, L.G. A smooth particle mesh Ewald method. J. Chem. Phys. 1995, 103, 8577–8592. [Google Scholar] [CrossRef]
- Kabsch, W.; Sander, C. Dictionary of protein secondary structure. Pattern recognition of hydrogen-bonded and geometrical features. Biopolymers 1983, 22, 2577–2637. [Google Scholar] [CrossRef] [PubMed]
- Pettersen, E.F.; Goddard, T.D.; Huang, C.C.; Couch, G.S.; Greenblatt, D.M.; Meng, E.C.; Ferrin, T.E. UCSF Chimera—A visualization system for exploratory research and analysis. J. Comput. Chem. 2004, 25, 1605–1612. [Google Scholar] [CrossRef] [PubMed]
- Torrie, G.; Valleau, J. Nonphysical sampling distributions in Monte Carlo free-energy estimation: Umbrella sampling. J. Comput. Phys. 1997, 23, 187–199. [Google Scholar] [CrossRef]
- Kumar, S.; Rosenberg, J.M.; Bouzida, D.; Swendsen, R.H.; Kollman, P.A. The weighted histogram analysis method for free-energy calculations on biomolecules. I. The method. J. Comput. Chem. 1992, 13, 1011–1021. [Google Scholar] [CrossRef]
- Piggot, T.; Holdbrook, D.; Khalid, S. Electroporation of the E. coli and S. Aureus membranes: Molecular dynamics simulations of complex bacterial membranes. J. Phys. Chem. B 2011, 115, 13381–13388. [Google Scholar] [CrossRef] [PubMed]
- Humphrey, W.; Dalke, A.; Schulten, K. VMD: Visual molecular dynamics. J. Mol. Graph. 1996, 14, 33–38. [Google Scholar] [CrossRef]
Sample Availability: The files used to simulate the investigated peptides with the GROMACS software package are available from the authors. |


| Peptide (Sequence) | Net Charge (e) | Hydrophobicity (min) 2 | Aggregation in Water 3 | Antibacterial Activity (μM) 4 | LPS Permeabilization (%) 5 | Anti Endotoxin Activity (mg/kg) 6 |
|---|---|---|---|---|---|---|
| TempL (FVQWFSKFLGRIL-NH2) | +3 | 21 | 17 | 10 | 24 | 1.0 |
| Q3K-TempL (FVKWFSKFLGRIL-NH2) | +4 | 19 | 6.5 | 5 | 100 | 0.25 |
| Peptide | Solvent Accessible Surface (SAS) | Average COM Distance (nm) | RMSF (nm) | Helicity (%) | ||
|---|---|---|---|---|---|---|
| Peptide 1 (nm2) | Residue 3 2 (%) | Residue 5,8 2 (%) | ||||
| TempL | 79 ± 3 | 65 ± 4 | 24 ± 7 | 1.89 ± 0.04 | 0.26 ± 0.03 | 50 ± 7 |
| F5,8L-TempL | 79 ± 4 | --- | 25 ± 6 | 1.9 ± 0.1 | 0.22 ± 0.06 | 50 ± 7 |
| F5,8A-TempL | 88 ± 2 | --- | 29 ± 9 | 2.5 ± 0.1 | 0.35 ± 0.07 | 38 ± 4 |
| Q3K-TempL | 86 ± 6 | 86 ± 6 | --- | 2.4 ± 0.4 | 0.30 ± 0.09 | 51 ± 6 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Farrotti, A.; Conflitti, P.; Srivastava, S.; Ghosh, J.K.; Palleschi, A.; Stella, L.; Bocchinfuso, G. Molecular Dynamics Simulations of the Host Defense Peptide Temporin L and Its Q3K Derivative: An Atomic Level View from Aggregation in Water to Bilayer Perturbation. Molecules 2017, 22, 1235. https://doi.org/10.3390/molecules22071235
Farrotti A, Conflitti P, Srivastava S, Ghosh JK, Palleschi A, Stella L, Bocchinfuso G. Molecular Dynamics Simulations of the Host Defense Peptide Temporin L and Its Q3K Derivative: An Atomic Level View from Aggregation in Water to Bilayer Perturbation. Molecules. 2017; 22(7):1235. https://doi.org/10.3390/molecules22071235
Chicago/Turabian StyleFarrotti, Andrea, Paolo Conflitti, Saurabh Srivastava, Jimut Kanti Ghosh, Antonio Palleschi, Lorenzo Stella, and Gianfranco Bocchinfuso. 2017. "Molecular Dynamics Simulations of the Host Defense Peptide Temporin L and Its Q3K Derivative: An Atomic Level View from Aggregation in Water to Bilayer Perturbation" Molecules 22, no. 7: 1235. https://doi.org/10.3390/molecules22071235