Synthesis, Antitumor Evaluation and Molecular Docking of New Morpholine Based Heterocycles
Abstract
:1. Introduction
2. Results and Discussion
2.1. Chemistry
2.2. Cytotoxic Activity
Examination of the SAR Leads to the Following Conclusions
- The results revealed that all the tested compounds showed inhibitory activity to the tumor cell lines in a concentration dependent manner.
- The activities of the synthesized compounds depend on the structural skeleton and electronic environment of the molecules.
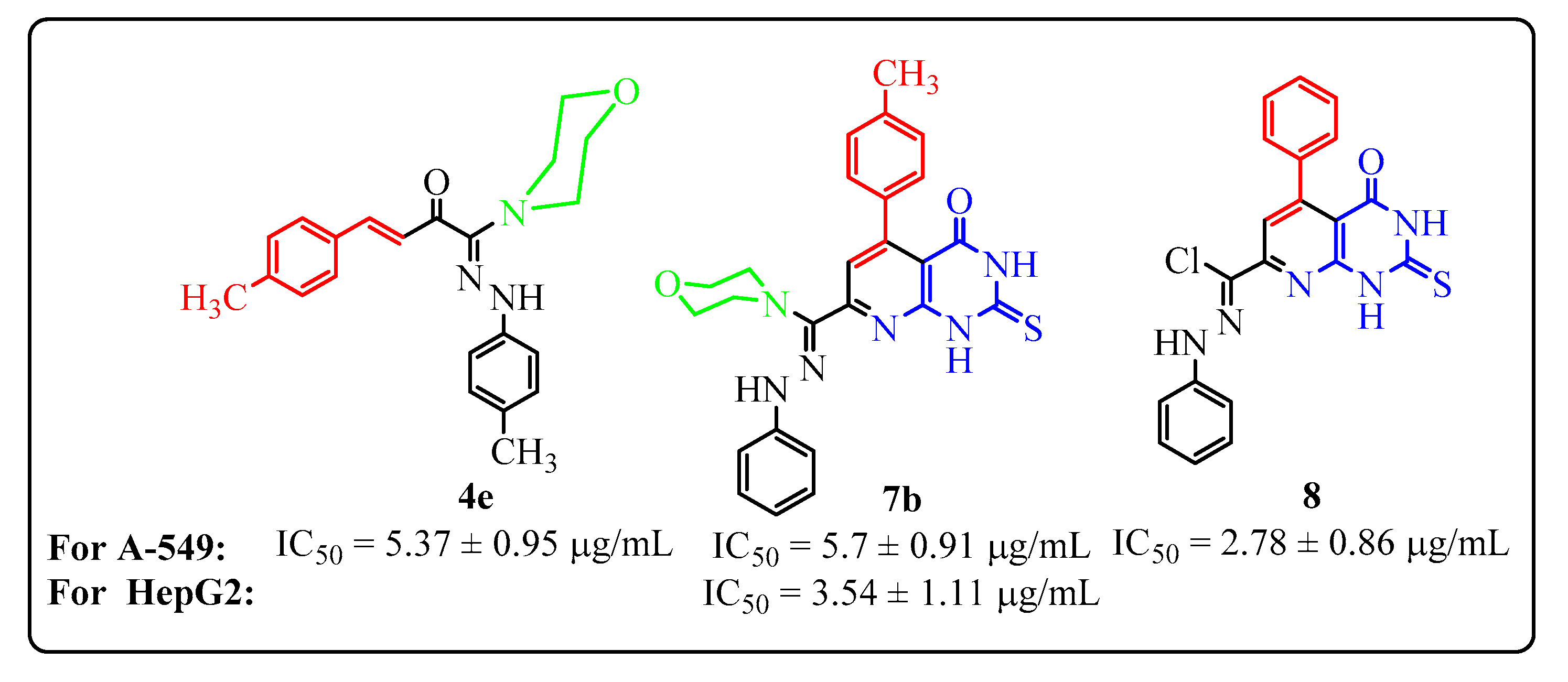
- Compounds 8, 4e and 7b were the most active (IC50 values of 2.78 ± 0.86, 5.37 ± 0.95 and 5.70 ± 0.91 μg/mL, respectively) against the lung carcinoma cell line (A-549), compared with cisplatin reference drug with IC50 value of 0.95 ± 0.90 μg/mL (Figure 1), while the remaining compounds have moderate inhibitory activity (IC50 = 6.79 ± 1.11 − 26.8 ± 0.75 µg/mL).
- Compounds 7b, 7c, 10d and 4f were the most active (IC50 value of 3.54 ± 1.11, 8.42 ± 1.15, 8.72 ± 0.89 and 9.78 ± 0.78 μg/mL, respectively) against the human hepatocellular carcinoma cell line (HepG-2), compared with the reference drug cisplatin with an IC50 value of 1.40 ± 1.1μg/mL (Figure 1). The other compounds have moderate inhibitory activity (IC50 = 12.4 ± 0.98 − 29.9 ± 0.93 µg/mL).
- Among the morpholinylchalcone derivatives, the dimethylchalcone 4e is the most active one against the A549 (IC50 = 5.37 ± 0.95 μg/mL) line, while the methylchlorochalcone 4f is the most active one against the HepG-2 cell line (A549) (IC50 = 9.78 ± 0.78 μg/mL).
- For pyrido[2,3-d][1,2,4]triazolo[4,3-a]pyrimidin-5(1H)-ones 10a–d: Compounds 10c and 10d (substituted with COOEt group at position 3) have more in vitro inhibitory activity than compounds 10a and 10b (substituted with a COCH3 group at position 3). Also compound 10d is more active than 10c where the p-substitution with a methyl group increases the activity via its +I effect.
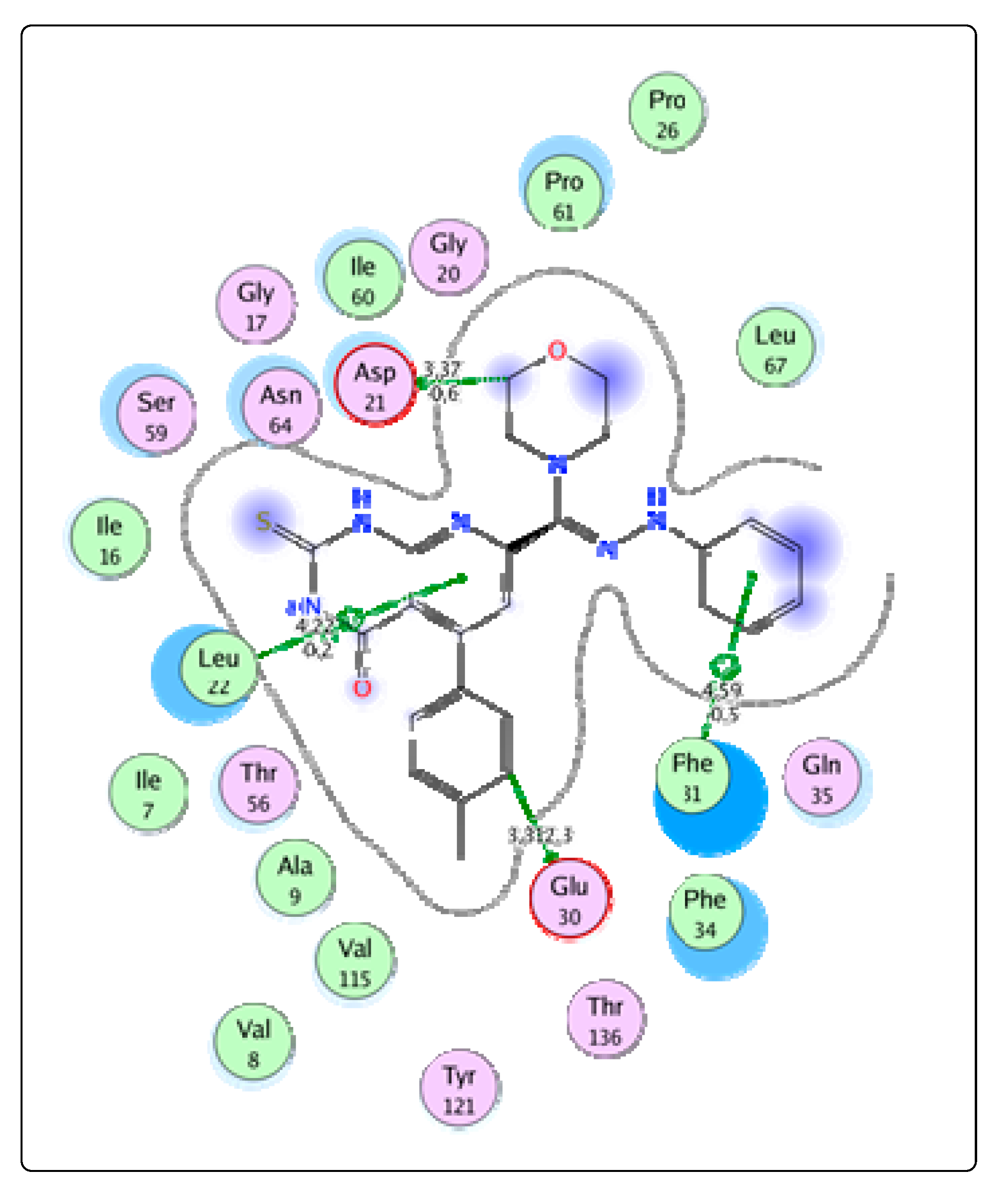
2.3. Molecular Docking
Bioactivity and ADME Toxicity
3. Experimental
3.1. General Information
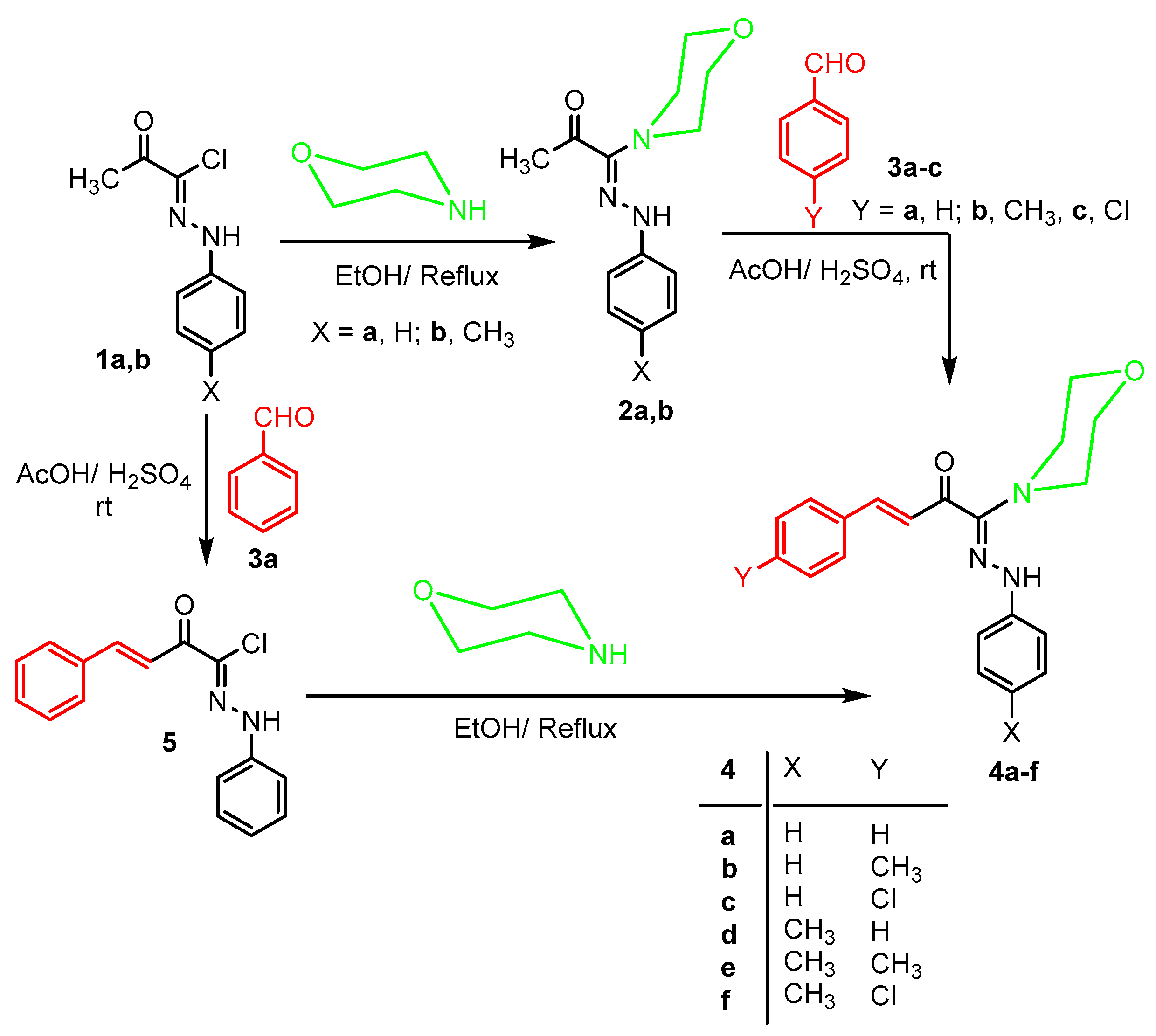
3.1.1. Synthesis of Chalcones 4a–f
3.1.2. Synthesis of 2-Oxo-N,4-diphenylbut-3-enehydrazonoyl Chloride (5)
3.1.3. Alternative Synthesis of 4a
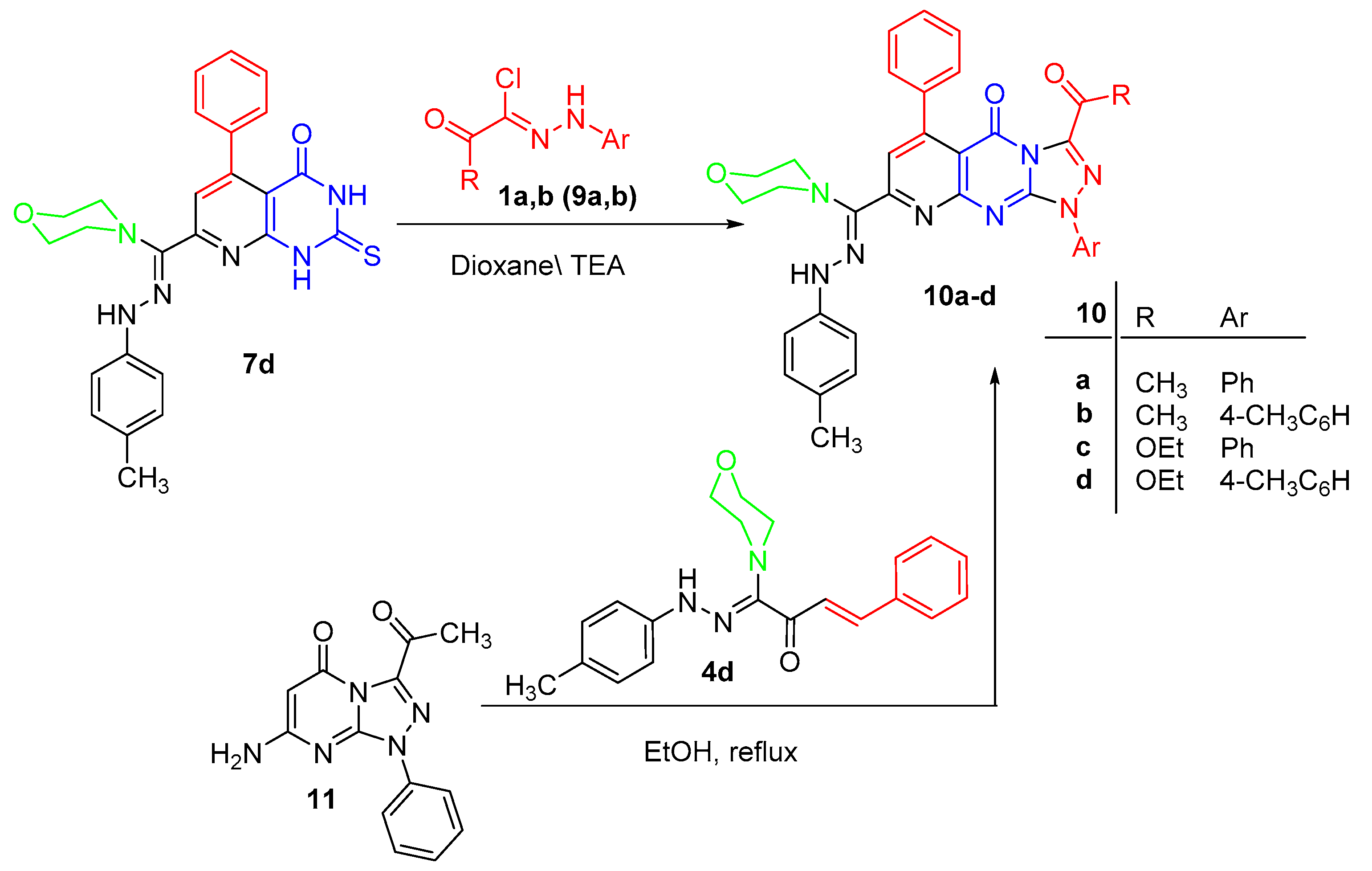
3.1.4. Synthesis of Thiones 7a–e
3.1.5. Alternative Synthesis of 7a
3.1.6. Synthesis of Pyrido[2,3-d][1,2,4]triazolo[4,3-a]pyrimidin-5(1H)-ones 10a–d
3.1.7. Alternate Synthesis of 10a
3.2. Anticancer Activity
3.3. Molecular Modeling
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Abbas, I.M.; Gomha, S.M.; Elaasser, M.M.; Bauomi, M.A. Synthesis and biological evaluation of new pyridines containing imidazole moiety as antimicrobial and anticancer agents. Turk. J. Chem. 2015, 39, 334–346. [Google Scholar] [CrossRef]
- Abdallah, M.A.; Riyadh, S.M.; Abbas, I.M.; Gomha, S.M. Synthesis and biological activities of 7-arylazo-7H-pyrazolo[5,1-c][1,2,4]triazolo-6(5H)-ones and 7-arylhydrazono-7H-[1,2,4]triazolo[3,4-b][1,3,4] thiadiazines. J. Chin. Chem. Soc. 2005, 52, 987–994. [Google Scholar] [CrossRef]
- Gomha, S.M.; Abdel-Aziz, H.M.; Khalil, K.D. Synthesis and SAR study of the novel thiadiazole-imidazole derivatives as new anti-cancer agents. Chem. Pharm. Bull. 2016, 64, 1356–1363. [Google Scholar] [CrossRef] [PubMed]
- Gomha, S.M.; Abdel-Aziz, H.A. Synthesis of new heterocycles derived from 3-(3-methyl-1H-indol-2-yl)-3-oxopropanenitrile as potent antifungal agents. Bull. Korean Chem. Soc. 2012, 33, 2985–2990. [Google Scholar] [CrossRef]
- Gomha, S.M.; Abdel-Aziz, H.A. Synthesis of new functionalized derivatives of indolo[2,3-e][1,2,4]-triazolo-[4,5-b]-1,2,4-triazine. J. Serb. Chem. Soc. 2013, 78, 1119–1125. [Google Scholar] [CrossRef]
- Gomha, S.M. A facile one-pot synthesis of 6,7,8,9-tetrahydrobenzo[4,5]thieno[2,3-d]-1,2,4-triazolo[4,5-a]pyrimidin-5-ones. Monatsh. Chem. 2009, 140, 213–220. [Google Scholar] [CrossRef]
- Gomha, S.M.; Riyadh, S.M. Synthesis of triazolo[4,3-b][1,2,4,5]tetrazines and triazolo[3,4-b][1,3,4]thiadiazines using chitosan as ecofriendly catalyst under microwave irradiation. Arkivoc 2009, 11, 58–68. [Google Scholar]
- Abbas, I.M.; Riyadh, S.M.; Abdallah, M.A.; Gomha, S.M. A novel route to tetracyclic fused tetrazines and thiadiazines. J. Heterocycl. Chem. 2006, 43, 935–942. [Google Scholar] [CrossRef]
- Gomha, S.M.; Badrey, G.; Abdalla, M.M.; Arafa, R.K. Novel Anti-HIV-1 NNRTIs Based on a Pyrazolo[4,3-d]isoxazole Backbone Scaffold: Design, synthesis and exploration of molecular basis of action. MedChemComm 2014, 5, 1685–1692. [Google Scholar] [CrossRef]
- Gomha, S.M.; Abdelrazek, F.M.; Abdulla, M.M. Synthesis of new functionalised derivatives of [1,2,4]triazolo[4,3-a]pyrimidine and pyrimido[2,1-b][1,3,5]thiadiazine as aromatase inhibitors. J. Chem. Res. 2015, 39, 425–429. [Google Scholar]
- Plech, T.; Wujec, M.; Siwek, A.; Osikowska, U.; Malm, A. Synthesis and antimicrobial activity of thiosemicarbazides, s-triazoles and their Mannich bases bearing 3-chlorophenyl moiety. Eur. J. Med. Chem. 2011, 46, 241–248. [Google Scholar] [CrossRef] [PubMed]
- Bayrak, H.; Demirbas, H.; Karaoglu, S.A.; Demirbas, N. Synthesis of some new 1,2,4-triazoles, their Mannich and Schiff bases and evaluation of their antimicrobial activities. Eur. J. Med. Chem. 2009, 44, 1057–1066. [Google Scholar] [CrossRef] [PubMed]
- Alberto, I.; Juan, R.; Marcela, R.; Carlos, E.; Jairo, Q.; Rodrigo, A.; Manuel, N.; Justo, C.; María, V.R.; Susana, A.Z.; et al. Synthesis, antifungal and antitumor activity of novel (Z)-5-hetarylmethylidene-1,3-thiazol-4-ones and (Z)-5-ethylidene-1,3-thiazol-4-ones. Molecules 2013, 18, 5482–5497. [Google Scholar]
- Brown, G.R.; Foubister, A.J.; Forster, G.; Stribling, D. (S)-3-[(Benzyloxy)methyl]morpholine Hydrochloride: A Nonstimulant Appetite Suppressant without Conventional Neurotransmitter Releasing Properties. J. Med. Chem. 1986, 29, 1288–1290. [Google Scholar] [CrossRef] [PubMed]
- Ihmaid, S.; Al-Rawi, J.; Bradley, C.; Angove, M.J.; Robertson, M.N.; Clark, R.L. Synthesis, structural elucidation, DNA-PK inhibition, homology modelling and anti-platelet activity of morpholino-substituted-1,3-naphth-oxazines. Bioorg. Med. Chem. 2011, 19, 3983–3994. [Google Scholar] [CrossRef] [PubMed]
- Sunny, M.; Shabana, I.K.; Diwan, S.R. 4-Aminoquinoline-triazine-based hybrids with improved in vitro antimalarial activity against CQ-sensitive and CQ-resistant strains of plasmodium falciparum. Chem. Biol. Drug Des. 2013, 81, 625–630. [Google Scholar]
- Cordeu, L.; Cubedo, E.; Bandres, E.; Rebollo, A.; Saenz, X.; Chozas, H.; Dominguez, M.V.; Echeverria, M.; Mendivil, B.; Sanmartin, C.; et al. Biological profile of new apoptotic agents based on 2,4-pyrido[2,3-d]pyrimidine derivatives. Bioorg. Med. Chem. 2007, 15, 1659–1669. [Google Scholar] [CrossRef] [PubMed]
- Font, M.; Gonzalez, A.; Palop, J.A.; Sanmartin, C. New insights into the structural requirements for pro-apoptotic agents based on 2,4-diaminoquinazoline, 2,4-diaminopyrido[2,3-d]pyrimidine and 2,4-diaminopyrimidine derivatives. Eur. J. Med. Chem. 2011, 46, 3887–3899. [Google Scholar] [CrossRef] [PubMed]
- Dorsey, J.F.; Jove, R.; Kraker, A.J.; Wu, J. The pyrido[2,3-d]pyrimidine derivative PD180970 inhibits p210Bcr-Abl tyrosine kinase and induces apoptosis of K562 leukemic cells. Cancer Res. 2000, 60, 3127–3131. [Google Scholar] [PubMed]
- Deyanov, A.B.; Niyazov, R.K.; Nazmetdivov, F.Y.; Syropyatov, B.Y.; Kolla, V.E.; Konshin, M.E. Synthesis and biological activity of amides and nitriles of 2-arylami-no-5-carboxy(carbethoxy)-6-methylnicotinic acids and 1-aryl-6-carbethoxy-7-methyl-4-oxo-1,4-dihydro- pyrido[2,3-d]pyrimidines. Pharm. Chem. J. 1991, 25, 248–250. [Google Scholar] [CrossRef]
- Grivsky, E.M.; Lee, S.; Sigel, C.W.; Duch, D.S.; Nichol, C.A. Synthesis and antitumor activity of 2,4-diamino-6-(2,5-dimethoxybenzyl)-5-methylpyrido[2,3-d]pyrimidine. J. Med. Chem. 1980, 23, 327–329. [Google Scholar] [CrossRef] [PubMed]
- Thompson, A.M.; Bridges, A.J.; Fry, D.W.; Kraker, A.J.; Denny, W.A. Tyrosine kinase inhibitors.7.7-ami-no-4-(phenylamino)-and7-amino-4-[(phenylmethyl)amino]pyrido[4,3-d]pyrimidines: A new class of inhibitors of the tyrosine kinase activity of the epidermal growth factor receptor. J. Med. Chem. 1995, 38, 3780–3788. [Google Scholar] [CrossRef] [PubMed]
- Donkor, I.O.; Klein, C.L.; Liang, L.; Zhu, N.; Bradley, E.; Clark, A.M. Synthesis and antimicrobial activity of some 6,7-annulated pyrido[2,3-d]pyrimidines. J. Pharm. Sci. 1995, 84, 661–664. [Google Scholar] [CrossRef] [PubMed]
- Pastor, A.; Alajarin, R.; Vaquero, J.J.; Alvarez-Builla, J.; Casa-Juana, M.F.; Sunkel, C.; Priego, J.G.; Fonseca, I.; Sanz-Aparicio, J. Synthesis and structure of new pyrido[2,3-d]pyrimidine derivatives with calcium channel antagonist activity. Tetrahedron 1994, 50, 8085–8098. [Google Scholar] [CrossRef]
- Agarwal, A.; Ashutosh, R.; Goyal, N.; Chauhan, P.M.S.; Gupta, S. Dihydropyrido[2,3-d]pyrimidines as a new class of antileishmanial agents. Bioorg. Med. Chem. 2005, 13, 6678–6684. [Google Scholar] [CrossRef] [PubMed]
- Monge, A.; Merino, V.M.; Sanmartin, C.; Fernandez, F.J.; Ochoa, M.C.; Berllver, C.; Artigas, P.; Alvarez, E.F. 2-Arylamino-4-oxo-3,4-dihydropyrido-[2,3-d]pyrimidines: Synthesis and diuretic activity. Eur. J. Med. Chem. 1989, 24, 24–29. [Google Scholar] [CrossRef]
- Gomha, S.M.; Ahmed, S.M.; Abdelhamid, A.O. Synthesis and cytotoxicity evaluation of some novel thiazoles, thiadiazoles, and pyrido[2,3-d][1,2,4]triazolo[4,3-a]pyrimidin-5(1H)-one incorporating triazole moiety. Molecules 2015, 20, 1357–1376. [Google Scholar] [CrossRef] [PubMed]
- Gomha, S.M.; Khalil, K.D. A convenient ultrasound-promoted synthesis and cytotoxic activity of some new thiazole derivatives bearing a coumarin nucleus. Molecules 2012, 17, 9335–9347. [Google Scholar] [CrossRef] [PubMed]
- Gomha, S.M.; Abbas, I.M.; Elaasser, M.M.; Mabrouk, B.K.A. Synthesis, molecular docking and pharmacological study of pyrimido-thiadiazinones and its bis-derivatives. Lett. Drug Des. Discov. 2017, 14, 434–443. [Google Scholar] [CrossRef]
- Gomha, S.M.; Eldebss, T.M.A.; Abdulla, M.M.; Mayhoub, A.S. Diphenylpyrroles: Novel p53 Activators. Eur. J. Med. Chem. 2014, 82, 472–479. [Google Scholar] [CrossRef] [PubMed]
- Gomha, S.M.; Dawood, K.M. Synthesis of novel indolizine, pyrrolo[1,2-a]quinoline, and 4,5-dihydrothiophene derivatives via nitrogen ylides and their antimicrobial evaluation. J. Chem. Res. 2014, 38, 515–519. [Google Scholar] [CrossRef]
- Gomha, S.M.; Eldebss, T.M.A.; Badrey, M.G.; Abdulla, M.M.; Mayhoub, A.S. Novel 4-heteroaryl-antipyrines as DPP-IV Inhibitors. Chem. Biol. Drug Des. 2015, 86, 1292–1303. [Google Scholar] [CrossRef] [PubMed]
- Gomha, S.M.; Edrees, M.M.; Altalbawy, F.M.A. Synthesis and characterization of some new bis-pyrazolyl-thiazoles incorporating the thiophene moiety as potent anti-tumor agents. Int. J. Mol. Sci. 2016, 17, 1499. [Google Scholar] [CrossRef] [PubMed]
- Hassaneen, H.M.; Abdelhamid, A.O.; Shawali, A.S.; Pagni, R. A Study of the effect of nitro group in the synthesis of pyrazoles and thiadiazolines from hydrazidoyl halides. Heterocycles 1982, 19, 319–326. [Google Scholar]
- Shawali, S.; Gomha, S.M. A New entry for short and regioselective synthesis of [1,2,4]triazolo[4,3-b][1,2,4]-triazin-7(1H)-one. Adv. Synth. Catal. 2000, 342, 599–604. [Google Scholar] [CrossRef]
- Gomha, S.M.; Riyadh, S.M.; Mahmmoud, E.A.; Elaasser, M.M. Synthesis and anticancer activities of thiazoles, 1,3-thiazines, and thiazolidine using chitosan-grafted-poly(vinylpyridine) as basic catalyst. Heterocycles 2015, 91, 1227–1243. [Google Scholar]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Sharma, M.; Chauhan, P.M.S. Dihydrofolate reductase as a therapeutic target for infectious diseases: Opportunities and challenges. Future Med. Chem. 2012, 4, 1335–1365. [Google Scholar] [CrossRef] [PubMed]
- Daina, A.; Zoete, V. A boiled-egg to predict gastrointestinal absorption and brain penetration of small molecules. ChemMedChem 2016, 11, 1117–1121. [Google Scholar] [CrossRef] [PubMed]
- Lipinski, C.A.; Lombardo, F.; Dominy, B.W.; Feeney, P.J. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv. Drug Deliv. Rev. 2001, 46, 3–25. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds 4a,c,e,f, 5, 7a–c, 8 and 10a–d are available from the authors. |


| Tested Compounds | Tumor Cell Lines | Tested Compounds | Tumor Cell Lines | ||
|---|---|---|---|---|---|
| A-549 | HepG2 | A-549 | HepG2 | ||
| 4a | 16.3 ± 1.31 | 21.1 ± 0.91 | 7c | 6.79 ± 1.11 | 8.42 ± 1.15 |
| 4c | 24.0 ± 1.21 | 20.0 ± 1.23 | 8 | 2.78 ± 0.86 | 29.9 ± 0.93 |
| 4e | 5.37 ± 0.95 | 15.68 ± 1.12 | 10a | 24.47 ± 1.23 | 27.68 ± 1.31 |
| 4f | 7.38 ± 0.82 | 9.78 ± 0.78 | 10b | 26.8 ± 0.75 | 17.7 ± 0.73 |
| 5 | 10.3 ± 0.91 | 12.4 ± 0.98 | 10c | 15.2 ± 1.42 | 14.9 ± 1.14 |
| 7a | 9.41 ± 0.79 | 13.9 ± 0.77 | 10d | 12.2 ± 0.88 | 8.72 ± 0.89 |
| 7b | 5.7 ± 0.91 | 3.54 ± 1.11 | Cisplatin | 0.95 ± 0.9 | 1.4 ± 1.1 |
| Compound | 8 |
|---|---|
| Molecular weight | 472.56 g/mol |
| Num. rotatable bonds | 5 |
| Num. H-bond acceptors | 4 |
| Num. H-bond donors | 3 |
| TPSA | 130.49 Å2 |
| GI absorption | High |
| BBB permeant | No |
| P-gp substrate | Yes |
| CYP1A2 inhibitor | No |
| Log Kp (skin permeation) | −6.43 cm/s |
| Lipinski | Yes; 0 violation |
| PAINS | 0 alert |
| Leadlikeness | No; 2 violations: MW >350, XLOGP3 >3.5 |
| Synthetic accessibility | 3.76 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Muhammad, Z.A.; Edrees, M.M.; Faty, R.A.M.; Gomha, S.M.; Alterary, S.S.; Mabkhot, Y.N. Synthesis, Antitumor Evaluation and Molecular Docking of New Morpholine Based Heterocycles. Molecules 2017, 22, 1211. https://doi.org/10.3390/molecules22071211
Muhammad ZA, Edrees MM, Faty RAM, Gomha SM, Alterary SS, Mabkhot YN. Synthesis, Antitumor Evaluation and Molecular Docking of New Morpholine Based Heterocycles. Molecules. 2017; 22(7):1211. https://doi.org/10.3390/molecules22071211
Chicago/Turabian StyleMuhammad, Zeinab A., Mastoura M. Edrees, Rasha A. M. Faty, Sobhi M. Gomha, Seham S. Alterary, and Yahia N. Mabkhot. 2017. "Synthesis, Antitumor Evaluation and Molecular Docking of New Morpholine Based Heterocycles" Molecules 22, no. 7: 1211. https://doi.org/10.3390/molecules22071211







