N-Heterocyclic Carbene Coinage Metal Complexes of the Germanium-Rich Metalloid Clusters [Ge9R3]− and [Ge9RI2]2− with R = Si(iPr)3 and RI = Si(TMS)3
Abstract
:1. Introduction
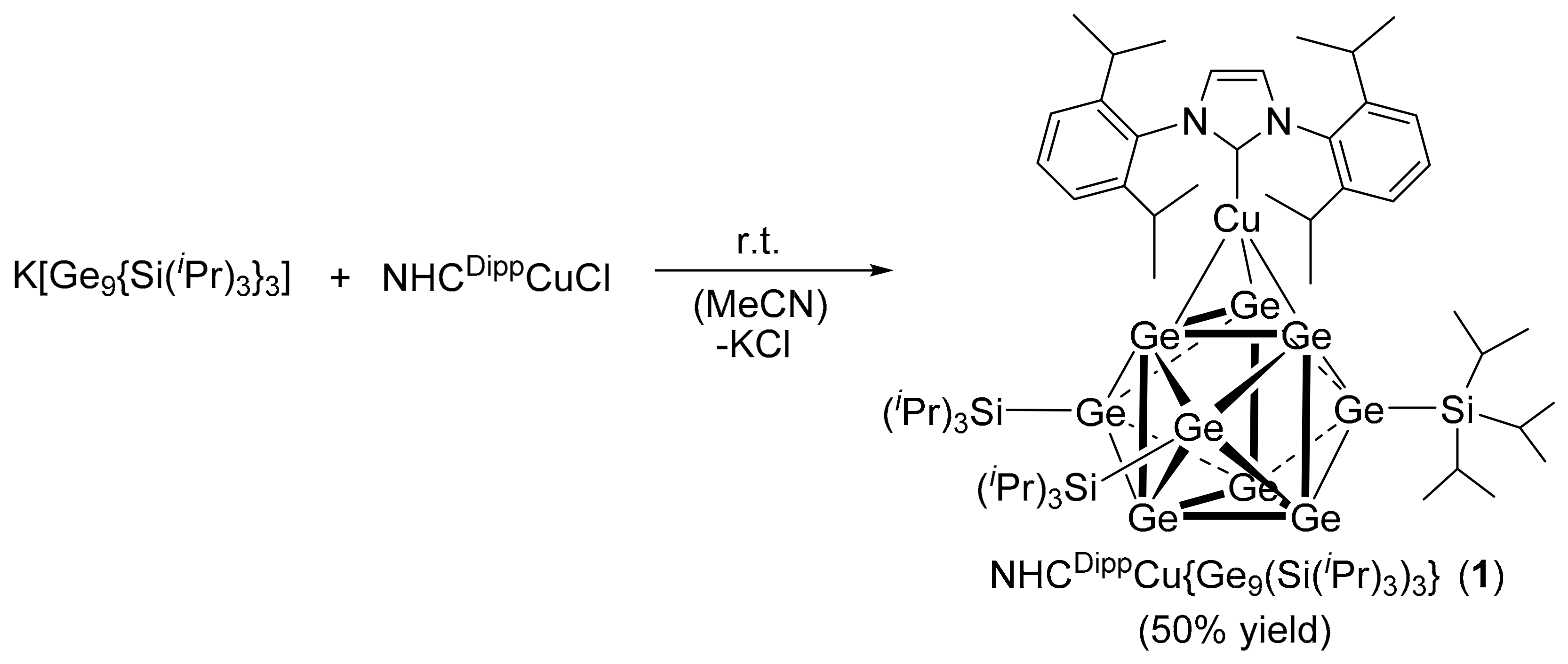
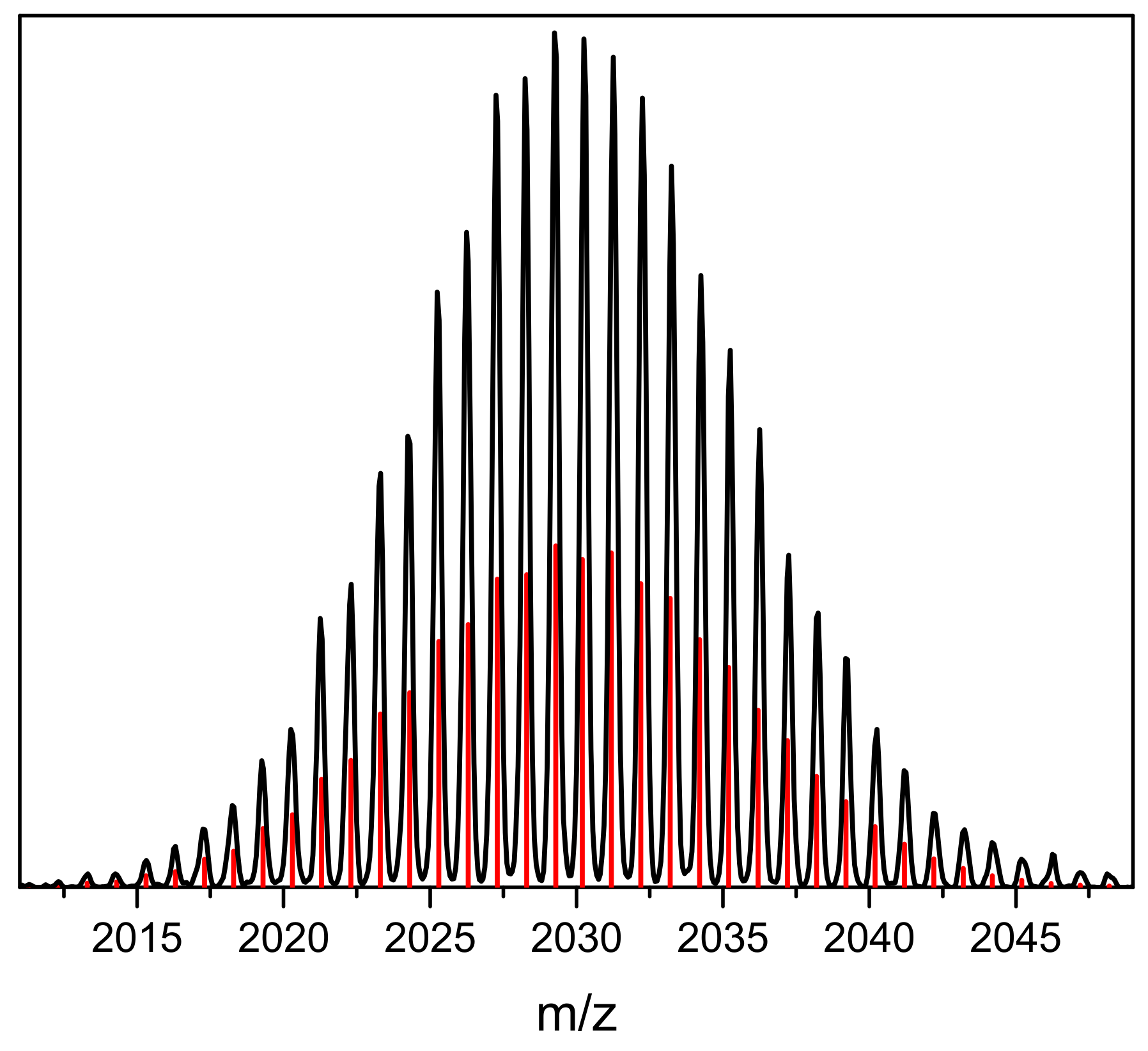
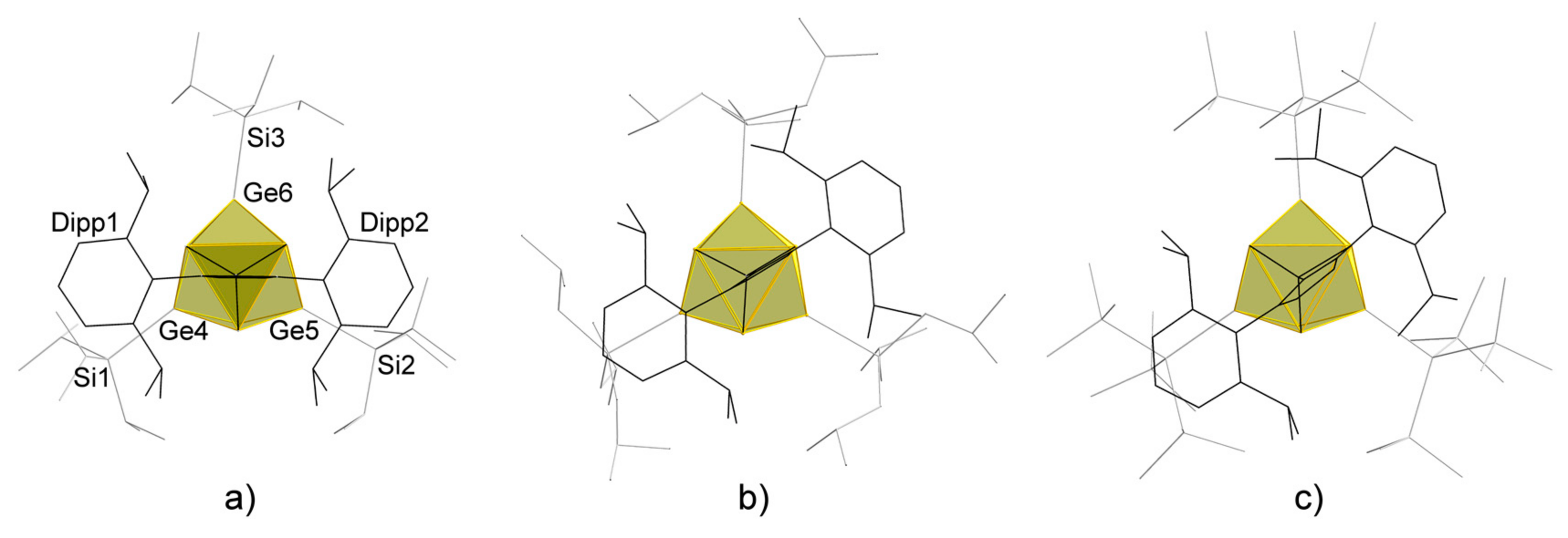
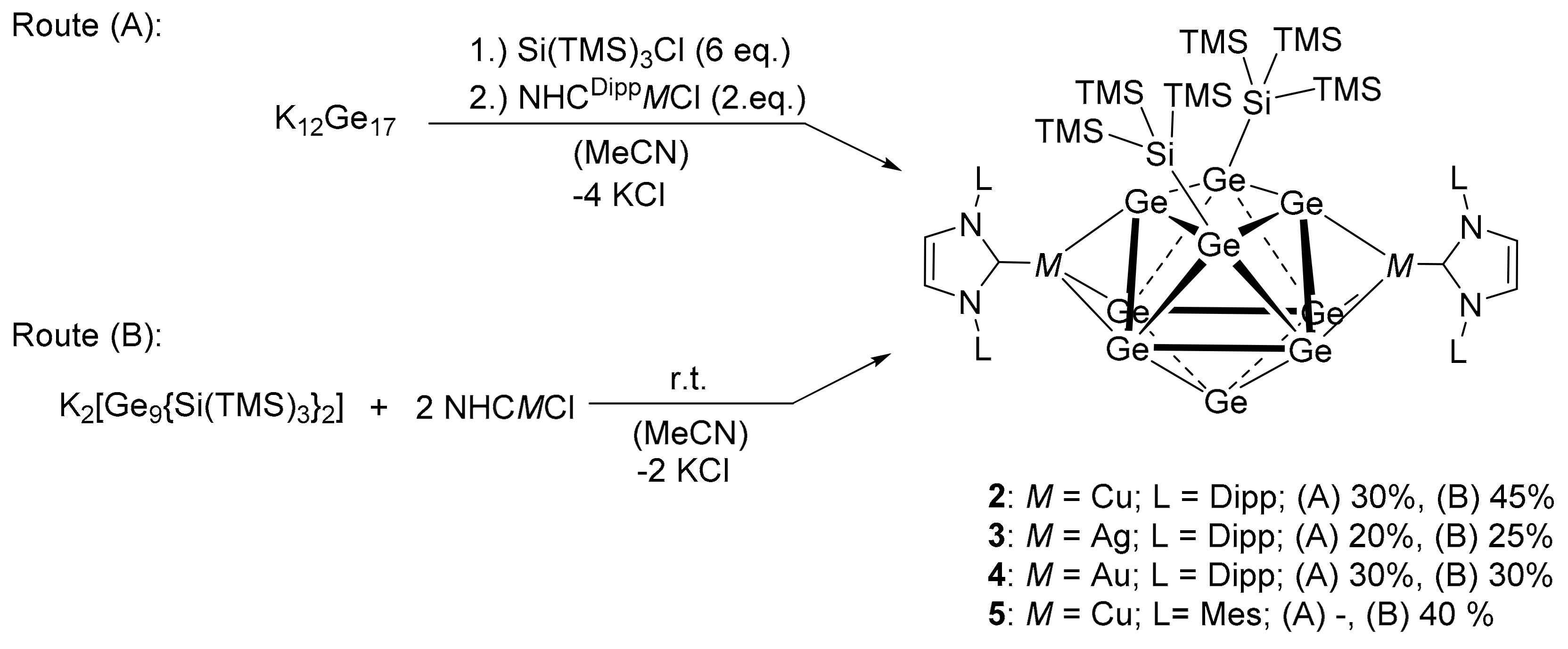
2. Results and Discussion
3. Materials and Methods
3.1. General
3.2. Single Crystal Structure Determination
3.3. NMR Spectroscopy
3.4. Electron Spray Ionization Mass Spectrometry (ESI-MS)
3.5. Elemental Analyses (EA)
3.6. Syntheses
NHCDippCu{η3-Ge9(Si(iPr)3)3} (1)
(NHCDippCu)2{η3-Ge9(Si(TMS)3)2} (2)
(NHCDippAg)2{η3-Ge9(Si(TMS)3)2} (3)
(NHCDippAu)2{η3-Ge9(Si(TMS)3)2} (4)
(NHCMesCu)2{η3-Ge9(Si(TMS)3)2} (5)
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Eichhorn, B.W.; Haushalter, R.C.; Pennington, W.T. Synthesis and Structure of closo-Sn9Cr(CO)34-: The First Member in a New Class of Polyhedral Clusters. J. Am. Chem. Soc. 1988, 110, 8704. [Google Scholar] [CrossRef]
- Scharfe, S.; Kraus, F.; Stegmaier, S.; Schier, A.; Fässler, T.F. Zintl Ions, Cage Compounds, and Intermetalloid Clusters of Group 14 and Group 15 Elements. Angew. Chem. Int. Ed. 2011, 50, 3630. [Google Scholar] [CrossRef] [PubMed]
- Sevov, S.C.; Goicoechea, J.M. Chemistry of Deltahedral Zintl Clusters. Organometallics 2006, 25, 5678. [Google Scholar] [CrossRef]
- Benda, C.B.; Waibel, M.; Fässler, T.F. On the Formation of Intermetalloid Clusters: Titanocene(III)diammine as a Versatile Reactant Toward Nonostannide Clusters. Angew. Chem. Int. Ed. 2015, 54, 522. [Google Scholar] [CrossRef]
- Scharfe, S.; Fässler, T.F. Varying Bonding Modes of the Zintl Ion [Ge9]4− in CuI Complexes: Syntheses and Structures of [Cu(η4-Ge9)(PR3)]3− (R: iPr, Cy) and [Cu(η4-Ge9)(η1-Ge9)]7−. Eur. J. Inorg. Chem. 2010, 1207. [Google Scholar] [CrossRef]
- Goichechea, J.M.; Sevov, S.C. Organozinc Derivatives of Delthedral Zintl Ions: Synthesis and Characterization of closo-[E9Zn(C6H5)]3− (E: Si, Ge, Sn, Pb). Organometallics 2006, 25, 4530. [Google Scholar] [CrossRef]
- Mayer, K.; Jantke, L.-A.; Schulz, S.; Fässler, T.F. Retention of the Zn-Zn bond in [Ge9Zn-ZnGe9]6− and Formation of [(Ge9Zn)-(Ge9)-(ZnGe9)]8- and Polymeric [− (Ge9Zn)2−−]. Angew Chem. Int. Ed. 2017, 56, 2350. [Google Scholar] [CrossRef] [PubMed]
- Scharfe, S.; Fässler, T.F.; Stegmaier, S.; Hoffmann, S.D.; Ruhland, K. [Cu@Sn9]3− and [Cu@Pb9]3−: Intermetalloid Clusters with Endohedral Cu Atoms in Spherical Environments. Chem. Eur. J. 2008, 14, 4479. [Google Scholar] [CrossRef] [PubMed]
- Waibel, M.; Kraus, F.; Scharfe, S.; Wahl, B.; Fässler, T.F. [(MesCu)2(η3-Si4)]4−: A Mesitylcopper Stabilized Tetrasilicide Tetraanion. Angew. Chem. Int. Ed. 2010, 49, 6611. [Google Scholar] [CrossRef] [PubMed]
- Stegmaier, S.; Waibel, M.; Henze, A.; Jantke, L.-A.; Karttunen, A.J.; Fässler, T.F. Soluble Zintl Phases A14ZnGe16 (A: K, Rb) Featuring [(η3-Ge4)Zn(η2-Ge4)]6− and [Ge4]4- Clusters and the Isolation of [(MesCu)2(η3,η3-Ge4)]4−: The Missing Link in the Solution Chemistry of Tetrahedral Group 14 Element Zintl Clusters. J. Am. Chem. Soc. 2012, 134, 14450. [Google Scholar] [CrossRef] [PubMed]
- Spiekermann, A.; Hoffmann, S.D.; Kraus, F.; Fässler, T.F. [Au3Ge18]5−: A Gold-Germanium Cluster with Remarkable Au-Au Interactions. Angew. Chem. Int. Ed. 2007, 46, 1638. [Google Scholar] [CrossRef] [PubMed]
- Spiekermann, A.; Hoffmann, S.D.; Fässler, T.F.; Krossing, I.; Preiss, U. [Au3Ge45]5--A Binary Anion Containing a {Ge45} Cluster. Angew. Chem. Int. Ed. 2007, 46, 5310. [Google Scholar] [CrossRef] [PubMed]
- Geitner, F.S.; Klein, W.; Fässler, T.F. On the Formation of the Intermetalloid Cluster [AgSn18]7−—The Reactivity of Coinage Metal NHC Compounds towards [Sn9]4−. Dalton Trans. 2017, 46, 5796. [Google Scholar] [CrossRef] [PubMed]
- Kysliak, O.; Schnepf, A. {Ge9[Si(SiMe3)3]2}-: A Starting Point for Mixed Substituted Metalloid Germanium Clusters. Dalton Trans. 2016, 45, 2404. [Google Scholar] [CrossRef] [PubMed]
- Schnepf, A. {Ge9[Si(SiMe3)3]3}-: A Soluble Polyhedral Ge9 Cluster Stabilized by only Three Silyl Groups. Angew Chem. Int. Ed. 2003, 42, 2624. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Sevov, S.C. Rational Synthesis of {Ge9[Si(SiMe3)3]3}- From Its Parent Zintl Ion Ge94−. Inorg. Chem. 2012, 51, 2706. [Google Scholar] [CrossRef] [PubMed]
- Kysliak, O.; Kunz, T.; Schnepf, A. Metalloid Ge9R3− Clusters with Various Silyl Substituents: From Shielded to Open Cluster Cores. Eur. J. Inorg. Chem. 2017, 805. [Google Scholar] [CrossRef]
- Mayer, K.; Schiegerl, L.J.; Fässler, T.F. On the Reactivity of Silyated Ge9 Clusters: Synthesis and Characterization of [ZnCp*(Ge9{Si(SiMe3)3}3], [CuPiPr3(Ge9{Si(SiMe3)3}3], and [(CuiPr3)4{Ge9(SiPh3)2}2]. Chem. Eur. J. 2016, 22, 18794. [Google Scholar] [CrossRef] [PubMed]
- Schiegerl, L.J.; Geitner, F.S.; Fischer, C.; Klein, W.; Fässler, T.F. Functionalization of [Ge9] with Small Silanes: [Ge9(SiR3)3]− (R: iBu, iPr, Et) and the Structures of (CuNHCDipp)[Ge9{Si(iBu)3}3], (K-18c6)Au[Ge9{Si(iBu)3}3]2 and (K-18c6)2[Ge9{Si(iBu)3}2]. Z. Anorg. Allg. Chem. 2016, 642, 1419. [Google Scholar] [CrossRef]
- Kysliak, O.; Schrenk, C.; Schnepf, A. {Ge9[Si(SiMe3)2(SiPh3)]3}-: Ligand Modification in Metalloid Germanium Cluster Chemistry. Inorg. Chem. 2015, 54, 7083. [Google Scholar] [CrossRef] [PubMed]
- Schenk, C.; Schnepf, A. [AuGe18{Si(SiMe3)3}6]-: A Soluble Au-Ge Cluster on the Way to a Molecular Cable? Angew. Chem. Int. Ed. 2007, 46, 5314–5316. [Google Scholar] [CrossRef] [PubMed]
- Schenk, C.; Henke, F.; Santiso-Quinones, G.; Krossing, I.; Schnepf, A. [Si(SiMe3)3]6Ge18M (M: Cu, Ag, Au): Metalloid Cluster Compounds as Unusual Buliding Blocks for a Supramolecular Chemistry. Dalton Trans. 2008, 4436. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Sevov, S.C. Coordination of Tri-Substituted Nona-Germanium Clusters to Cu(I) and Pd(0). Inorg. Chem. 2015, 54, 8121. [Google Scholar] [CrossRef] [PubMed]
- Geitner, F.S.; Fässler, T.F. Introducing Tetrel Zintl Ions to N-Heterocyclic Carbenes—Synthesis of Coinage Metal NHC Complexes of [Ge9{Si(SiMe3)3}3]−. Eur. J. Inorg. Chem. 2016, 2688. [Google Scholar] [CrossRef]
- Hintermann, L. Expedient Syntheses of the N-Heterocyclic Carbene Precursor Imidazolium Salts IPr·HCl, IMes·HCl and IXy·HCl. Beilstein J. Org. Chem. 2007, 3, 22. [Google Scholar] [CrossRef] [PubMed]
- Santoro, O.; Collado, A.; Slawin, A.M.Z.; Nolan, S.P.; Cazin, C.S.J. A General Synthesis Route to [Cu(X)(NHC)] (NHC = N-Heterocyclic Carbene, X = Cl, Br, I) Complexes. Chem. Commun. 2013, 49, 10483. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Frémont, P.; Scott, N.M.; Stevens, E.D.; Ramnial, T.; Lightbody, O.C.; Macdonald, C.L.B.; Clyburne, J.A.C.; Abernethy, C.D.; Nolan, S.P. Synthesis of Well-Defined N-Heterocyclic Carbene Silver(I) Complexes. Organometallics 2005, 24, 6301. [Google Scholar] [CrossRef]
- Collado, A.; Gomez-Suarez, A.; Martin, A.R.; Slawin, A.M.Z.; Nolan, S.P. Straightforward Synthesis of [Au(NHC)(X)] (NHC = N-Heterocyclic Carbene, X = Cl, Br, I) Complexes. Chem. Commun. 2013, 49, 5541. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G. Crystal Structure Refinement with SHELXL. Acta Cryst. Sect. C 2015, 71, 3. [Google Scholar] [CrossRef] [PubMed]
- Spek, A. Single Crystal Structure Validation with the Programm PLATON. J. Appl. Cryst. 2003, 36, 7. [Google Scholar] [CrossRef]
- Fulmer, G.R.; Miller, A.J.M.; Sherden, N.H.; Gottlieb, H.E.; Nudelman, A.; Stoltz, B.M.; Bercaw, J.E.; Goldberg, K.I. NMR Chemical Shifts of Trace Impurities: Common Laboratory Solvents, Organics, and Gases in Deuterated Solvents Relevant to the Organometallic Chemist. Organometallics 2010, 29, 2176. [Google Scholar] [CrossRef]
Sample Availability: Samples of all compounds are not available from the authors. |




| Distances [Å] | 1 | R = Si(iBu)3 [19] | R = Si(TMS)3 [24] |
|---|---|---|---|
| d1(Cu-Ge1) | 2.4914(8) | 2.4911(6) | 2.4943(9) |
| d2(Cu-Ge2) | 2.541(1) | 2.4933(6) | 2.5188(9) |
| d3(Cu-Ge3) | 2.5661(8) | 2.5594(6) | 2.5660(9) |
| dmean(Cu-Ge) a | 2.5328(9) | 2.5146(6) | 2.5263(9) |
| d(Cu-C1) b | 1.951(3) | 1.943(3) | 1.957(5) |
| angles [deg.] | |||
| ctp-Cu-C1 c | 177.67(4) | 172.77(2) | 173.59(1) |
| Distances [Å] | 2 | |
|---|---|---|
| A | B | |
| d1(Cu-Ge) | 2.601(1) | 2.532(1) |
| d2(Cu-Ge) | 2.532(1) | 2.582(1) |
| d3(Cu-Ge) | 2.405(1) | 2.413(1) |
| dmean(Cu-Ge) a | 2.513(1) | 2.509(1) |
| d(Cu-Ccarbene) | 1.941(5) | 1.913(5) |
| angles [deg.] | ||
| ctp-Cu-Ccarbene b | 162.88(2) | 163.92(2) |
| Compound | 1 | 2 |
|---|---|---|
| formula | C54H99Ge9Cu1N2Si3·2 tol | C72H128Ge9Cu2N4Si8·4 tol. |
| fw (g·mol−1) | 1761.73 | 2423.42 |
| space group (no.) | P1 (2) | P1 (2) |
| a (Å) | 12.377(3) | 15.102(2) |
| b (Å) | 13.387(3) | 16.831(3) |
| c (Å) | 26.468(5) | 25.659(5) |
| α (deg.) | 77.69(3) | 108.951(9) |
| β (deg.) | 78.76(3) | 101.847(8) |
| γ (deg.) | 77.58(3) | 90.837(8) |
| V (Å3) | 4133.0(2) | 6013.8(2) |
| Z | 2 | 2 |
| T (K) | 150(2) | 100(2) |
| λ (Å) | Mo-Kα | Mo-Kα |
| ρcalcd (g·cm−3) | 1.416 | 1.338 |
| μ (mm−1) | 3.553 | 2.680 |
| collected reflections | 62327 | 90837 |
| independent reflections | 14985 | 21064 |
| Rint/Rδ | 0.0263/0.0187 | 0.0914/0.0943 |
| parameters/restraints | 776/0 | 1273/366 |
| R1 (I > 2 σ(I)/all data) | 0.0330/0.0414 | 0.0468/0.0985 |
| wR2 (I > 2 σ(I)/all data) | 0.0801/0.0854 | 0.0953/0.1126 |
| goodness of fit | 1.084 | 0.998 |
| max./min. diff. el. density (e·Å−3) | 0.710/−0.757 | 0.827/–0.653 |
| CCDC | 1553930 | 1553929 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Geitner, F.S.; Giebel, M.A.; Pöthig, A.; Fässler, T.F. N-Heterocyclic Carbene Coinage Metal Complexes of the Germanium-Rich Metalloid Clusters [Ge9R3]− and [Ge9RI2]2− with R = Si(iPr)3 and RI = Si(TMS)3. Molecules 2017, 22, 1204. https://doi.org/10.3390/molecules22071204
Geitner FS, Giebel MA, Pöthig A, Fässler TF. N-Heterocyclic Carbene Coinage Metal Complexes of the Germanium-Rich Metalloid Clusters [Ge9R3]− and [Ge9RI2]2− with R = Si(iPr)3 and RI = Si(TMS)3. Molecules. 2017; 22(7):1204. https://doi.org/10.3390/molecules22071204
Chicago/Turabian StyleGeitner, Felix S., Michael A. Giebel, Alexander Pöthig, and Thomas F. Fässler. 2017. "N-Heterocyclic Carbene Coinage Metal Complexes of the Germanium-Rich Metalloid Clusters [Ge9R3]− and [Ge9RI2]2− with R = Si(iPr)3 and RI = Si(TMS)3" Molecules 22, no. 7: 1204. https://doi.org/10.3390/molecules22071204






