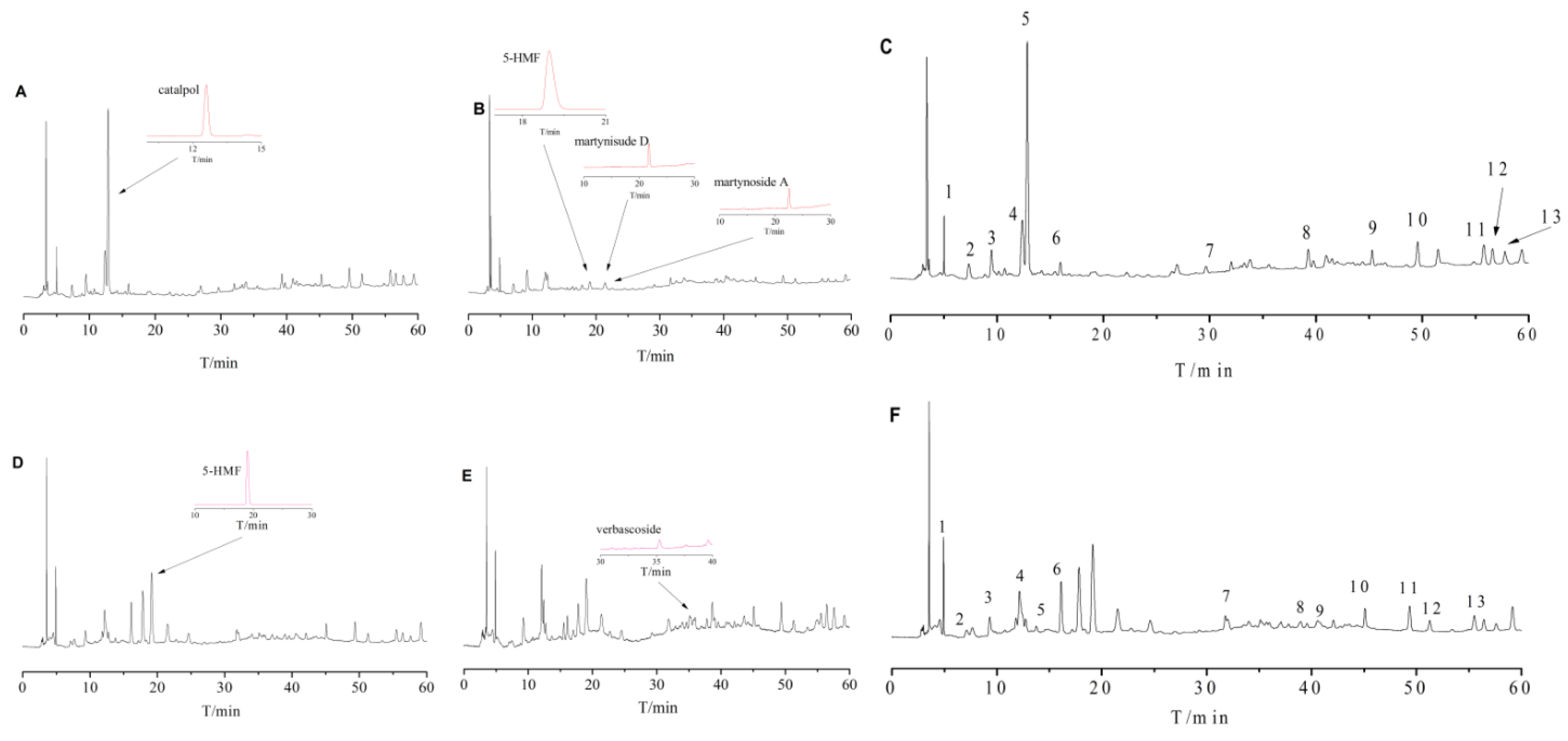
2.1. The Diverse HPLC Fingerprints of Shu Dihuang with Different Degrees of Processing and Adjuvants
HPLC chromatographic conditions were optimized based on references and actual experiments. The HPLC chromatograms of Shu Dihuang whole extract had unsteady baselines and the reproducibility was bad, which was mainly due to the complex chemical composition changing as a result of the different steaming and drying times and processing adjuvants. Also, there was no peak after 140 min and some peaks around 20 min were not completely separated. To simplify the HPLC chromatographic fingerprint and time frame, different parts of the extract were separated and the collection time was shortened. The petroleum ether, chloroform, ethyl acetate, n-butyl alcohol and water fractions extracted from test samples were assessed with HPLC chromatographic fingerprint in many preliminary experiments, and water fraction had the best reproducibility. Therefore, in this experiment we mainly used the water fraction for HPLC chromatographic fingerprinting.
For the methodology validation, the RSD values calculated from the relative retention time and relative peak area were less than 3%, which demonstrated that the instrumental and sampling precision were good, and the extraction method and chromatographic conditions were appropriate.
With SP and SWP as references, 13 common peaks were determined at 205 nm and 284 nm detection wavelengths, as shown in
Figure 1E,F. Because of the different processing procedures of Shu Dihuang, the HPLC chromatographic fingerprints were also very diverse. The overlaid HPLC chromatograms of Shu Dihuang are shown in
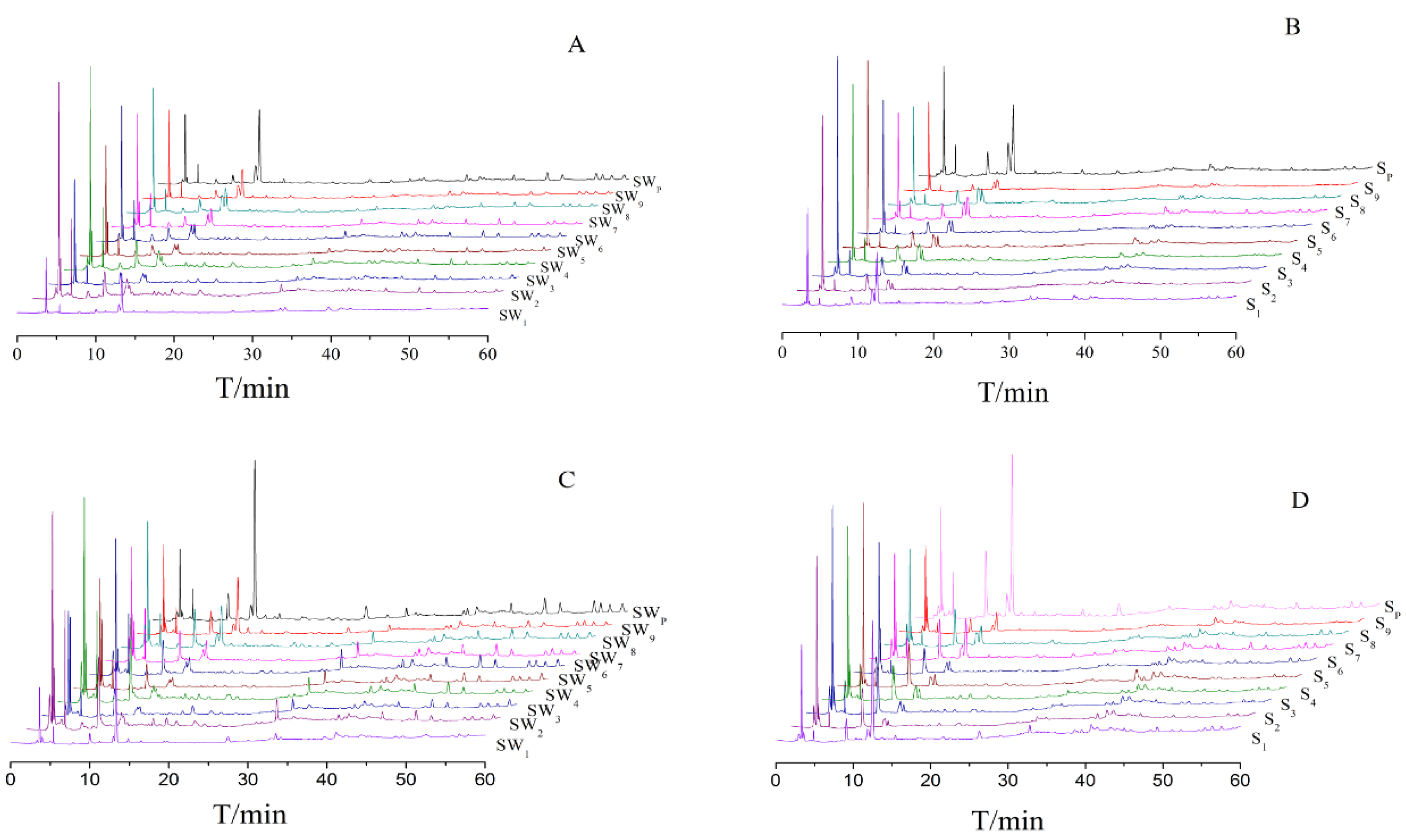
Figure 2. Their similarities were shown in
Table 1,
Table 2,
Table 3 and
Table 4. With Shu Dihuang (SWP) as the reference (
Table 3 and
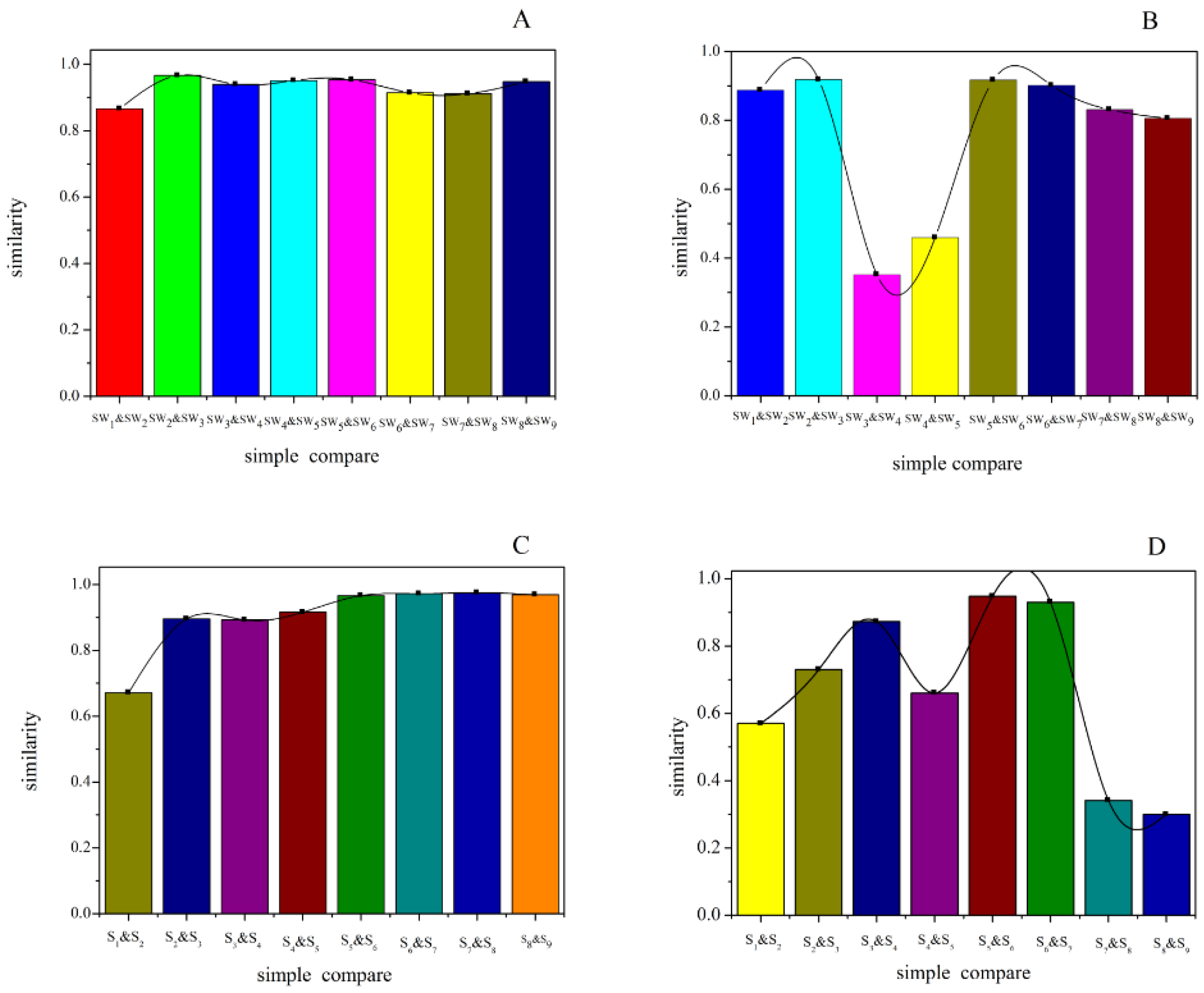
Table 4), the similarities were 0.442–0.969 and 0.472–0.949 at 205 and 284 nm detection wavelengths, respectively. When compared with the generated common peak HPLC chromatogram (R), SW3 had higher similarity values (0.972 and 0.949) at both 205 and 284 nm detection wavelength. For the relative similarity at 205 nm, there was higher similarity between SW2 and SW9 (greater than 0.9), and lower similarity between SW2 and SW1 (0.866) (
Figure 3). SW4 and SW5 both had higher similarities with the other samples, and they also had relatively high similarity with SWP and R.
For the relative similarity at 284 nm, the two smallest values appeared between SW4 and SW3, and SW5 and SW4 (0.352 and 0.459, respectively), and other samples’ relative similarities were all greater than 0.8. As shown in
Table 3, the similarities of SW5 with the other samples were higher than SW4. Compared with SWP and R, SW5 also had higher similarity. Overall, SW5 had higher similarity than SW4.
With Shu Dihuang (SP) as the reference (
Table 4 and
Table 5), similarities were 0.813–0.972 and 0.497–0.854 at 205 and 284 nm detection wavelengths, respectively. When compared with the generated common peak HPLC chromatogram (R), S5 (at 205 nm) and S3 (at 284 nm) had high similarities, which were 0.972 and 0.854. For the relative similarity at 205 nm, the smallest similarity appeared between S1 and S2 (0.671), and the other samples’ relative similarities were all greater than 0.9. S4 and S5 both had higher similarities with the other samples. But most of the other samples had higher similarities with S5. For the relative similarity at 284 nm, there were two small similarity values between S9 and S8 (0.3), and S8 and S7 (0.341). Similarly, S5 had higher similarity compared with the other samples.
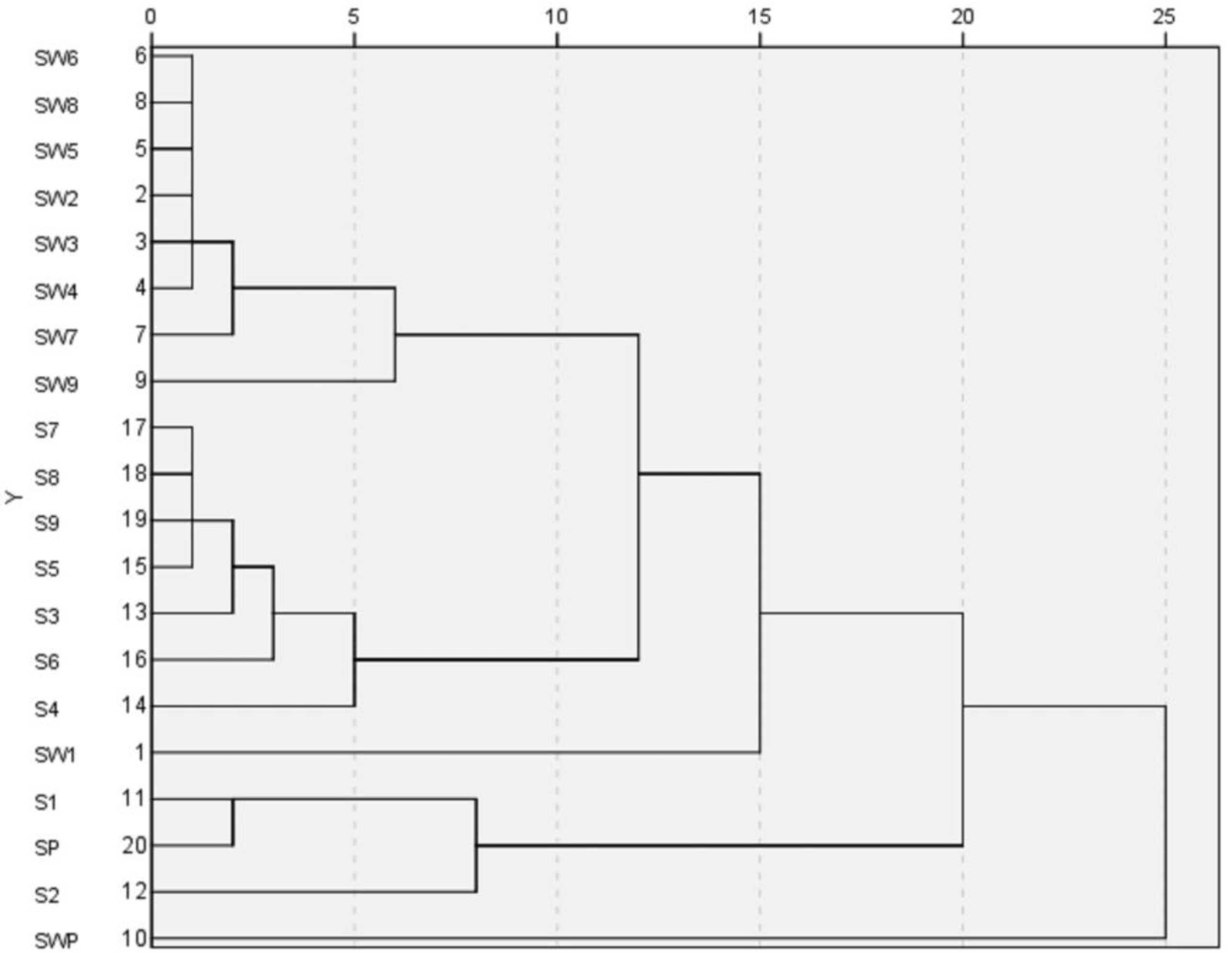
HCA is a way to structure a complicated set of observations into unique and mutually exclusive subject groups (clusters) that are similar to each other with respect to certain characteristics. The HCA results of S1~S9, SW1~SW9, SP and SWP of Shu Dihuang determined at 285 nm were shown in
Figure 4. Overall, it was clear that Shu Dihuang and Shu Dihuang processed with yellow rice wine were clustered separately into two groups, indicating that the processing adjuvant (yellow rice wine) caused changes in the composition and/or content of components. This further reflected the differences seen in relative similarity. SW5 and most of the samples processed with yellow rice wine were clustered into the same group, including SW6, SW8, SW2, SW3, SW4, SW7 and SW9. Similarly, S5, S7, S8, S9, S3, S6 and S4 were clustered into another group. Moreover, this result was consistent with the previous results.
In summary, the overall chemical composition of Shu Dihuang was affected by steaming and polishing cycles and processing adjuvant (yellow rice wine). Catalpol, a kind of cycloalkene ether terpenoid, was detected at 205 nm, while 5-HMF and verbascoside were mainly detected at 284 nm. The related studies had shown that the content of cycloalkene ether terpenoids was reduced while the content of 5-HMF was increased as the degree of processing increased, and 5-HMF was an important indicator for the quality of Shu Dihuang [
14,
15]. Therefore, the correlation result at 285 nm detection wavelength was a critical parameter. This also led to the low number of common peaks (13) and the absence of reference substances in common peaks. Based on the results from comparing the effects of steaming and polishing times and processing adjuvants, we could draw the conclusion that the overall chemical composition experienced a great change from three to five cycles of steaming and drying, and five cycles was a simple processing method of Shu Dihuang which obeyed the ancient processing theory. These results also suggested that the degree of processing must be taken into account when preparing Shu Dihuangtan.
2.2. The Pyrolysis and Combustion Characteristics of Shu Dihuang with Different Degrees of Processing and Adjuvants
The TG-DTG curves of Shu Dihuang are shown in
Figure 5, and the pyrolysis parameters and combustion characteristics are listed in
Table 5. Three different pyrolysis and combustion stages were observed: drying and dehydration, pyrolysis and combustion, carbonization and combustion. Since the pyrolysis and combustion characteristics were used to simulate the preparation process of Shu Dihuangtan, we focused more on the pyrolysis and combustion stage.
In the pyrolysis and combustion stagef, two obvious thermal weight loss phases and two thermal weight loss rate peaks appeared for Shu Dihuang with different steaming and drying cycles and adjuvants. For Shu Dihuang processed with yellow rice wine, the initial temperature and the final temperature in these two phases were 151 ± 8.16 °C to 266.67 ± 1.56 °C and 266.67 ± 1.56 °C to 386.67 ± 4.32 °C, respectively. Moreover, the intensities of the two thermal weight loss rate peaks were 6.07 ± 1.16%·min−1 and 2.74 ± 0.15%·min−1, and they appeared at 210.33 ± 4.32 °C and 294.89 ± 1.66 °C, respectively. Moreover, the pyrolysis-combustion characteristics of Shu Dihuang processed with no adjuvant were similar. The initial temperature and final temperature in the two phases were 158.22 ± 6.75 °C to 266.33 ± 5.37 °C and 266.33 ± 5.37 °C to 378.56 ± 4.30 °C, respectively. The intensities of the thermal weight loss rate peaks were 7.25 ± 1.38%·min−1 and 2.80 ± 0.14%·min−1, and they appeared at 211.33 ± 2.62 °C and 290.89 ± 2.51 °C, respectively.
Along with the increase of steaming and polishing cycles, both the mean intensity of the two thermal weight loss rate peaks and the temperature interval (initial and final temperature) decreased in the pyrolysis and combustion stage. For Shu Dihuang processed with yellow rice wine, the initial and the final temperatures for SW2 were respectively continuous within the range of 163 °C and 396 °C, and the intensities of the thermal weight loss rate peaks were 7.68%·min
−1 and 2.78%·min
−1. The initial and the final temperatures for SW5 were between 144 °C and 385 °C, and the intensities of thermal weight loss rate peaks were 5.84%·min
−1 and 2.77%·min
−1. For SW8, the initial temperature was 145 °C and the final temperature was 381 °C, and the intensities of thermal weight loss rate peaks were 4.88%·min
−1 and 2.96%·min
−1. For Shu Dihuang processed without adjuvant, the temperature interval for S2 started at 163 °C and finished at 396 °C, and the intensities of thermal weight loss rate peaks were 8.20%·min
−1 and 3.00%·min
−1. The initial and final temperatures for S5 were continuously within 148 °C and 377 °C, and the intensities of thermal weight loss rate peaks were 7.55%·min
−1 and 2.46%·min
−1. For S8, the initial temperature was 158 °C and the final temperature was 373 °C, and the intensities of thermal weight loss rate peaks were 5.86%·min
−1 and 2.68%·min
−1. However, overall, the differences of thermal weight loss and thermal weight loss rate were very small from SW5 to SW9. Also, the peak temperature of thermal weight loss rate of SW5 was similar to SW6-SW9 (
Figure 5), demonstrating that the trends for Shu Dihuang processed with or without adjuvant were similar, so we hold the opinion that five cycles represent a relatively good processing method.
For Shu Dihuang processed with the same steaming and polishing cycles, the pyrolysis-combustion characteristics were also different because of the processing adjuvants (with yellow rice wine or not). The intensity of the thermal weight loss rate peak was less for the Shu Dihuang processed with yellow rice wine compared to the Shu Dihuang processed without adjuvants at the same steaming and polishing cycle. Meanwhile, the Shu Dihuang processed with yellow rice wine spanned a smaller temperature interval during its thermal weight loss.
To summarize, the degree of processing and adjuvant are important conditions in the carbonization process for preparing Shu Dihuangtan. The intensity of thermal weight loss rate peak near 210.33 ± 4.32 °C or 211.33 ± 2.62 °C was an important indicator of the degree of processing of Shu Dihuang. Moreover, the corresponding temperature of the thermal weight loss rate peak in the second stage of pyrolysis-combustion near 290.89 ± 2.51 °C was the upper temperature limit of carbonization of Shu Dihuang.
2.3 FTIR Diversity of Shu Dihuangtan
As shown in
Figure 6, there were nine common peaks in the FTIR spectra located at 3000, 2920, 1702, 1650, 1609, 1430, 785, 720 and 667 cm
−1, respectively. The Shu Dihuangtan peaks of (CS1~CS9, CSW1-~CSW9, CSP and CSWP) at 3000 cm
−1 were possibly due to the stretching vibration of ν
=C-H [
16], and the peak near 1650 cm
−1 was assigned to the stretching vibration of ν
=C-C [
17]. Combining these data with the out-of-plane bending vibration at 720 cm
−1 from the second derivative spectrum, we concluded that there was an olefin structure with a bis-substituted
cis single olefin in Shu Dihuangtan [
18]. Furthermore, the peak near 2920 cm
−1 was classified as the stretching vibration of -CH
3 and -CH
2 [
19], the peak at 1702 cm
−1 belonged to the carboxyl C=O stretching vibration, and the peaks at 1609 and 1430 cm
−1 were the characteristic absorption peaks of aromatic acidic material [
20]. Combining these data with the aromatic compound C–H vibration absorption peak at 785 cm
−1, we speculated that there was benzodiazepine material in Shu Dihuangtan [
21]. In addition, the peak at 667 cm
−1 was classified as the in-plane bending vibration of CO
2 [
22], and the peaks at 720 cm
−1 and 667 cm
−1 had significant differences in the second derivative spectrum. The peak intensities of CS5 and CSW5 were smaller than others, while the peak intensities of CS4 and CSW4 were much stronger.
With Shu Dihuangtan (CSP) as the reference (
Table 6), the similarities ranged from 0.285 to 0.871 (CS1 to CS9, processed with no adjuvants). The highest similarity was between CS1 and CSP (0.871), while the lowest similarity was between CS4 and CSP (0.285). For the similarities between any two samples, CS3 and CS9 were relatively higher. Meanwhile, the relative similarity between CS3 and CSP as well as the sample with several cycles of steaming and drying (generally nine cycles) in the ancient theory were both obviously high. For the relative similarities among CS1-CS9, as shown in
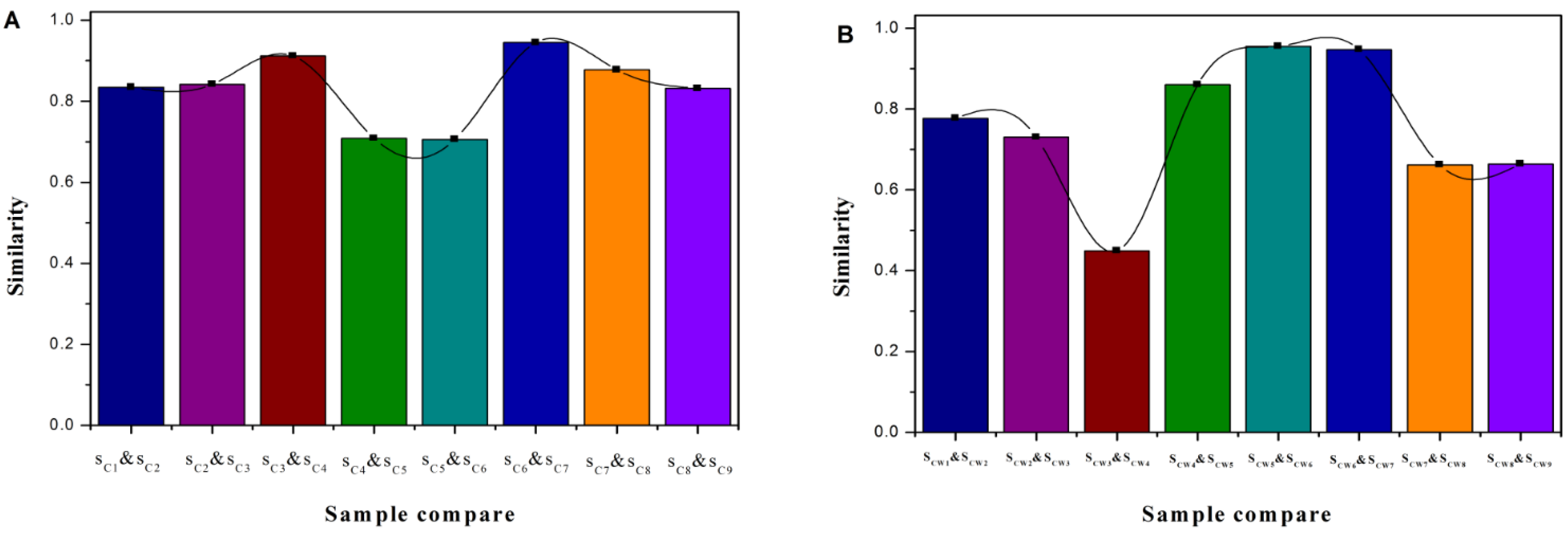
Figure 7, the highest two were CS4 and CS3 (0.912), and CS7 and CS6 (0.945); the lowest two were CS5 and CS4 (0.709), and CS6 and CS5 (0.706).
With Shu Dihuangtan (CSWP) as the reference (
Table 7), the similarities ranged from 0.623 to 0.951 (CSW1 to CSW9, processed with yellow rice wine). The highest similarity was between CSW7 and CSWP (0.951), while the lowest similarity was between CSW8 and CSP (0.623). For the similarities between any two samples, CSW4 and CSW9 were relatively higher, and CSW5 and CSW6 had the highest similarity (0.955). For the relative similarities among CSW1-CSW9, as shown in
Figure 6, the smallest was CSW4 and CSW3 (0.449), and the similarities among CSW4-CSW7 were kept at high levels.
Overall, all samples’ similarities were different and most of them were relatively small. The global chemical composition of Shu Dihuangtan was affected by both the degree of processing and adjuvant.