Functionalization of Endohedral Metallofullerenes with Reactive Silicon and Germanium Compounds †
Abstract
:1. Introduction
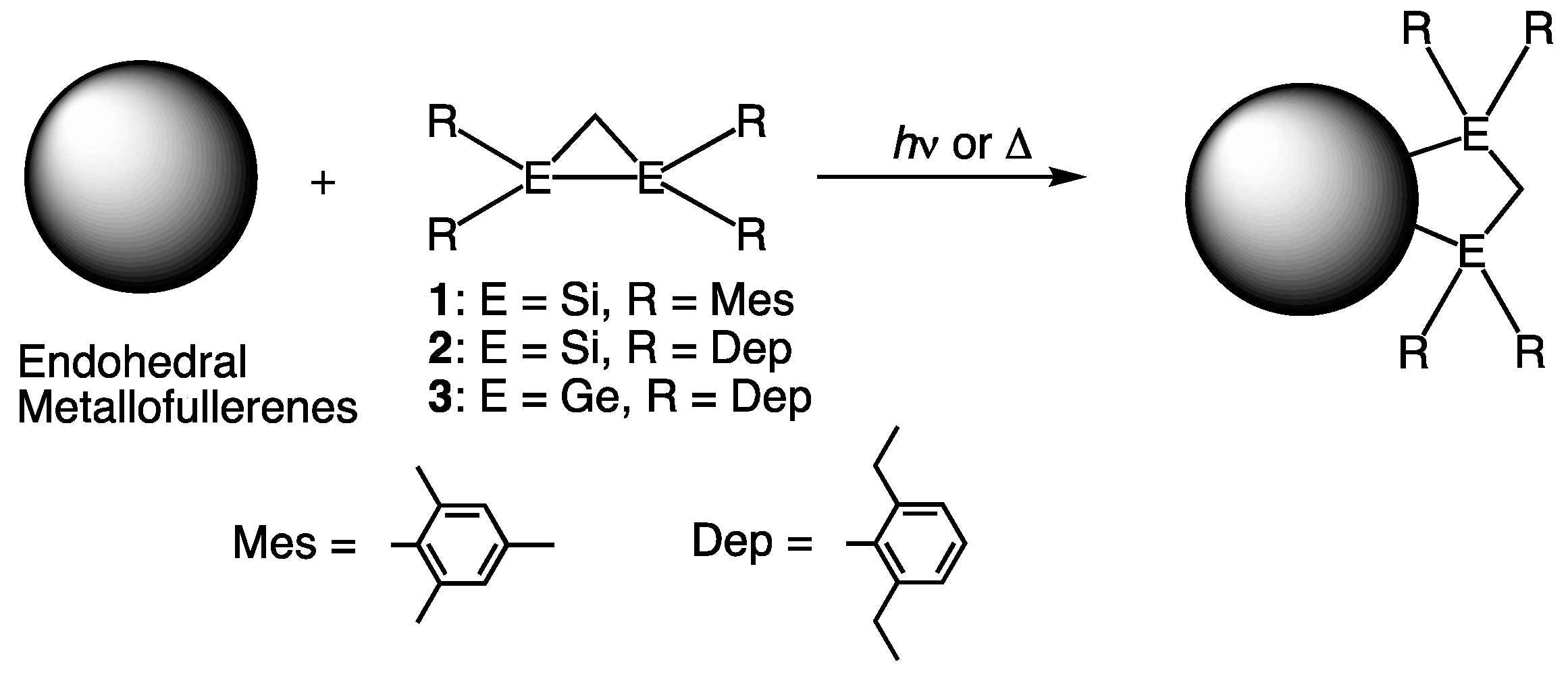
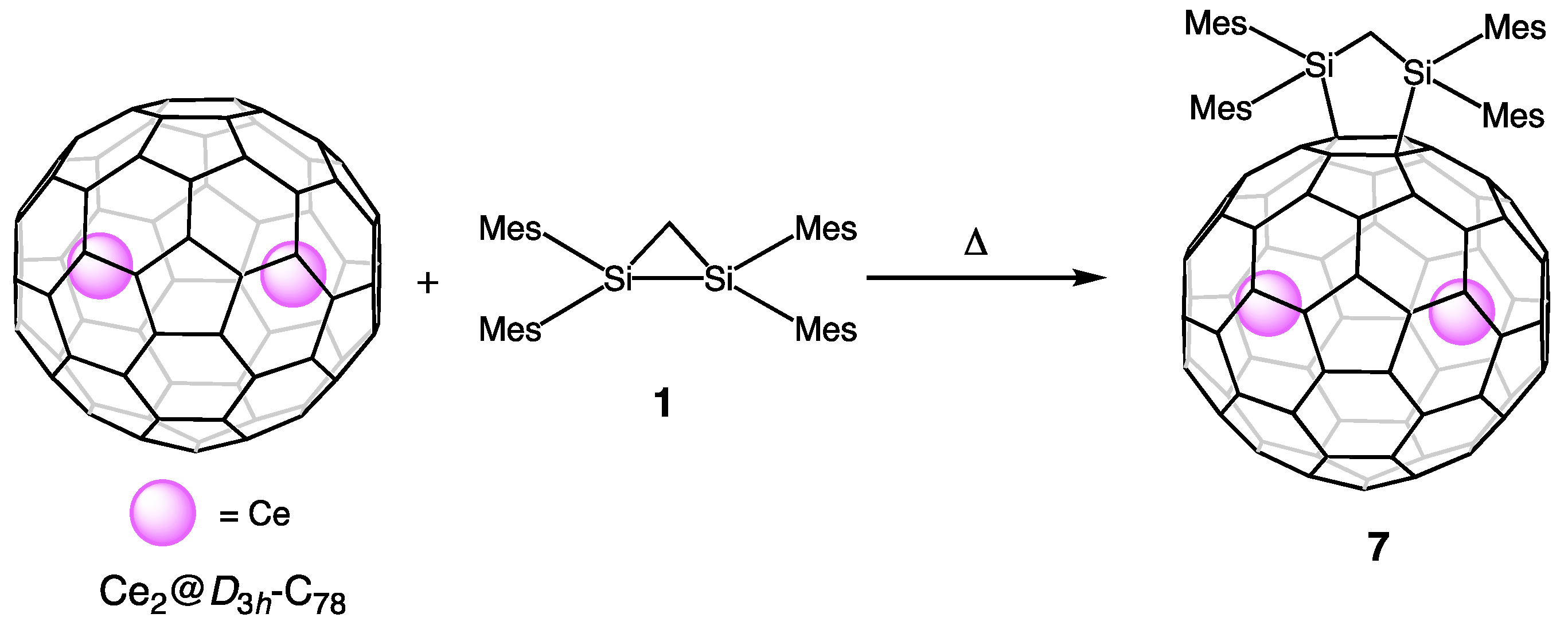
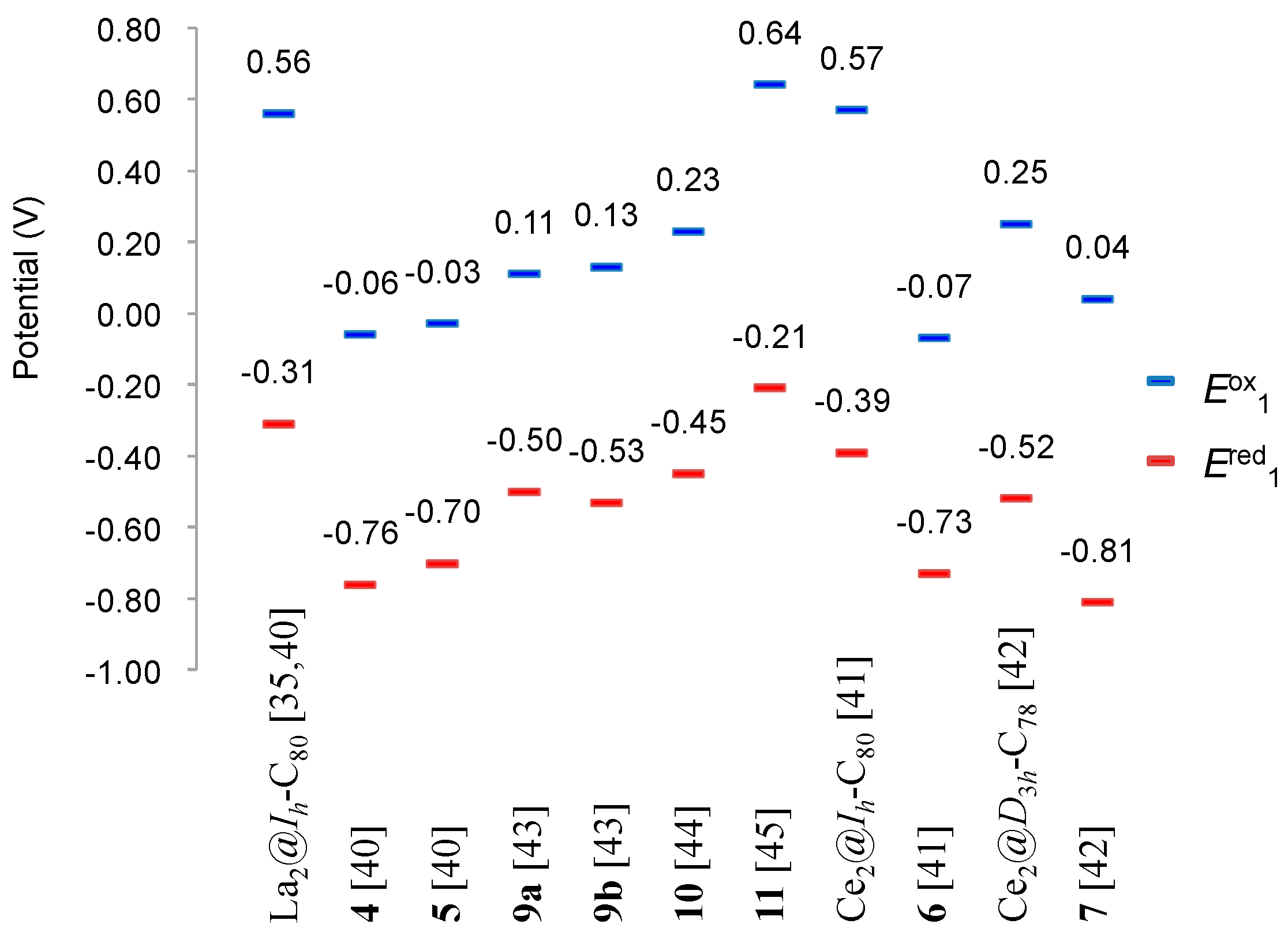
2. Comparative Studies of Reactions of EMFs with Disilirane
3. Reactions of La@C2v-C82 with Disilirane 1
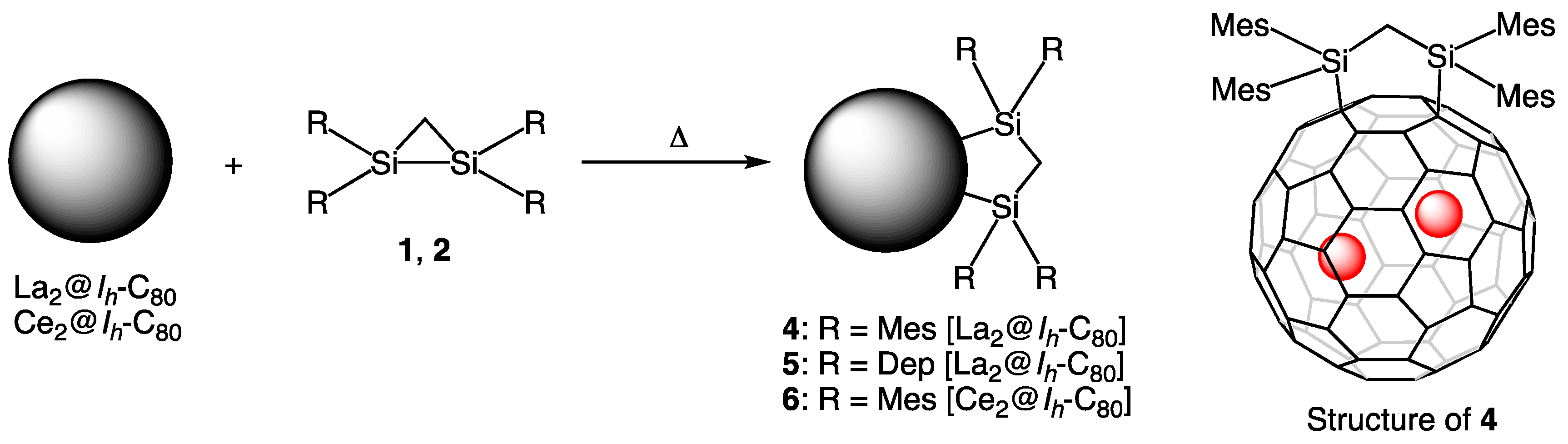
4. Reactions of La2@Ih-C80 and Related EMFs with Disiliranes 1 and 2
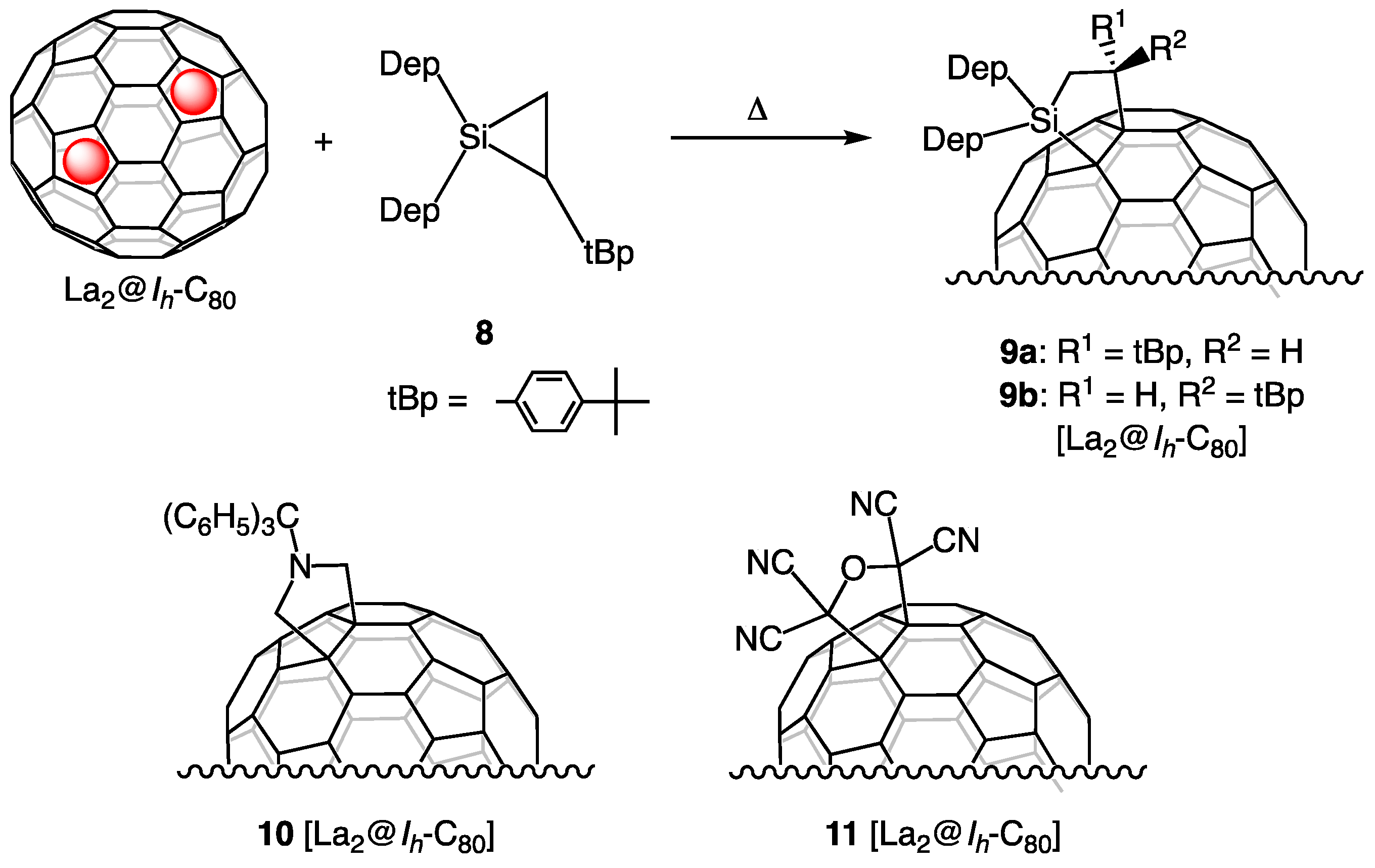
5. Reactions of La2@Ih-C80 with Silirane 8
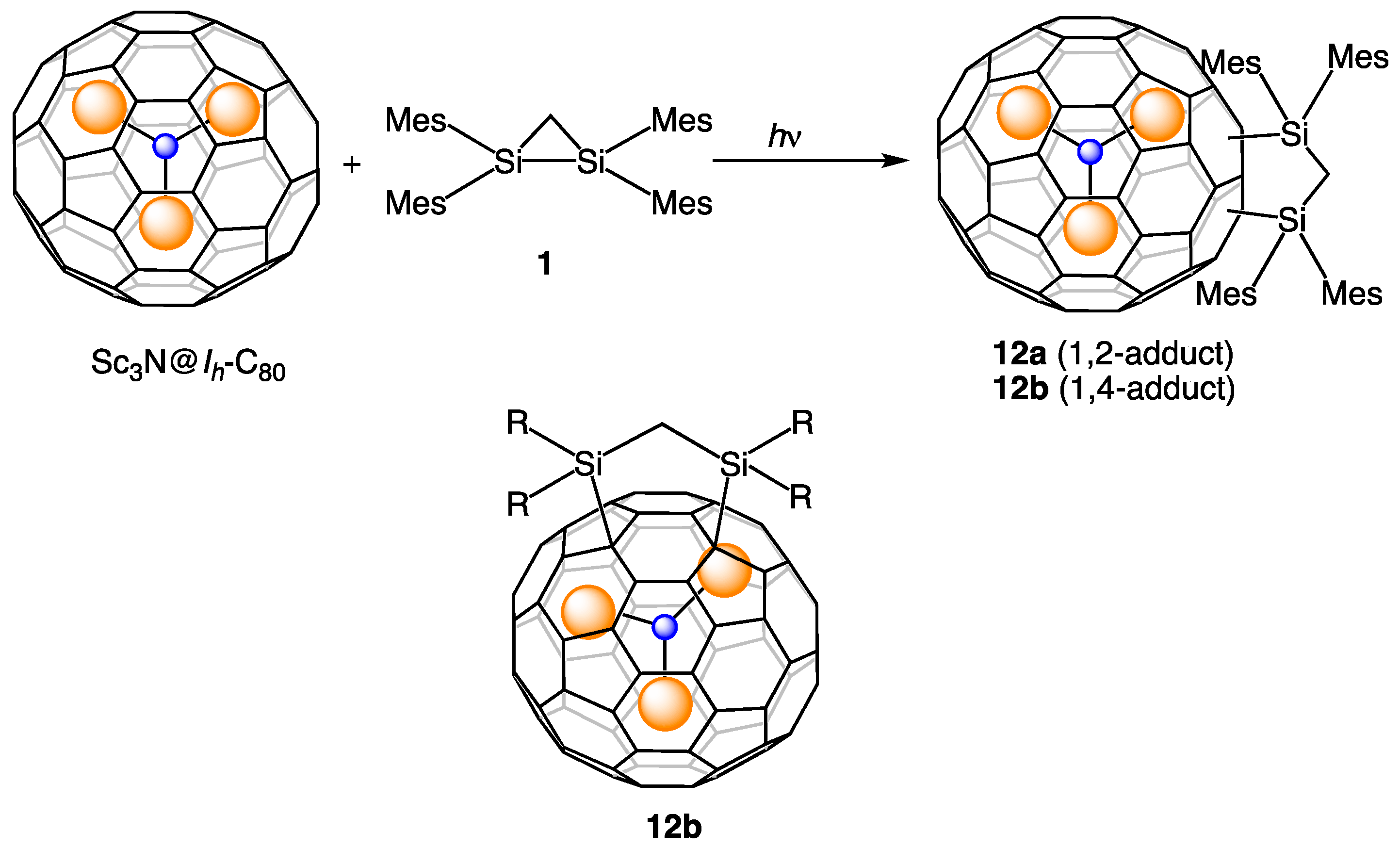
6. Reactions of Sc3N@Ih-C80 with Disilirane 1
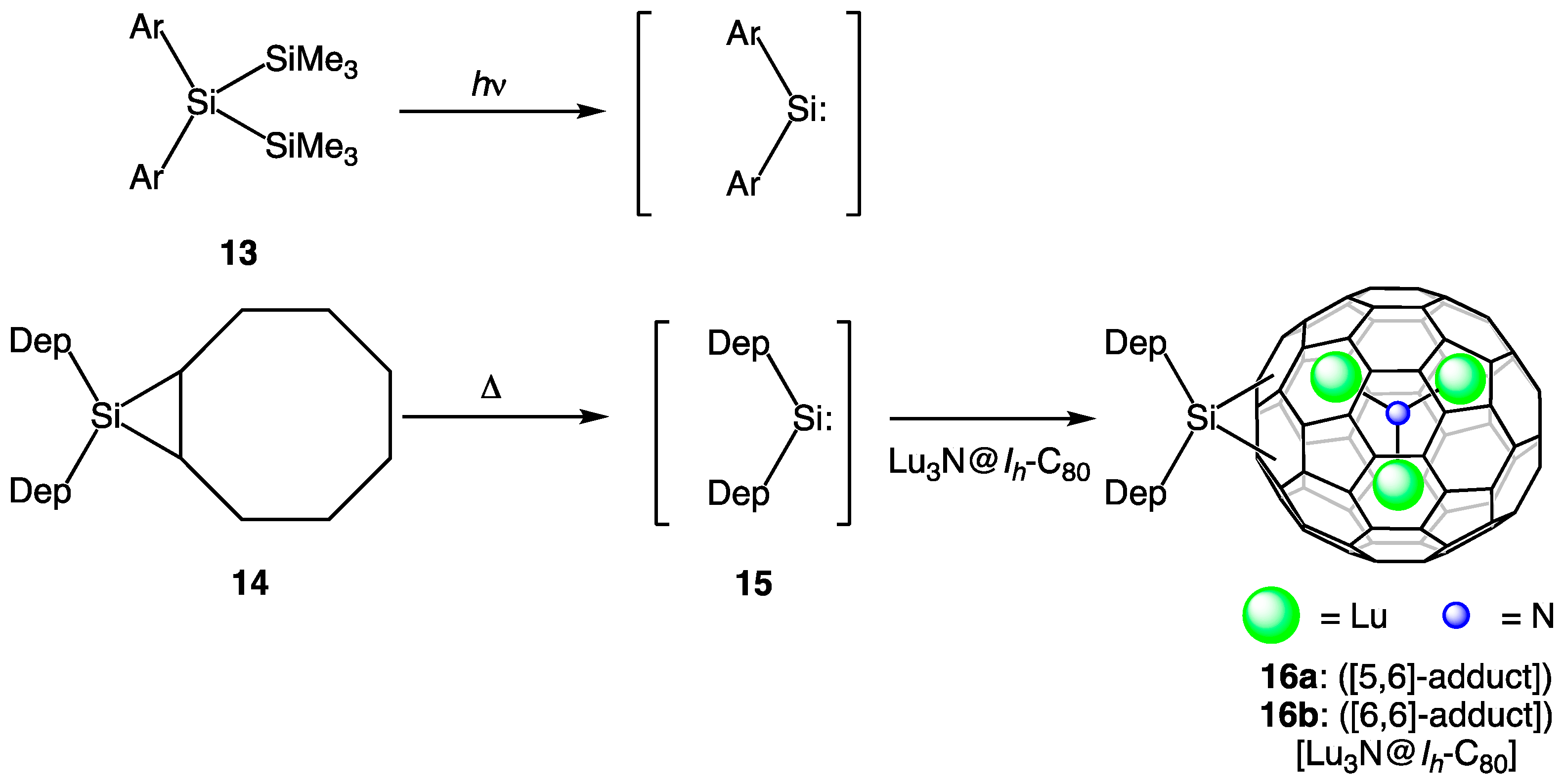
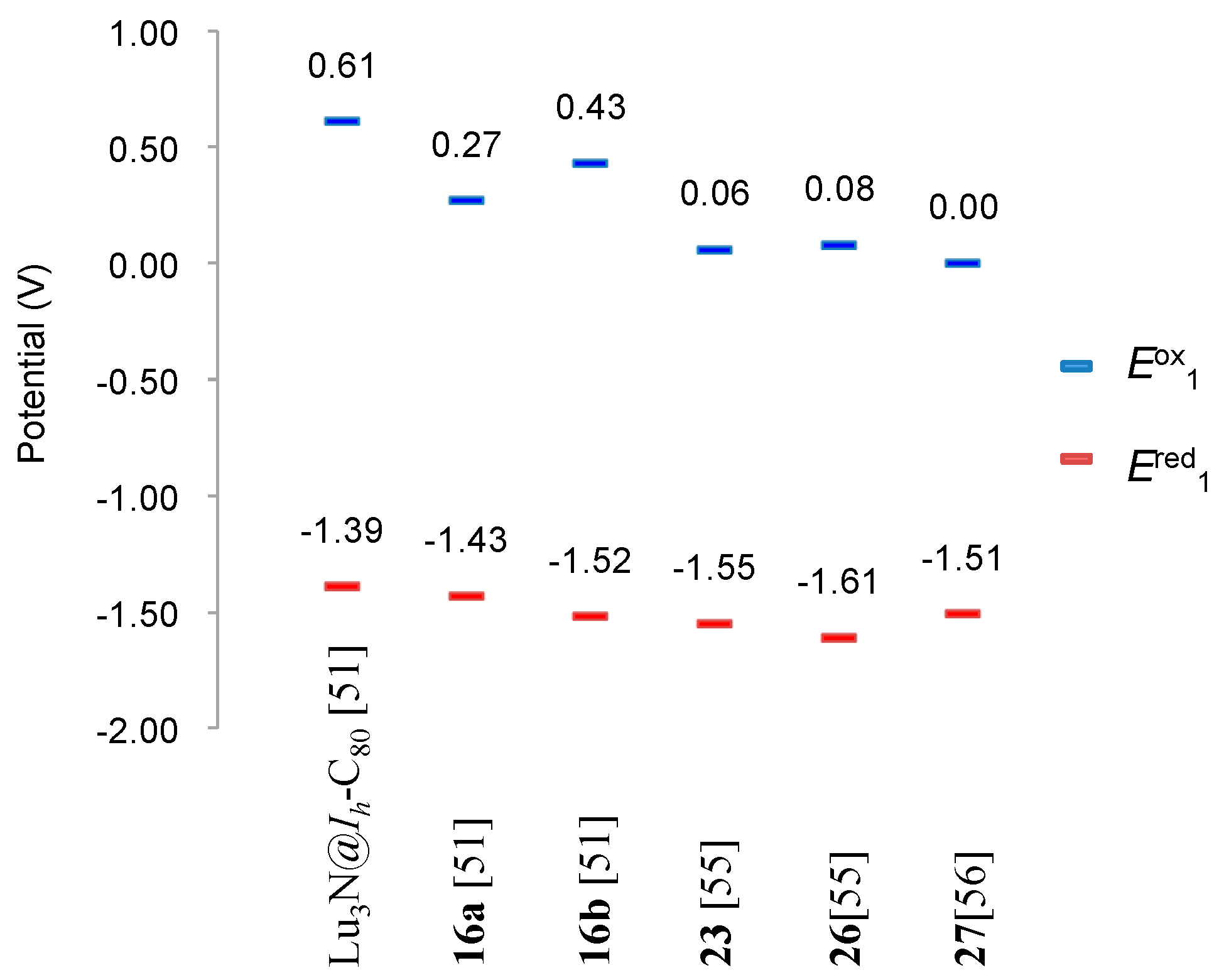
7. Reactions of Lu3N@Ih-C80 with Silylene
8. Reactions of Lu3N@Ih-C80 with Disilirane and Digermirane
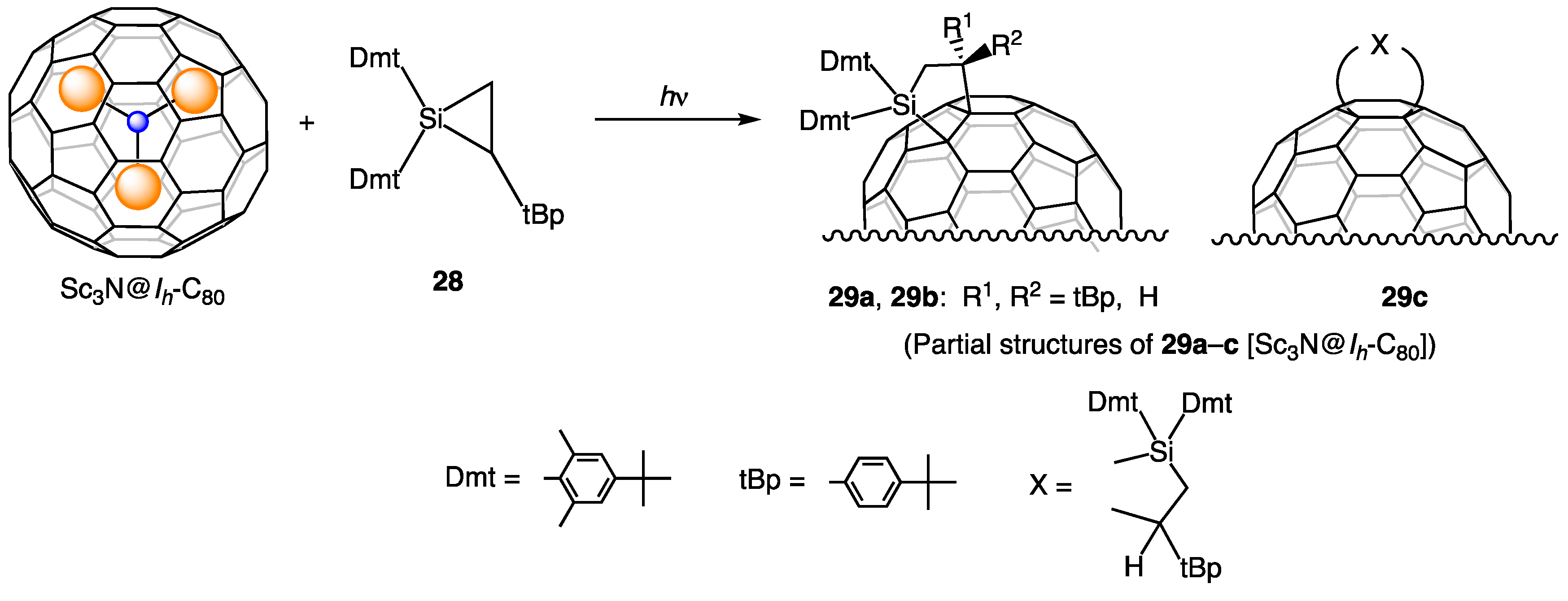
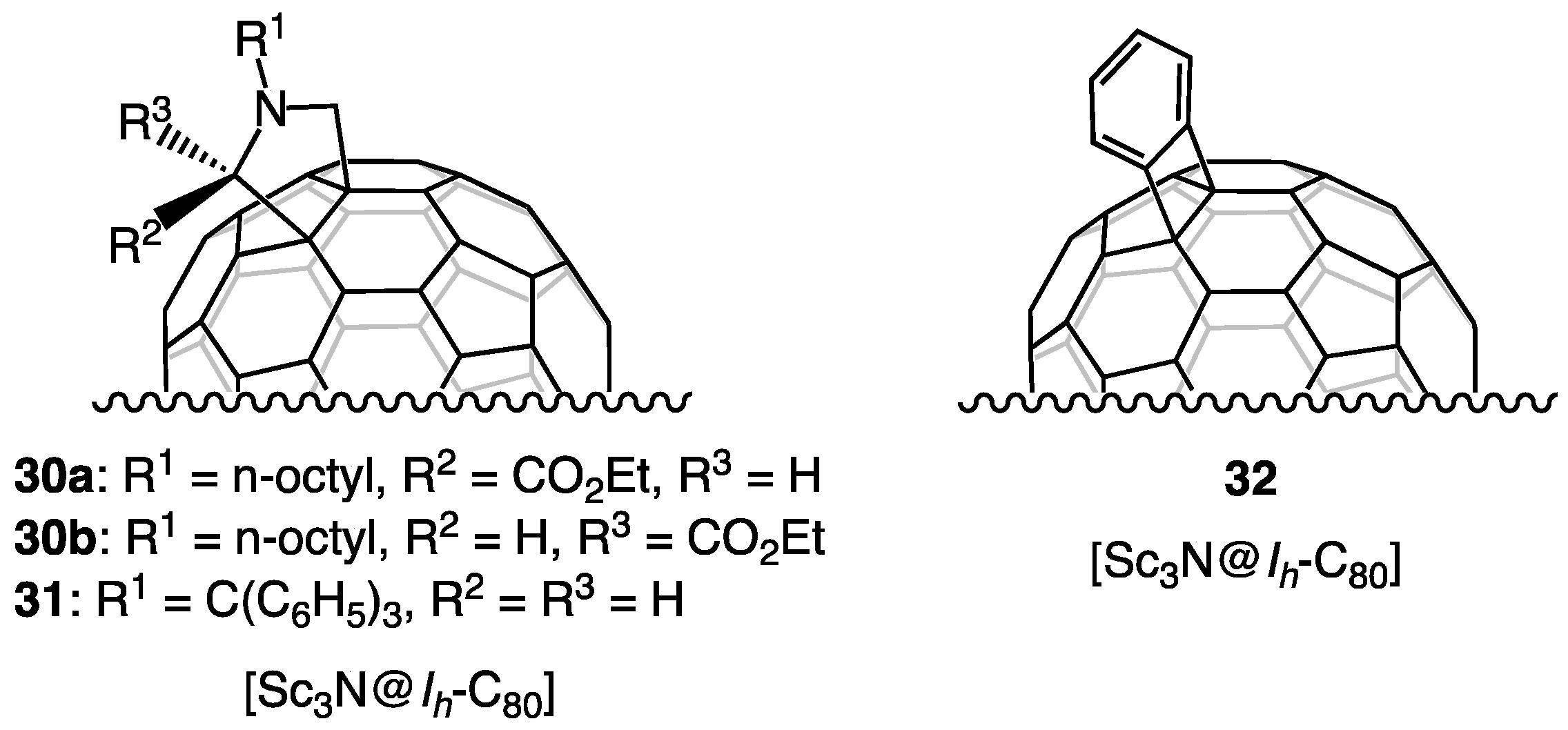
9. Reactions of Sc3N@Ih-C80 with Silirane 28
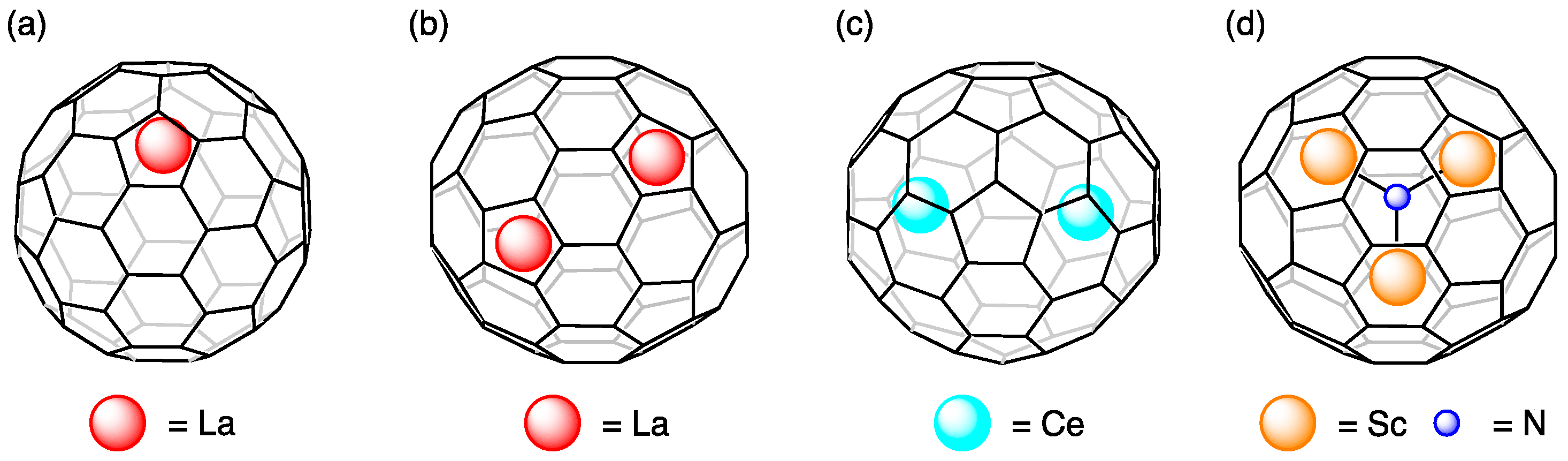
10. Dynamic Behaviors of the Encapsulated Atoms
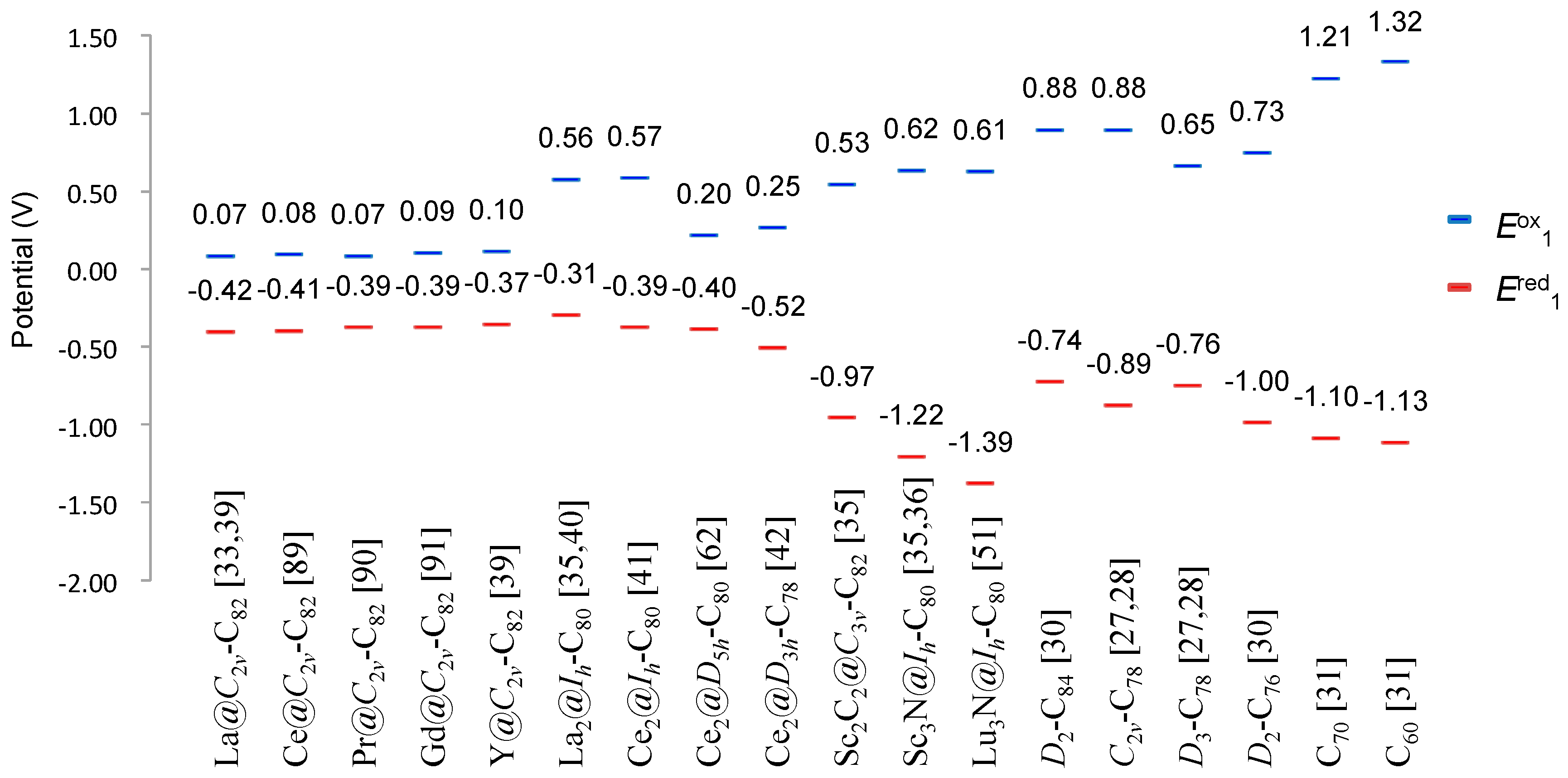
11. Potential Applications
12. Summary
Acknowledgments
Conflicts of Interest
References
- Endofullerenes: A New Family of Carbon Clusters; Akasaka, T.; Nagase, S. (Eds.) Kluwer: Dordrecht, The Netherlands, 2002. [Google Scholar]
- Dunsch, L.; Yang, S. Metal nitride cluster fullerenes: Their current state and future prospects endohedral fullerenes. Small 2007, 8, 1298–1320. [Google Scholar] [CrossRef] [PubMed]
- Chaur, M.N.; Melin, F.; Ortiz, A.L.; Echegoyen, L. Chemical, electrochemical, and structural properties of endohedral metallofullerenes. Angew. Chem. Int. Ed. 2009, 48, 7514–7538. [Google Scholar] [CrossRef] [PubMed]
- Yamada, M.; Akasaka, T.; Nagase, S. Endohedral metal atoms in pristine and functionalized fullerene cages. Acc. Chem. Res. 2010, 43, 92–102. [Google Scholar] [CrossRef] [PubMed]
- Chemistry of Nanocarbons; Akasaka, T.; Wudl, F.; Nagase, S. (Eds.) Wiley: Chichester, UK, 2010. [Google Scholar]
- Maeda, Y.; Tsuchiya, T.; Lu, X.; Takano, Y.; Akasaka, T.; Nagase, S. Current progress on the chemical functionalization and supramolecular chemistry of M@C82. Nanoscale 2011, 3, 2421–2429. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Akasaka, T.; Nagase, S. Chemistry of endohedral metallofullerenes: The role of metals. Chem. Commun. 2011, 47, 5942–5957. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Feng, L.; Akasaka, T.; Nagase, S. Current status and future developments of endohedral metallofullerenes. Chem. Soc. Rev. 2012, 41, 7723–7760. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Stevenson, S.; Dorn, H.C. Trimetallic nitride template endohedral metallofullerenes: Discovery, structural characterization, reactivity, and applications. Acc. Chem. Res. 2013, 46, 1458–1557. [Google Scholar] [CrossRef] [PubMed]
- Rivera-Nazario, D.M.; Pinzón, J.R.; Stevenson, S.; Echegoyen, L.A. Buckyball maracas: Exploring the inside and outside properties of endohedral fullerenes. J. Phys. Org. Chem. 2013, 26, 194–205. [Google Scholar] [CrossRef]
- Yamada, M.; Akasaka, T.; Nagase, S. Carbene additions to fullerenes. Chem. Rev. 2013, 113, 7209–7264. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Akasaka, T.; Nagase, S. Carbide cluster metallofullerenes: Structure, properties, and possible origin. Acc. Chem. Res. 2013, 46, 1627–1635. [Google Scholar] [CrossRef] [PubMed]
- Popov, A.A.; Yang, S.; Dunsch, L. Endohedral fullerenes. Chem. Rev. 2013, 113, 5989–6113. [Google Scholar] [CrossRef] [PubMed]
- Nagase, S. Theory and calculations of molecules containing heavier main group elements and fullerenes encaging transition metals: Interplay with experiment. Bull. Chem. Soc. Jpn. 2014, 87, 167–195. [Google Scholar] [CrossRef]
- Yamada, M.; Akasaka, T. Emergence of highly elaborated π-space and extending its functionality based on nanocarbons: New vistas in the fullerene world. Bull. Chem. Soc. Jpn. 2014, 87, 1289–1314. [Google Scholar] [CrossRef]
- Bock, H.; Solouki, B. Photoelectron spectra of silicon compounds. In The Chemistry of Organosilicon Compounds; Patai, S., Rappoport, Z., Eds.; Wiley: Chichester, UK, 1989; Part 1; Chapter 9; pp. 555–653. [Google Scholar]
- Traylor, T.G.; Hanstein, W.; Berwin, H.J.; Clinton, N.A.; Brown, R.S. Vertical stabilization of cations by neighboring σ bonds. General considerations. J. Am. Chem. Soc. 1971, 93, 5715–5725. [Google Scholar] [CrossRef]
- Hosomi, A. Characteristics in the reactions of allylsilanes and their applications to versatile synthetic equivalents. Acc. Chem. Res. 1988, 21, 200–206. [Google Scholar] [CrossRef]
- Fleming, I.; Barbero, A.; Walter, D. Stereochemical control in organic synthesis using silicon-containing compounds. Chem. Rev. 1997, 97, 2063–2192. [Google Scholar] [CrossRef] [PubMed]
- Akasaka, T.; Ando, W.; Kobayashi, K.; Nagase, S. Reaction of C60 with silylene, the first fullerene silirane derivative. J. Am. Chem. Soc. 1993, 115, 1605–1606. [Google Scholar] [CrossRef]
- Akasaka, T.; Ando, W.; Kobayashi, K.; Nagase, S. Photochemical [2 + 3] cycloaddition of C60 with disilirane. J. Am. Chem. Soc. 1993, 115, 10366–10367. [Google Scholar] [CrossRef]
- Suzuki, T.; Maruyama, Y.; Akasaka, T.; Ando, W.; Kobayashi, K.; Nagase, S. Redox properties of organofullerenes. J. Am. Chem. Soc. 1994, 116, 1359–1363. [Google Scholar] [CrossRef]
- Akasaka, T.; Mitsuhida, E.; Ando, W.; Kobayashi, K.; Nagase, S. Adduct of C70 at the equatorial belt: Photochemical cycloaddition with disilirane. J. Am. Chem. Soc. 1994, 116, 2627–2628. [Google Scholar] [CrossRef]
- Akasaka, T.; Mitsuhida, E.; Ando, W.; Kobayashi, K.; Nagase, S. Regioselective addition of silylene onto [70] fullerene. J. Chem. Soc. Chem. Commun. 1995, 1529–1530. [Google Scholar] [CrossRef]
- Akasaka, T.; Maeda, Y.; Wakahara, T.; Okamura, M.; Fujitsuka, M.; Ito, O.; Kobayashi, K.; Nagase, S.; Kako, M.; Nakadaira, Y.; et al. Novel Metal-free bis-silylation: C60-sensitized reaction of disilirane with benzonitrile. Org. Lett. 1999, 1, 1509–1512. [Google Scholar] [CrossRef]
- Akasaka, T.; Suzuki, T.; Maeda, Y.; Ara, M.; Wakahara, T.; Kobayashi, K.; Nagase, S.; Kako, M.; Nakadaira, Y.; Fujitsuka, M.; et al. Photochemical bissilylation of C60 with disilane. J. Org. Chem. 1999, 64, 566–569. [Google Scholar] [CrossRef]
- Han, A.; Wakahara, T.; Maeda, Y.; Niino, Y.; Akasaka, T.; Yamamoto, K.; Kako, M.; Nakadaira, Y.; Kobayashi, K.; Nagase, S. Photochemical cycloaddition of C78 with disilirane. Chem. Lett. 2001, 974–975. [Google Scholar] [CrossRef]
- Wakahara, T.; Han, A.; Niino, Y.; Maeda, Y.; Akasaka, T.; Suzuki, T.; Yamamoto, K.; Kako, M.; Nakadaira, Y.; Kobayashi, K.; et al. Silylation of higher fullerenes. J. Mater. Chem. 2002, 12, 2061–2064. [Google Scholar] [CrossRef]
- Maeda, Y.; Rahman, G.M.A.; Wakahara, T.; Kako, M.; Okamura, M.; Sato, S.; Akasaka, T.; Kobayashi, K.; Nagase, S. Synthesis and characterization of tetrakis-silylated C60 isomers. J. Org. Chem. 2003, 68, 6791–6794. [Google Scholar] [CrossRef] [PubMed]
- Han, A.H.; Wakahara, T.; Maeda, Y.; Akasaka, T.; Fujitsuka, M.; Ito, O.; Yamamoto, K.; Kako, M.; Kobayashi, K.; Nagase, S. A new method for separating the D3 and C2v isomers of C78. New J. Chem. 2009, 33, 497–500. [Google Scholar] [CrossRef]
- Wakahara, T.; Kako, M.; Maeda, Y.; Akasaka, T.; Kobayashi, K.; Nagase, S. Synthesis and characterization of cyclic silicon compounds of fullerenes. Curr. Org. Chem. 2003, 7, 927–943. [Google Scholar] [CrossRef]
- Nagatsuka, J.; Sugitani, S.; Kako, M.; Nakahodo, T.; Mizorogi, N.; Ishitsuka, M.O.; Maeda, Y.; Tsuchiya, T.; Akasaka, T.; Gao, X.; et al. Photochemical addition of C60 with siliranes: Synthesis and characterization of carbosilylated and hydrosilylated C60 derivatives. J. Am. Chem. Soc. 2010, 132, 12106–12120. [Google Scholar] [CrossRef] [PubMed]
- Akasaka, T.; Kato, T.; Kobayashi, K.; Nagase, S.; Yamamoto, K.; Funasaka, H.; Takahashi, T. Exohedral adducts of La@C82. Nature 1995, 374, 600–601. [Google Scholar] [CrossRef]
- Akasaka, T.; Kato, T.; Nagase, S.; Kobayashi, K.; Yamamoto, K.; Funasaka, H.; Takahashi, T. Chemical derivatization of endohedral metallofullerene La@C82 with digermirane. Tetrahedron 1996, 52, 5015–5020. [Google Scholar] [CrossRef]
- Akasaka, T.; Nagase, S.; Kobayashi, K.; Suzuki, T.; Kato, T.; Kikuchi, K.; Achiba, Y.; Yamamoto, K.; Funasaka, H.; Takahashi, T. Synthesis of the first adducts of the dimetallofullerenes La2@C80 and Sc2@C84 by addition of a disilirane. Angew. Chem. Int. Ed. 1995, 34, 2139–2141. [Google Scholar] [CrossRef]
- Iiduka, Y.; Ikenaga, O.; Sakuraba, A.; Wakahara, T.; Tsuchiya, T.; Maeda, Y.; Nakahodo, T.; Akasaka, T.; Kako, M.; Mizorogi, N.; et al. Chemical reactivity of Sc3N@C80 and La2@C80. J. Am. Chem. Soc. 2005, 127, 9956–9957. [Google Scholar] [CrossRef] [PubMed]
- Wakahara, T.; Iiduka, Y.; Ikenaga, O.; Nakahodo, T.; Sakuraba, A.; Tsuchiya, T.; Maeda, Y.; Kako, M.; Akasaka, T.; Yoza, K.; et al. Characterization of the bis-silylated endofullerene Sc3N@C80. J. Am. Chem. Soc. 2006, 128, 9919–9925. [Google Scholar] [CrossRef] [PubMed]
- Maeda, Y.; Miyashita, J.; Hasegawa, T.; Wakahara, T.; Tsuchiya, T.; Feng, L.; Lian, Y.; Akasaka, T.; Kobayashi, K.; Nagase, S.; et al. Chemical reactivities of the cation and anion of M@C82 (M = Y, La, and Ce). J. Am. Chem. Soc. 2005, 127, 2143–2146. [Google Scholar] [CrossRef] [PubMed]
- Yamada, M.; Feng, L.; Wakahara, T.; Tsuchiya, T.; Maeda, Y.; Lian, Y.; Kako, M.; Akasaka, T.; Kato, T.; Kobayashi, K.; et al. Synthesis and characterization of exohedrally silylated M@C82 (M = Y and La). J. Phys. Chem. B 2005, 109, 6049–6051. [Google Scholar] [CrossRef] [PubMed]
- Wakahara, T.; Yamada, M.; Takahashi, S.; Nakahodo, T.; Tsuchiya, T.; Maeda, Y.; Akasaka, T.; Kako, M.; Yoza, K.; Horn, E.; et al. Two-dimensional hopping motion of encapsulated La atoms in silylated La2@C80. Chem. Commun. 2007, 2680–2682. [Google Scholar] [CrossRef] [PubMed]
- Yamada, M.; Wakahara, T.; Tsuchiya, T.; Maeda, Y.; Akasaka, T.; Kako, M.; Yoza, K.; Horn, E.; Mizorogi, N.; Kobayashi, K.; et al. Positional control of encapsulated atoms inside a fullerene cage by exohedral addition. J. Am. Chem. Soc. 2005, 127, 14570–14571. [Google Scholar] [CrossRef] [PubMed]
- Yamada, M.; Wakahara, T.; Tsuchiya, T.; Maeda, Y.; Kako, M.; Akasaka, T.; Yoza, K.; Horn, E.; Mizorogi, N.; Nagase, S. Location of the metal atoms in Ce2@C78 and its bis-silylated derivative. Chem. Commun. 2008, 558–560. [Google Scholar] [CrossRef] [PubMed]
- Yamada, M.; Minowa, M.; Sato, S.; Kako, M.; Slanina, Z.; Mizorogi, N.; Tsuchiya, T.; Maeda, Y.; Nagase, S.; Akasaka, T. Thermal carbosilylation of endohedral dimetallofullerene La2@Ih-C80 with silirane. J. Am. Chem. Soc. 2010, 132, 17953–17960. [Google Scholar] [CrossRef] [PubMed]
- Yamada, M.; Wakahara, T.; Nakahodo, T.; Tsuchiya, T.; Maeda, Y.; Akasaka, T.; Yoza, K.; Horn, E.; Mizorogi, N.; Nagase, S. Synthesis and structural characterization of endohedral pyrrolidinodimetallofullerene: La2@C80(CH2)2NTrt. J. Am. Chem. Soc. 2006, 128, 1402–1403. [Google Scholar] [CrossRef] [PubMed]
- Yamada, M.; Minowa, M.; Sato, S.; Slanina, Z.; Tsuchiya, T.; Maeda, Y.; Nagase, S.; Akasaka, T. Regioselective cycloaddition of La2@Ih-C80 with tetracyanoethylene oxide: Formation of an endohedral dimetallofullerene adduct featuring enhanced electron-accepting character. J. Am. Chem. Soc. 2011, 133, 3796–3799. [Google Scholar] [CrossRef] [PubMed]
- Shustova, N.B.; Popov, A.A.; Mackey, M.A.; Coumbe, C.E.; Phillips, J.P.; Stevenson, S.; Strauss, S.H.; Boltalina, O.V. Radical trifluoromethylation of Sc3N@C80. J. Am. Chem. Soc. 2007, 129, 11676–11677. [Google Scholar] [CrossRef] [PubMed]
- Shu, C.Y.; Slebodnick, C.; Xu, L.S.; Champion, H.; Fuhrer, T.; Cai, T.; Reid, J.E.; Fu, W.; Harich, K.; Dorn, H.C.; et al. Highly Regioselective derivatization of trimetallic nitride templated endohedral metallofullerenes via a facile photochemical reaction. J. Am. Chem. Soc. 2008, 130, 17755–17760. [Google Scholar] [CrossRef] [PubMed]
- Ross, R.B.; Cardona, C.M.; Guldi, D.M.; Sankaranarayanan, S.G.; Reese, M.O.; Kopidakis, N.; Peet, J.; Walker, B.; Bazan, G.C.; Van Keuren, E.; et al. Endohedral fullerenes for organic photovoltaic devices. Nat. Mater. 2009, 8, 208–212. [Google Scholar] [CrossRef] [PubMed]
- Ross, R.B.; Cardona, C.M.; Swain, F.B.; Guldi, D.M.; Sankaranarayanan, S.G.; Van Keuren, E.; Holloway, B.C.; Drees, M. Tuning conversion efficiency in metallo endohedral fullerene-based organic photovoltaic devices. Adv. Funct. Mater. 2009, 19, 2332–2337. [Google Scholar] [CrossRef]
- Gaspar, P.P.; West, R. Silylenes. In The Chemistry of Organosilicon Compounds; Rappoport, Z., Apeloig, Y., Eds.; Wiley: Chichester, UK, 1998; Volume 2, Part 3; Chapter 43; pp. 2463–2568. [Google Scholar]
- Sato, K.; Kako, M.; Suzuki, M.; Mizorogi, N.; Tsuchiya, T.; Olmstead, M.M.; Balch, A.L.; Akasaka, T.; Nagase, S. Synthesis of silylene-bridged endohedral metallofullerene Lu3N@Ih-C80. J. Am. Chem. Soc. 2012, 134, 16033–16039. [Google Scholar] [CrossRef] [PubMed]
- Pinzón, J.R.; Zuo, T.; Echegoyen, L. Synthesis and electrochemical studies of Bingel–Hirsch derivatives of M3N@Ih-C80 (M = Sc, Lu). Chem. Eur. J. 2010, 16, 4864–4869. [Google Scholar] [CrossRef] [PubMed]
- Li, F.F.; Rodríguez-Fortea, A.; Poblet, J.M.; Echegoyen, L. Reactivity of metallic nitride endohedral metallofullerene anions: Electrochemical synthesis of a Lu3N@Ih-C80 derivative. J. Am. Chem. Soc. 2011, 133, 2760–2765. [Google Scholar] [CrossRef] [PubMed]
- Yamada, M.; Abe, T.; Saito, C.; Yamazaki, T.; Sato, S.; Mizorogi, N.; Slanina, Z.; Uhlík, F.; Suzuki, M.; Maeda, Y.; et al. Adamantylidene addition to M3N@Ih-C80 (M = Sc, Lu) and Sc3N@D5h-C80: Synthesis and crystallographic characterization of the [5,6]-open and [6,6]-open adducts. Chem. Eur. J. 2017, 23, 6552–6561. [Google Scholar] [CrossRef] [PubMed]
- Sato, K.; Kako, M.; Mizorogi, N.; Tsuchiya, T.; Akasaka, T.; Nagase, S. Bis-silylation of Lu3N@Ih-C80: Considerable variation in the electronic structures. Org. Lett. 2012, 14, 5908–5911. [Google Scholar] [CrossRef] [PubMed]
- Kako, M.; Miyabe, M.; Sato, K.; Suzuki, M.; Mizorogi, N.; Wang, W.-W.; Yamada, M.; Maeda, Y.; Olmstead, M.M.; Balch, A.L.; et al. Preparation, structural determination, and characterization of electronic properties of bis-silylated and bis-germylated Lu3N@Ih-C80. Chem. Eur. J. 2015, 21, 16411–16420. [Google Scholar] [CrossRef] [PubMed]
- Kako, M.; Sugiura, T.; Akasaka, T. Photochemical addition of silirane to endohedral metallofullerene: Electronic properties of carbosilylated Sc3N@Ih-C80. Phosphorus Sulfur Silicon Relat. Elem. 2016, 191, 201–206. [Google Scholar] [CrossRef]
- Maeda, Y.; Kimura, M.; Ueda, C.; Yamada, M.; Kikuchi, T.; Suzuki, M.; Wang, W.; Mizorogi, N.; Karousis, N.; Tagmatarchis, N.; et al. Isolation and characterization of [5,6]-pyrrolidino-Sc3N@Ih-C80 diastereomers. Chem. Commun. 2014, 50, 12552–12555. [Google Scholar] [CrossRef] [PubMed]
- Cai, T.; Slebodnick, C.; Xu, L.; Harich, K.; Glass, T.E.; Chancellor, C.; Fettinger, J.C.; Olmstead, M.M.; Balch, A.L.; Gibson, H.W.; et al. A pirouette on a metallofullerene sphere: Interconversion of isomers of N-tritylpyrrolidino Ih Sc3N@C80. J. Am. Chem. Soc. 2006, 128, 6486–6492. [Google Scholar] [CrossRef] [PubMed]
- Chen, N.; Pinzón, J.R.; Echegoyen, L. Influence of the encapsulated clusters on the electrochemical behaviour of endohedral fullerene derivatives: Comparative study of N-tritylpyrrolidino derivatives of Sc3N@Ih-C80 and Lu3N@Ih-C80. ChemPhysChem 2011, 12, 1422–1425. [Google Scholar] [CrossRef] [PubMed]
- Li, F.F.; Pinzón, J.R.; Mercado, B.Q.; Olmstead, M.M.; Balch, A.L.; Echegoyen, L. [2 + 2] Cycloaddition reaction to Sc3N@Ih-C80. The formation of very stable [5,6]-and [6,6]-adducts. J. Am. Chem. Soc. 2011, 133, 1563–1571. [Google Scholar] [CrossRef] [PubMed]
- Yamada, M.; Mizorogi, N.; Tsuchiya, T.; Akasaka, T.; Nagase, S. Synthesis and characterization of the D5h isomer of the endohedral dimetallofullerene Ce2@C80: Two-dimensional circulation of encapsulated metal atoms inside a fullerene cage. Chem. Eur. J. 2009, 15, 9486–9493. [Google Scholar] [CrossRef] [PubMed]
- Akasaka, T.; Nagase, S.; Kobayashi, K.; Wälchli, M.; Yamamoto, K.; Funasaka, H.; Kako, M.; Hoshino, T.; Erata, T. First evidence for circular motion of metal atoms in endohedral dimetallofullerenes. Angew. Chem. Int. Ed. Engl. 1997, 36, 1643–1645. [Google Scholar] [CrossRef]
- Feng, L.; Suzuki, M.; Mizorogi, N.; Lu, X.; Yamada, M.; Akasaka, T.; Nagase, S. Mapping the metal positions inside spherical C80 Cages: Crystallographic and theoretical studies of Ce2@D5h-C80 and Ce2@Ih-C80. Chem. Eur. J. 2013, 19, 988–993. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, M.; Mizorogi, N.; Yang, T.; Uhlík, F.; Slanina, Z.; Zhao, X.; Yamada, M.; Maeda, Y.; Hasegawa, T.; Nagase, S.; et al. La2@Cs(17490)-C76: A new non-IPR dimetallic metallofullerene featuring unexpectedly weak metal–pentalene interactions. Chem. Eur. J. 2013, 19, 17125–17130. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, K.; Nagase, S.; Maeda, Y.; Wakahara, T.; Akasaka, T. La2@C80: Is the circular motion of two La atoms controllable by exohedral addition? Chem. Phys. Lett. 2003, 374, 562–566. [Google Scholar] [CrossRef]
- Miller, R.D.; Michil, J. Polysilane high polymers. Chem. Rev. 1989, 89, 1359–1410. [Google Scholar] [CrossRef]
- West, R. Polysilanes. In The Chemistry of Organosilicon Compounds; Patai, S., Rappoport, Z., Eds.; Wiley: Chichester, UK, 1989; Part 2; Chapter 19; pp. 1207–1240. [Google Scholar]
- Fujino, M. Photoconductivity in organopolysilanes. Chem. Phys. Lett. 1987, 136, 451–453. [Google Scholar] [CrossRef]
- Wang, Y.; West, R.; Yuan, C.-H. Fullerene-doped polysilane photoconductor. J. Am. Chem. Soc. 1993, 115, 3844–3855. [Google Scholar] [CrossRef]
- Kepler, R.G.; Cahill, P.A. Photoinduced charge transfer and charge carrier generation in polysilane films containing C60 molecules. Appl. Phys. Lett. 1933, 63, 1552–1554. [Google Scholar] [CrossRef]
- Brynda, E.; Nešpůrek, S.; Schnabel, W. Photoconductivity of doped poly(methylphenylsilane). Chem. Phys. 1993, 175, 459–465. [Google Scholar] [CrossRef]
- Watanabe, A.; Tsutsumi, Y.; Matsuda, M. Effect of Si-skeleton dimensionality on optical and electrical properties of poly(methylpenylsilylene) and poly(phenylsilyne). Synth. Met. 1995, 74, 191–196. [Google Scholar] [CrossRef]
- Seki, S.; Shibata, H.; Yoshida, Y.; Ishigure, K.; Tagawa, S. Radiation effects on hole drift mobility in polysilanes. Radiat. Phys. Chem. 1997, 49, 389–393. [Google Scholar] [CrossRef]
- Furukawa, K.; Ebata, K. Preparation and single molecule structure of electroactive polysilane end-grafted on a crystalline silicon surface. Appl. Phys. Lett. 2000, 77, 4289–4291. [Google Scholar] [CrossRef]
- Nešpůrek, S.; Herden, V.; Kunst, M.; Schnabel, W. Microwave photoconductivity and polaron formation in poly[methyl(phenyl)silylene]. Synth. Met. 2000, 109, 309–313. [Google Scholar] [CrossRef]
- Grozema, F.C.; Siebbeles, L.D.A.; Warman, J.M.; Seki, S.; Tagawa, S.; Scherf, U. Hole conduction along molecular wires: σ-bonded silicon versus π-bond-conjugated carbon. Adv. Mater. 2002, 14, 228–231. [Google Scholar] [CrossRef]
- Seki, S.; Koizumi, Y.; Kawaguchi, T.; Habara, H.; Tagawa, S. Dynamics of positive charge carriers on Si chains of polysilanes. J. Am. Chem. Soc. 2004, 126, 3521–3528. [Google Scholar] [CrossRef] [PubMed]
- Seki, S.; Sakurai, Y.; Maeda, K.; Kunimi, Y.; Tagawa, S. Negative resist material based on polysilanes for electron beam and X-Ray lithographies. Jpn. J. Appl. Phys. 2000, 39, 4225–4230. [Google Scholar] [CrossRef]
- Suzuki, H.; Hoshino, S.; Furukawa, K.; Ebata, K.; Yuan, C.-H.; Bleyl, I. Polysilane light-emitting diodes. Polym. Adv. Technol. 2000, 11, 460–467. [Google Scholar] [CrossRef]
- Watanabe, A.; Ito, O. Photoinduced electron transfer between C60 and polysilane studied by laser flash photolysis in the near-IR region. J. Phys. Chem. 1994, 98, 7736–7740. [Google Scholar] [CrossRef]
- Acharya, A.; Seki, S.; Saeki, A.; Koizumi, Y.; Tagawa, S. Study of transport properties in fullerene-doped polysilane films using flash photolysis time-resolved microwave technique. Chem. Phys. Lett. 2005, 404, 356–360. [Google Scholar] [CrossRef]
- Watanabe, A.; Ito, O.; Mochida, K. Photoinduced electron transfer from polygermane to C60 studied by laser flash photolysis. Organometallics 1995, 14, 4281–4285. [Google Scholar] [CrossRef]
- Lee, J.; Seoul, C.; Park, J.; Youk, J. Fullerene/poly(methylphenylsilane) (PMPS) organic photovoltaic cells. Synth. Met. 2004, 145, 11–14. [Google Scholar] [CrossRef]
- Rybak, A.; Jung, J.; Ciesielski, W.; Ulanski, J. Photovoltaic effect in novel polysilane with phenothiazine rings and its blends with fullerene. Mater. Sci. Poland 2006, 24, 527–534. [Google Scholar]
- Yoshida, K.; Oku, T.; Suzuki, A.; Akiyama, T.; Tokumitsu, K.; Nakamura, M.; Yamada, M. Fabrication and characterization of polysilane: PCBM bulk heterojunction solar cells. Cent. Eur. J. Eng. 2013, 3, 165–169. [Google Scholar] [CrossRef]
- Oku, T.; Nakagawa, J.; Iwase, M.; Kawashima, A.; Yoshida, K.; Suzuki, A.; Akiyama, T.; Tokumitsu, K.; Yamada, M.; Nakamura, M. Microstructures and photovoltaic properties of polysilane-based solar cells. Jpn. J. Appl. Phys. 2013, 52, 04CR07. [Google Scholar] [CrossRef]
- Wakahara, T.; Kondo, T.; Okamura, M.; Akasaka, T.; Hamada, Y.; Suzuki, T.; Kako, M.; Nakadaira, Y. Photochemical bis-silylation of C60: Synthesis of a novel C60 main chain polysilane. J. Organomet. Chem. 2000, 611, 78–84. [Google Scholar] [CrossRef]
- Wakahara, T.; Kobayashi, J.; Yamada, M.; Maeda, Y.; Tsuchiya, T.; Okamura, M.; Akasaka, T.; Wälchli, M.; Kobayashi, K.; Nagase, S.; et al. Characterization of Ce@C82 and its anion. J. Am. Chem. Soc. 2004, 126, 4883–4887. [Google Scholar] [CrossRef] [PubMed]
- Akasaka, T.; Okubo, S.; Kondo, M.; Maeda, Y.; Wakahara, T.; Kato, T.; Suzuki, T.; Yamamoto, K.; Kobayashi, K.; Nagase, S. Isolation and characterization of two Pr@C82 isomers. Chem. Phys. Lett. 2000, 319, 153–156. [Google Scholar] [CrossRef]
- Akasaka, T.; Nagase, S.; Kobayashi, K.; Suzuki, T.; Kato, T.; Yamamoto, K.; Funasaka, H.; Takahashi, T. Exohedral derivatization of an endohedral metallofullerene Gd@C82. J. Chem. Soc. Chem. Commun. 1995, 1343–1344. [Google Scholar] [CrossRef]



© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kako, M.; Nagase, S.; Akasaka, T. Functionalization of Endohedral Metallofullerenes with Reactive Silicon and Germanium Compounds. Molecules 2017, 22, 1179. https://doi.org/10.3390/molecules22071179
Kako M, Nagase S, Akasaka T. Functionalization of Endohedral Metallofullerenes with Reactive Silicon and Germanium Compounds. Molecules. 2017; 22(7):1179. https://doi.org/10.3390/molecules22071179
Chicago/Turabian StyleKako, Masahiro, Shigeru Nagase, and Takeshi Akasaka. 2017. "Functionalization of Endohedral Metallofullerenes with Reactive Silicon and Germanium Compounds" Molecules 22, no. 7: 1179. https://doi.org/10.3390/molecules22071179




