Synthesis and Structure of the Inclusion Complex {NdQ[5]K@Q[10](H2O)4}·4NO3·20H2O
Abstract
:1. Introduction
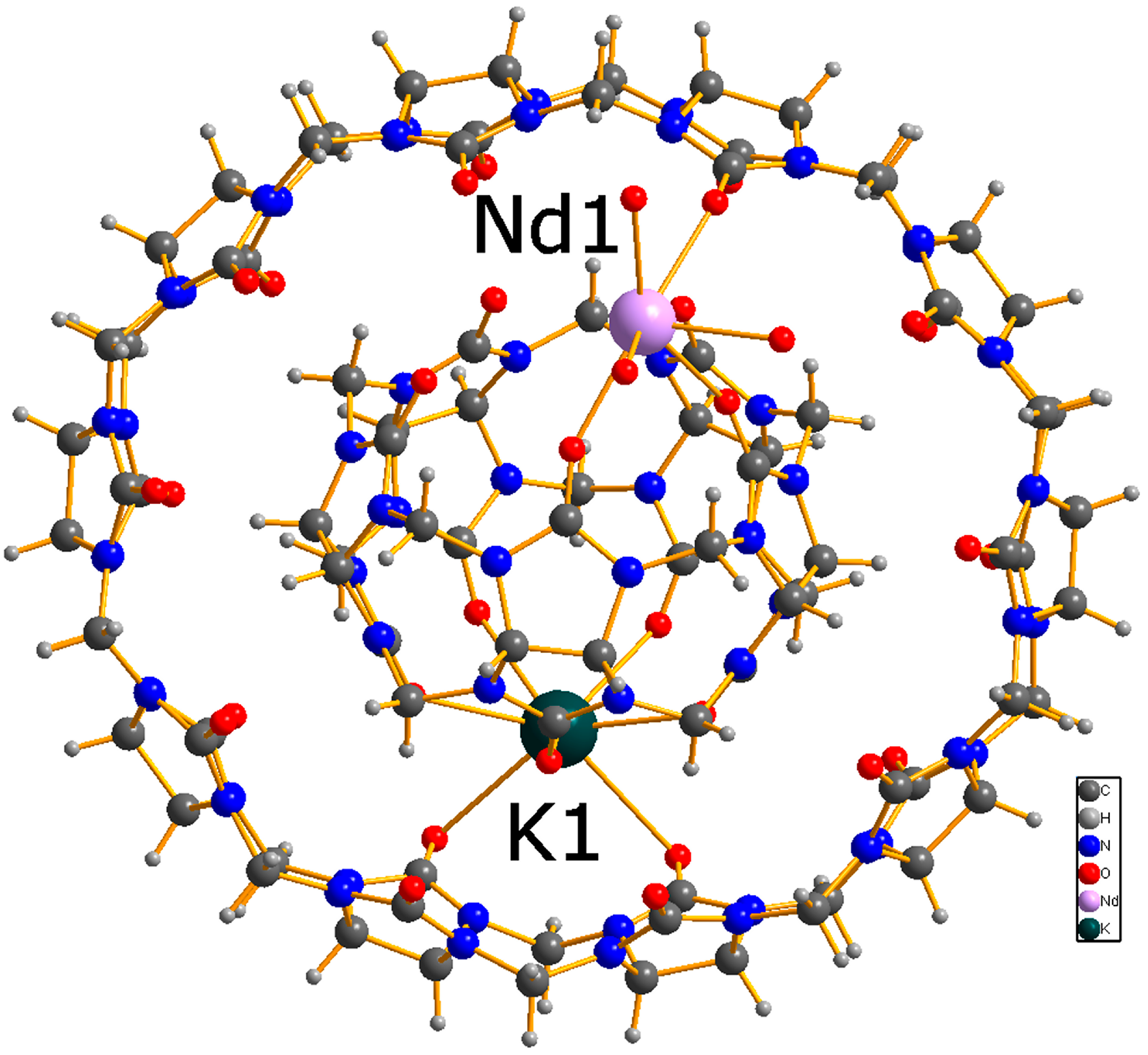
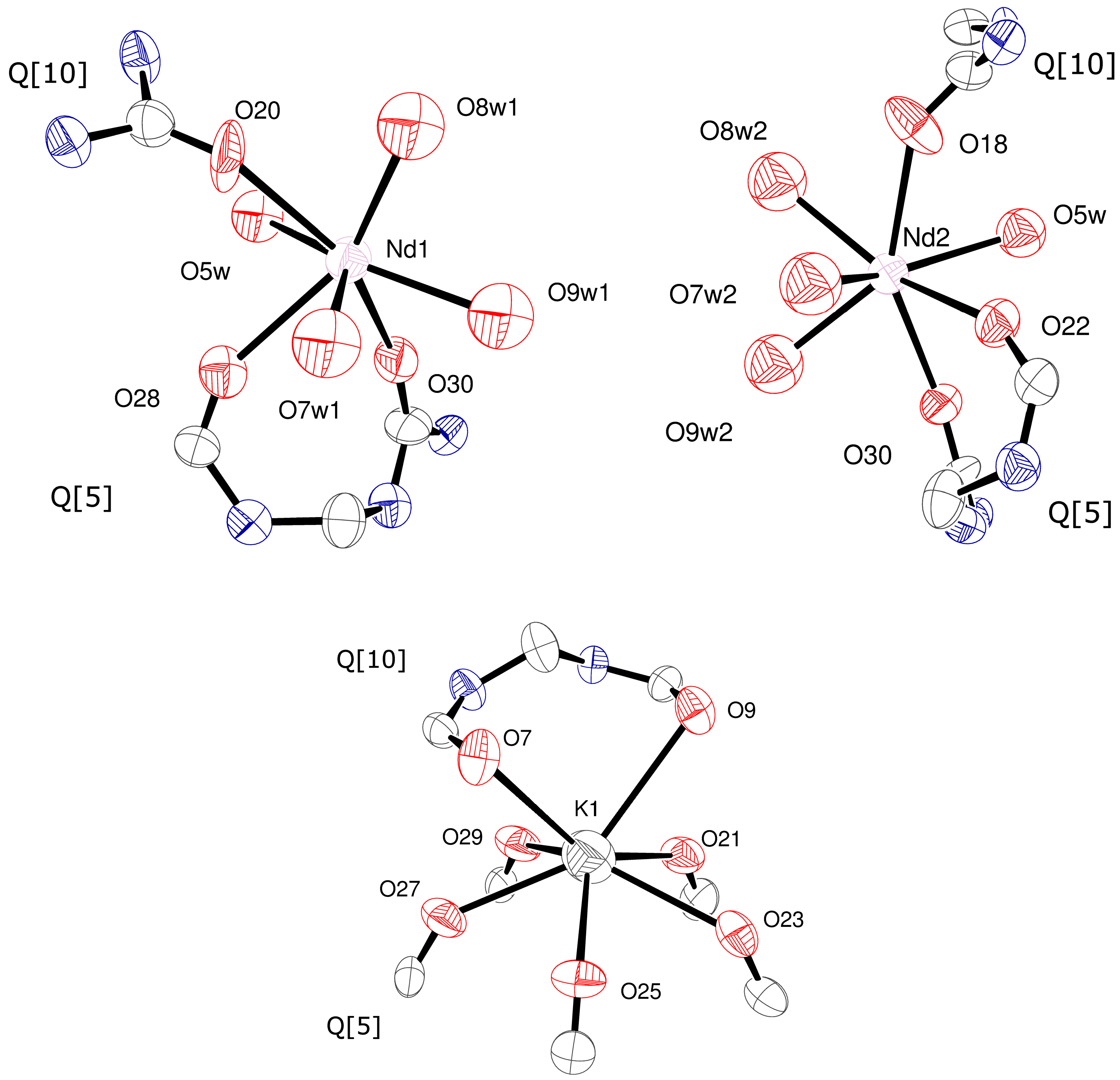
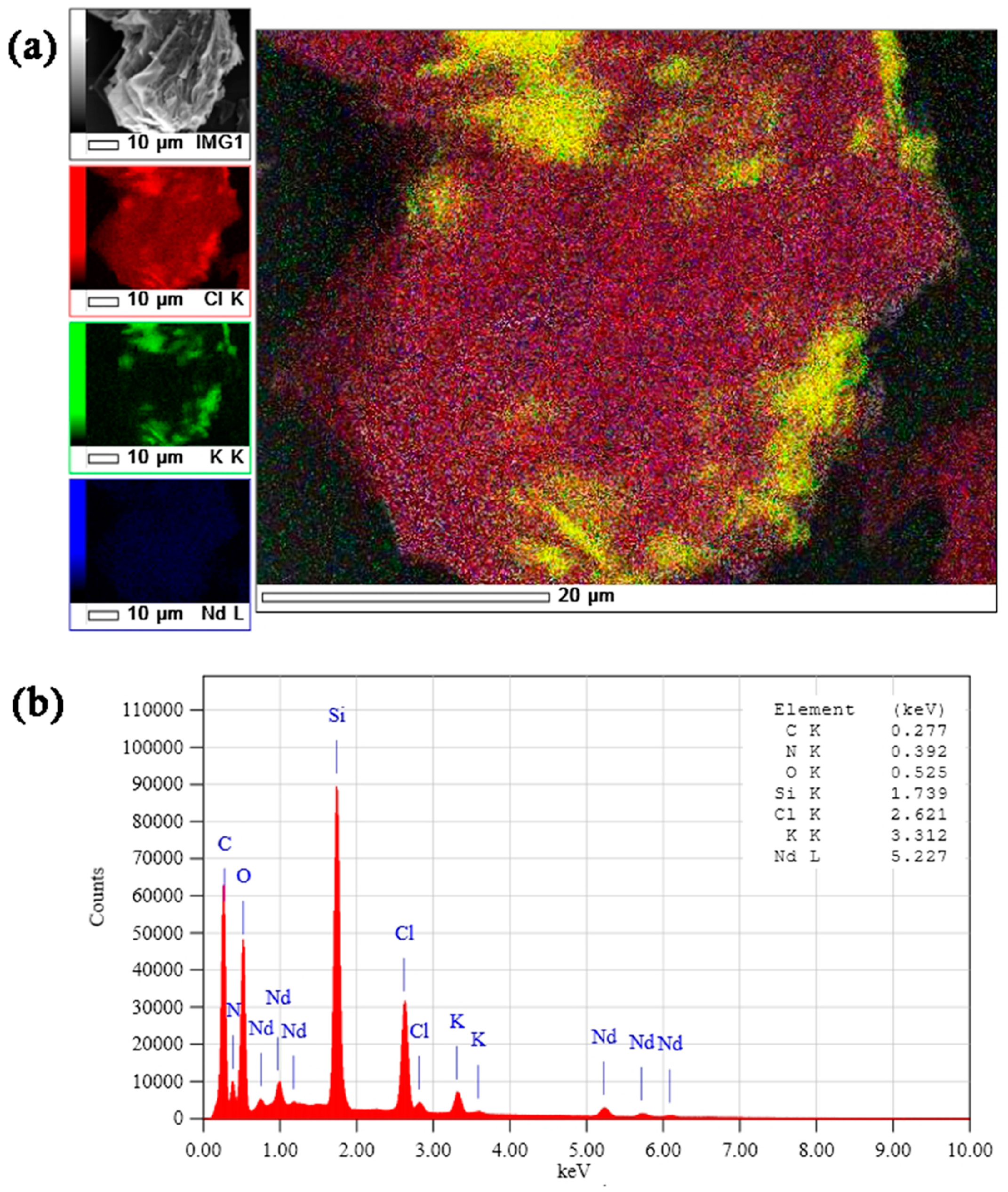
2. Results and Discussion
3. Materials and Methods
3.1. General
3.2. Measurements
3.3. Crystal Structure Determination
3.4. Synthesis of Complex 1
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Assaf, K.I.; Nau, W.M. Cucurbit[n]urils: From synthesis to high-affinity binding and catalysis. Chem. Soc. Rev. 2015, 44, 394–418. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.; Wang, F.; Zheng, B.; Huang, F. Stimuli-responsive supramolecular polymeric materials. Chem. Soc. Rev. 2012, 41, 6042–6065. [Google Scholar] [CrossRef] [PubMed]
- Ni, X.-L.; Xiao, X.; Cong, H.; Liang, L.-L.; Cheng, K.; Cheng, X.-J.; Ji, N.-N.; Zhu, Q.-J.; Xue, S.-F.; Tao, Z. Cucurbit[n]uril-based coordination chemistry: From simple coordination complexes to novel poly-dimensional coordination polymers. Chem. Soc. Rev. 2013, 42, 9480–9508. [Google Scholar] [CrossRef] [PubMed]
- Yu, G.; Jie, K.; Huang, F. Supramolecular amphiphiles based on host-guest molecular recognition motifs. Chem. Rev. 2015, 115, 7240–7303. [Google Scholar] [CrossRef] [PubMed]
- Ni, X.-L.; Xue, S.-F.; Tao, Z.; Zhu, Q.-J.; Lindoy, L.F.; Wei, G. Advances in the lanthanide metallosupramolecular chemistry of the cucurbit[n]urils. Coord. Chem. Rev. 2015, 287, 89–113. [Google Scholar] [CrossRef]
- Bai, Q.; Zhang, S.; Chen, H.; Sun, T.; Redshaw, C.; Zhang, J.-X.; Ni, X.-L.; Wei, G.; Tao, Z. Alkyl Substituted Cucurbit[6]uril Assisted Competitive Fluorescence Recognition of Lysine and Methionine in Aqueous Solution. Chem. Sel. 2017, 2, 2569–2573. [Google Scholar] [CrossRef]
- Day, A.I.; Blanch, R.J.; Arnold, A.P.; Lorenzo, S.; Lewis, G.R.; Dance, I. A Cucurbituril-based Gyroscane: A new Supramolecular Form. Angew. Chem. Int. Ed. 2002, 41, 275–277. [Google Scholar] [CrossRef]
- Isaacs, L.; Park, S.-K.; Liu, S.; Young, H.K.; Selvapalam, N.; Kim, Y.; Kim, H.; Zavalij, Y.; Kim, G.-H.; Lee, H.-S.; et al. The Inverted Cucurbit[n]uril family. J. Am. Chem. Soc. 2005, 127, 18000–18001. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.-X.; Lin, R.-L.; Long, L.-S.; Huang, R.-B.; Zheng, L.-S. A novel inclusion complex form between Q[10] host and Q[5] guest stabilized by potassium ion coordination. Inorg. Chem. Commun. 2008, 11, 1085–1087. [Google Scholar] [CrossRef]
- Day, A.I.; Arnold, A.P.; Blanch, R.J. Cucurbiturils and Methods for Binding Gases and Volatiles Using Cucurbiturils. U.S. Patent US006869466B2, 22 March 2005. [Google Scholar]
- Kim, J.; Jung, I.-S.; Kim, S.-Y.; Lee, E.; Kang, J.-K.; Sakamoto, S.; Yamaguchi, K.; Kim, K. New Cucurituril Homologues: Syntheses, Isolation, Characterization, and X-ray Crystal Structures of Cucurit[n]uril (n = 5, 7 and 8). J. Am. Chem. Soc. 2000, 122, 540–541. [Google Scholar] [CrossRef]
- Liu, S.; Yang, X.; Gong, W. Method for Preparing High-Purity Cucurbit[10]uril. CN Patent CN104557951, 19 April 2015. [Google Scholar]
- Mainicheva, E.A.; Tripolskaya, A.A.; Gerasko, O.A.; Naumov, D.Y.; Fedin, V.P. Synthesis and crystal structures of PrIII and NdIII complexes with the macrocyclic cavitand cucurbit[6]uril. Russ. Chem. Bull. 2006, 55, 1566–1573. [Google Scholar] [CrossRef]
- Thuéry, P. Second-sphere tethering of rare-earth ions to cucurbit[6]uril by iminodiacetic acid involving carboxylic group encapsulation. Inorg. Chem. 2010, 49, 9078–9085. [Google Scholar] [CrossRef] [PubMed]
- Thuéry, P. l-Cysteine as a chiral linker in lanthanide-cucurbit[6]uril one-dimensional assemblies. Inorg. Chem. 2011, 50, 10558–10560. [Google Scholar] [CrossRef] [PubMed]
- Thuéry, P. Lanthanide ion complexes with 2-, 3- or 4-sulfobenzoate and cucurbit[6]uril. Cryst. Growth Des. 2012, 12, 1632–1640. [Google Scholar] [CrossRef]
- Liang, L.-L.; Ni, X.-L.; Zhao, Y.; Chen, K.; Xiao, X.; Zhang, Y.-Q.; Redshaw, C.; Zhu, Q.-J.; Xue, S.-F.; Tao, Z. Construction of cucurbit[7]uril based tubular nanochannels incorporating associated [CdCl4]2− and lanthanide ions. Inorg. Chem. 2013, 52, 1909–1915. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Liang, L.-L.; Chen, K.; Zhang, T.; Xiao, X.; Zhang, Y.-Q.; Tao, Z.; Xue, S.-F.; Zhu, Q.-J. Inorganic anion-aided coordination of lanthanide metal ions to cucurbituril and supramolecular self-assembly: Potential applications in the separation of light lanthanides. CrystEngComm 2013, 15, 7987–7998. [Google Scholar] [CrossRef]
- Cheng, X.-J.; Ji, N.-N.; Zhao, Y.; Liang, L.-L.; Xiao, X.; Zhang, Y.-Q.; Xue, S.-F.; Zhu, Q.-J.; Tao, Z. [CdCl4]2− anion-induced coordination of Ln3+ to cucurbit[8]uril and the formation of supramolecular self-assemblies: Potential application in isolation of light lanthanides. CrystEngComm 2014, 16, 144–147. [Google Scholar] [CrossRef]
- Zhang, D.-Q.; Zhang, Y.-Q.; Xue, S.-F.; Tao, Z.; Xiao, X.; Zhu, Q.-J. Coordination of lanthanides in the inverted cucurbituril supramolecular assemblies formed in the presence of tetrachloride zincate anion: Potential applications for isolation of lighter lanthanides. Polyhedron 2015, 99, 147–155. [Google Scholar] [CrossRef]
- Yang, B.; Gao, Z.-Z.; Lu, J.-H.; Zhu, Q.-J.; Xue, S.-F.; Tao, Z.; Prior, T.J.; Redshaw, C.; Wei, G.; Xiao, X. Interaction of a symmetrical α,α′,δ,δ′-tetramethylcucurbit[6]uril with Ln3+: Potential applications for isolation of lanthanides. CrystEngComm 2016, 18, 5028–5035. [Google Scholar] [CrossRef]
- Liu, J.; Gu, Y.; Lin, R.; Yao, W.; Liu, X.; Zhu, J. Anion encapsulation by Ln(III)/K(I) heterobismetal-capped cucurbit[5]uril. Supramol. Chem. 2010, 22, 130–134. [Google Scholar] [CrossRef]
- Liang, L.-L.; Chen, K.; Feng, X.; Zhang, Y.-Q.; Zhu, Q.-J.; Xue, S.-F.; Tao, Z. Three cucurbit[5]uril-based heterometallic complexes. J. Mol. Struct. 2011, 1006, 87–90. [Google Scholar] [CrossRef]
- Han, B.-X.; Wang, C.-Z.; Chen, K.; Xiao, X.; Tao, Z.; Xue, S.-F.; Zhang, Y.-Q.; Zhu, Q.-J. Coordination and supramolecular assemblies of K+/Ln3+ to perhydroxycucurbit[5]uril in the presence of [PMo12O40]3−: Potential application in isolation of light lanthanides. CrystEngComm 2014, 16, 1615–1619. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Cryst. 2015, C71, 3–8. [Google Scholar]
- Sheldrick, G.M. A short history of SHELX. Acta Cryst. Sect. A 2008, 64, 112–122. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compound are not available from the authors. |

| Compound | 1 |
|---|---|
| Empirical formula | C90 H90 K N64 Nd O66 |
| Formula weight | 3313.58 |
| Crystal system | Orthorhombic |
| Space group | P c a 21 |
| a, Å | 18.998(7) |
| b, Å | 29.043(10) |
| c, Å | 26.562(9) |
| α, deg | 90 |
| β, deg | 90 |
| γ, deg | 90 |
| V, Å3 | 14656(9) |
| Z | 4 |
| Dcalcd, g cm−3 | 1.449 |
| T, K | 293(2) |
| μ, mm−1 | 0.492 |
| Unique reflns | 14709 |
| Obsd reflns | 10294 |
| Params | 1774 |
| Rint | 0.1265 |
| R[I > 2σ(I)] a | 0.0829 |
| wR[I > 2σ(I)] b | 0.2132 |
| R(all data) | 0.1178 |
| wR(all data) | 0.2466 |
| GOF on F2 | 1.020 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, L.X.; Kan, J.L.; Cong, H.; Prior, T.J.; Tao, Z.; Xiao, X.; Redshaw, C. Synthesis and Structure of the Inclusion Complex {NdQ[5]K@Q[10](H2O)4}·4NO3·20H2O. Molecules 2017, 22, 1147. https://doi.org/10.3390/molecules22071147
Chen LX, Kan JL, Cong H, Prior TJ, Tao Z, Xiao X, Redshaw C. Synthesis and Structure of the Inclusion Complex {NdQ[5]K@Q[10](H2O)4}·4NO3·20H2O. Molecules. 2017; 22(7):1147. https://doi.org/10.3390/molecules22071147
Chicago/Turabian StyleChen, Li Xia, Jing Lan Kan, Hang Cong, Timothy J. Prior, Zhu Tao, Xin Xiao, and Carl Redshaw. 2017. "Synthesis and Structure of the Inclusion Complex {NdQ[5]K@Q[10](H2O)4}·4NO3·20H2O" Molecules 22, no. 7: 1147. https://doi.org/10.3390/molecules22071147






