Substituted Caffeic and Ferulic Acid Phenethyl Esters: Synthesis, Leukotrienes Biosynthesis Inhibition, and Cytotoxic Activity
Abstract
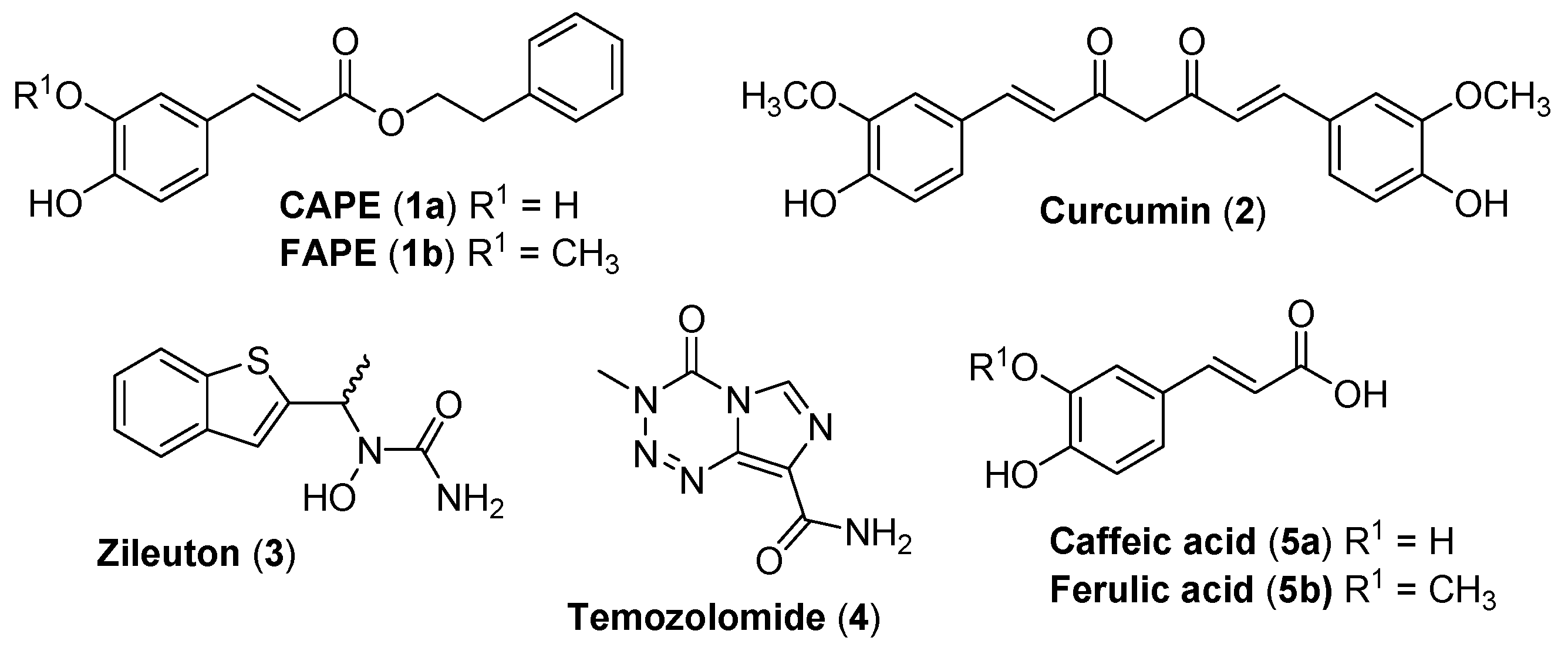
:1. Introduction
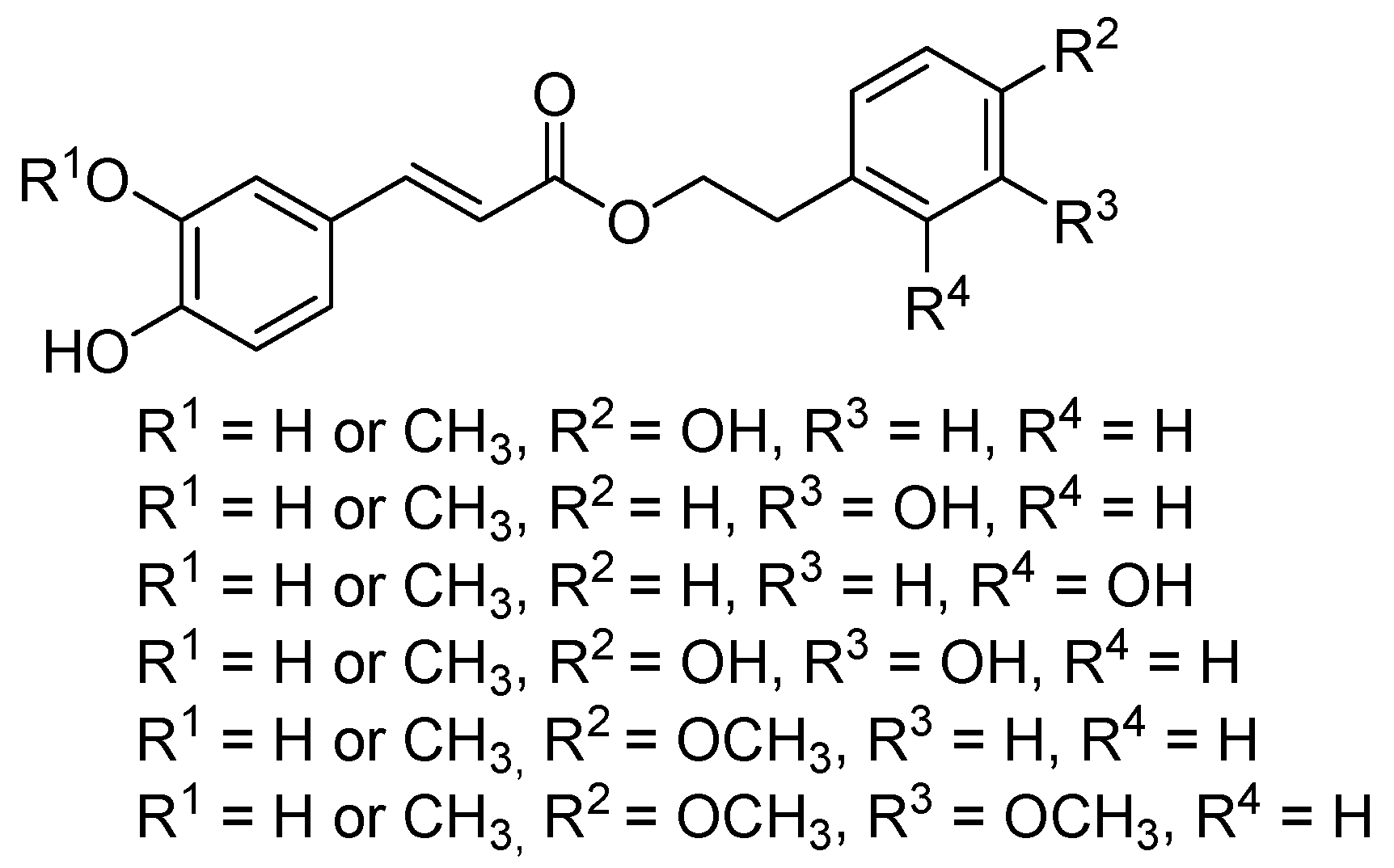
2. Results and Discussion
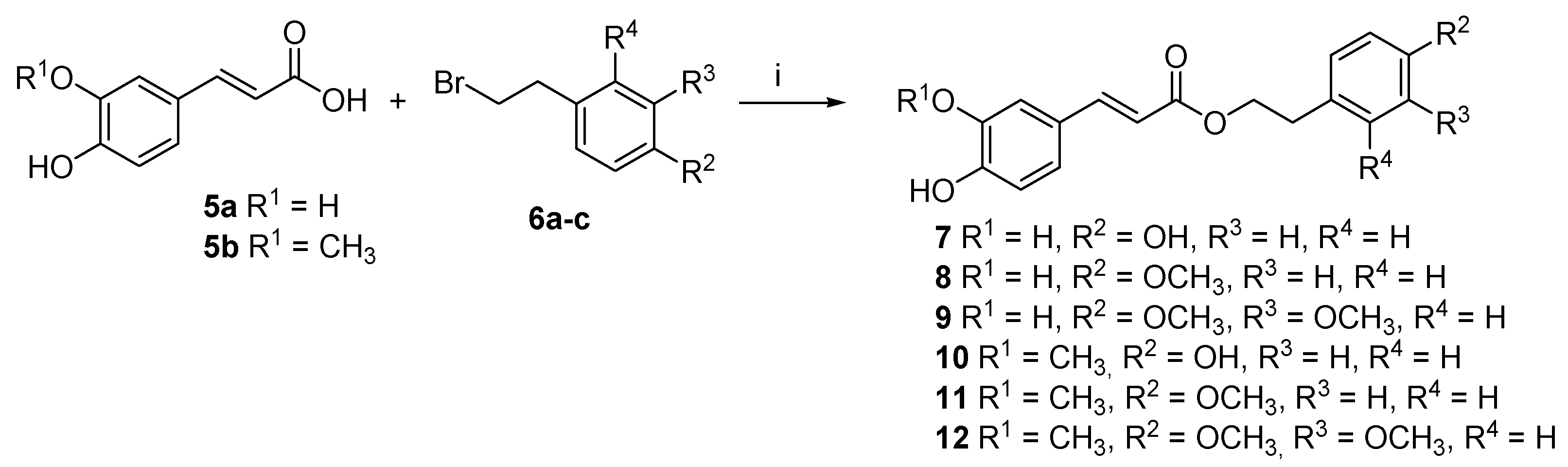
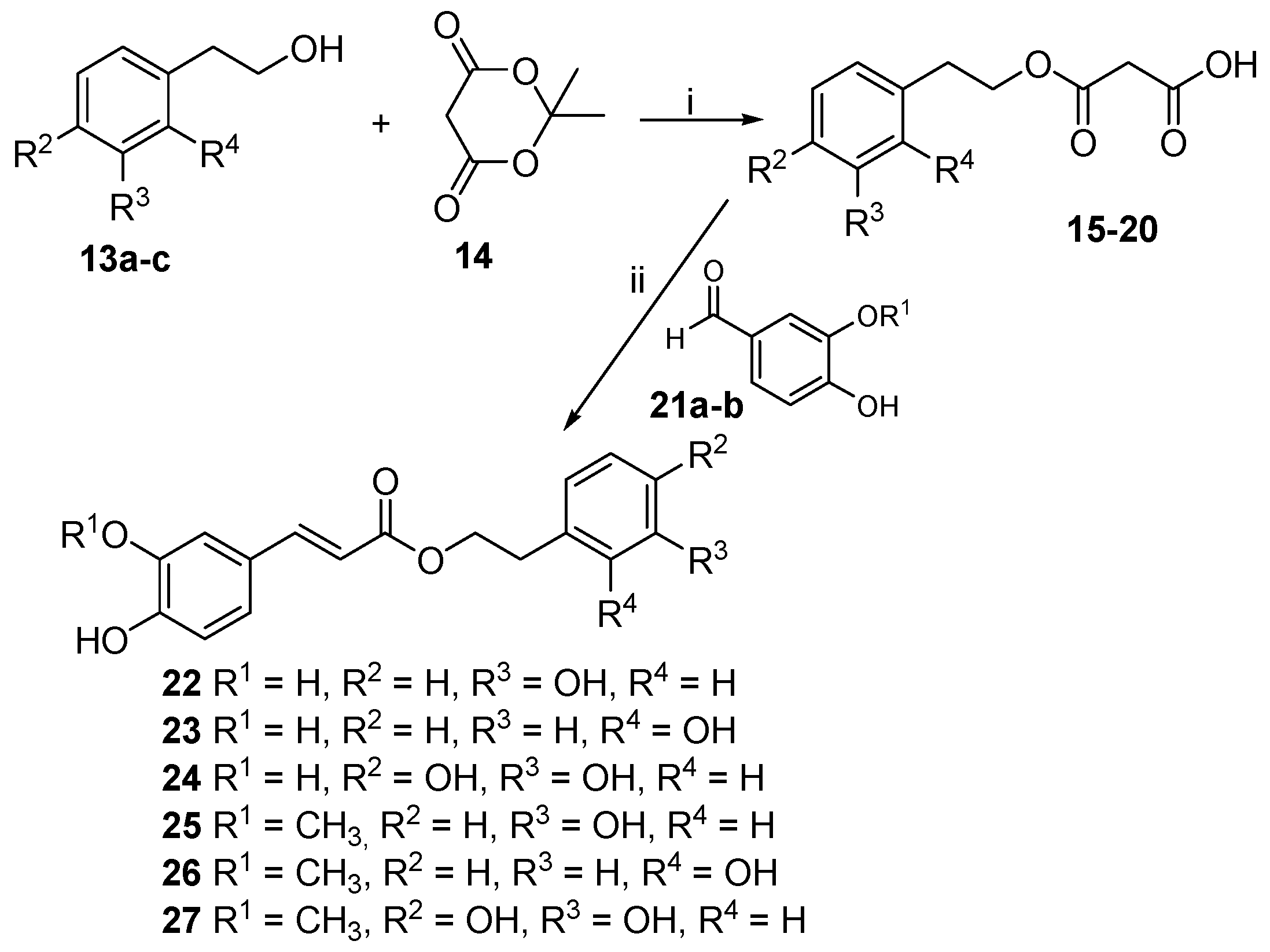
2.1. Chemistry
2.2. Biological Evaluation
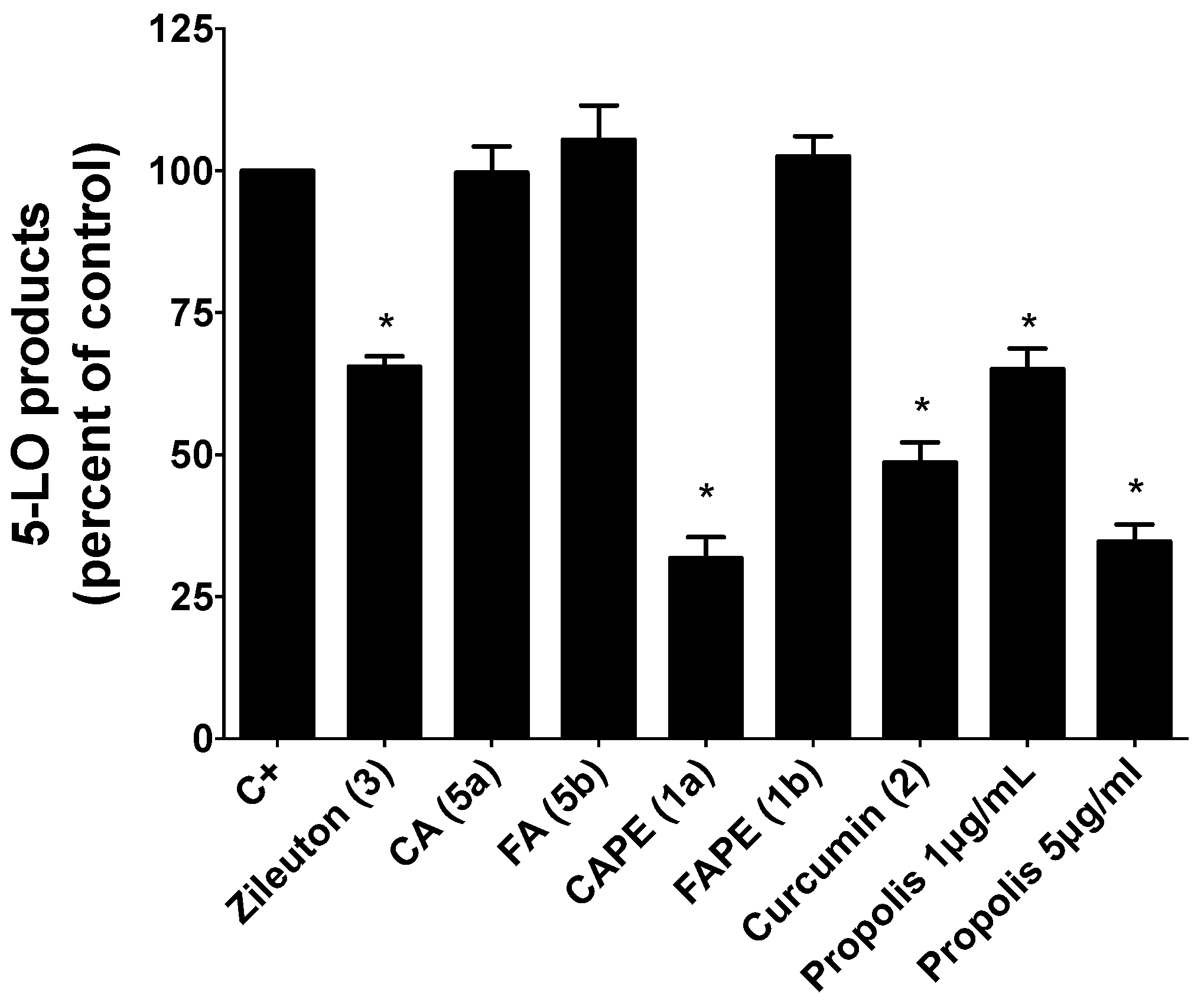
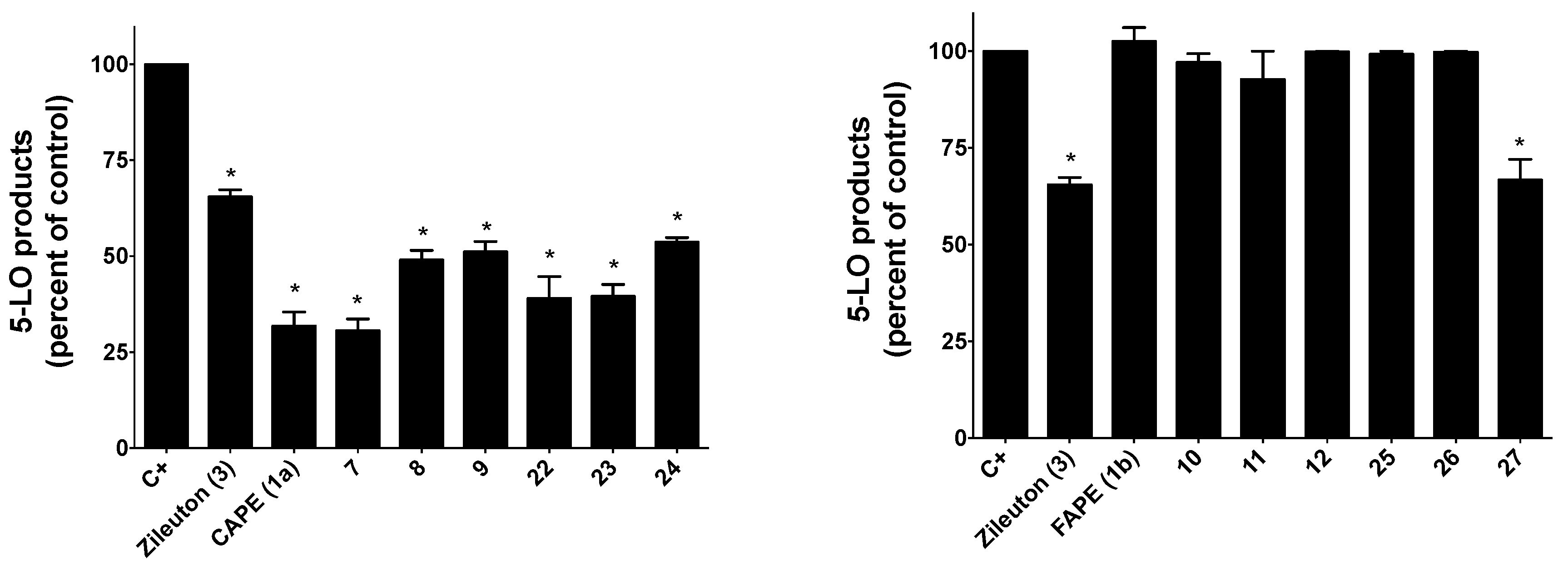
2.2.1. Inhibition of 5-LO Activity
2.2.2. Molecular Docking
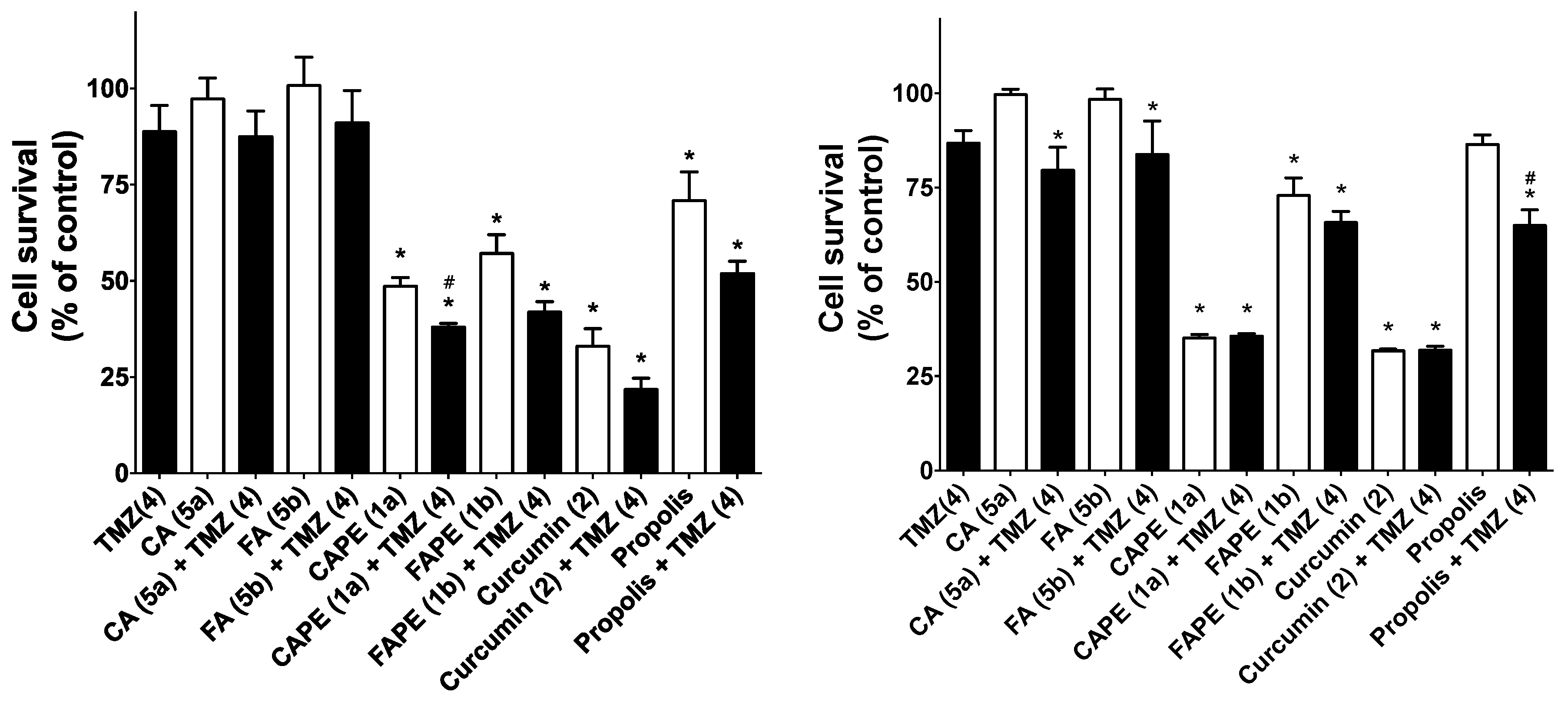
2.2.3. Cytotoxic Activity of Compounds
3. Conclusions
4. Experimental Section
4.1. Chemistry
4.1.1. General
4.1.2. Phenolic Acids Esterification: General Procedure (I)
4.1.3. Phenolic Acids Esterification: General Procedure (II)
4.1.4. Caffeic Acid 4-Hydroxyphenethyl Ester (7)
4.1.5. Ferulic Acid 4-Methoxyphenethyl Ester (8)
4.1.6. Caffeic Acid 3,4-Dimethoxyphenethyl Ester (9)
4.1.7. Ferulic Acid 4-Hydroxyphenethyl Ester (10)
4.1.8. Caffeic Acid 4-Methoxyphenethyl Ester (11)
4.1.9. Ferulic Acid 3,4-Dimethoxyphenethyl Ester (12)
4.1.10. Caffeic Acid 3-Hydroxyphenethyl Ester (22)
4.1.11. Caffeic Acid 2-Hydroxyphenethyl Ester (23)
4.1.12. Caffeic Acid 3,4-Dihydroxyphenethyl Ester (24)
4.1.13. Ferulic Acid 3-Hydroxyphenethyl Ester (25)
4.1.14. Ferulic Acid 2-Hydroxyphenethyl Ester (26)
4.1.15. Ferulic Acid 3,4-Dihydroxyphenethyl Ester (27)
4.2. 5-Lipoxygenase Activity Assay
4.3. Molecular Docking
4.4. Cytotoxic Effects of Compounds in Hs683 and LN319 Glioma Cells
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Preusser, M.; de Ribaupierre, S.; Wohrer, A.; Erridge, S.C.; Hegi, M.; Weller, M.; Stupp, R. Current concepts and management of glioblastoma. Ann. Neurol. 2011, 70, 9–21. [Google Scholar] [CrossRef] [PubMed]
- Brennan, C.W.; Verhaak, R.G.; McKenna, A.; Campos, B.; Noushmehr, H.; Salama, S.R.; Zheng, S.; Chakravarty, D.; Sanborn, J.Z.; Berman, S.H.; et al. The somatic genomic landscape of glioblastoma. Cell 2013, 155, 462–477. [Google Scholar] [CrossRef] [PubMed]
- Prados, M.D.; Byron, S.A.; Tran, N.L.; Phillips, J.J.; Molinaro, A.M.; Ligon, K.L.; Wen, P.Y.; Kuhn, J.G.; Mellinghoff, I.K.; de Groot, J.F.; et al. Toward precision medicine in glioblastoma: The promise and the challenges. Neuro-Oncology 2015, 17, 1051–1063. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Cohen, A.L.; Colman, H. Targeted therapeutics in patients with high-grade gliomas: Past, present, and future. Curr. Treat. Options Oncol. 2016, 17, 42. [Google Scholar] [CrossRef] [PubMed]
- Wen, P.Y.; Chang, S.M.; Lamborn, K.R.; Kuhn, J.G.; Norden, A.D.; Cloughesy, T.F.; Robins, H.I.; Lieberman, F.S.; Gilbert, M.R.; Mehta, M.P.; et al. Phase I/II study of erlotinib and temsirolimus for patients with recurrent malignant gliomas: North American Brain Tumor Consortium trial 04-02. Neuro-Oncology 2014, 16, 567–578. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.S.; Wang, X.; Lu, G.; Picinich, S.C. Cancer prevention by tea: Animal studies, molecular mechanisms and human relevance. Nat. Rev. Cancer 2009, 9, 429–439. [Google Scholar] [CrossRef] [PubMed]
- Negrão, R.; Duarte, D.; Costa, R.; Soares, R. Could platelet-accumulating polyphenols prevent tumour metastasis? Nat. Rev. Cancer 2011, 11, 685. [Google Scholar] [CrossRef] [PubMed]
- Garg, A.K.; Buchholz, T.A.; Aggarwal, B.B. Chemosensitization and radiosensitization of tumors by plant polyphenols. Antioxid. Redox Signal. 2005, 7, 1630–1647. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.L.; Liang, W.H.; Lee, Y.J.; Chuang, S.K.; Tseng, T.H. Antitumor progression potential of caffeic acid phenethyl ester involving p75(NTR) in C6 glioma cells. Chem. Biol. Interact. 2010, 188, 607–615. [Google Scholar] [CrossRef] [PubMed]
- Tseng, T.H.; Shen, C.H.; Huang, W.S.; Chen, C.N.; Liang, W.H.; Lin, T.H.; Kuo, H.C. Activation of neutral-sphingomyelinase, MAPKs, and p75 NTR-mediating caffeic acid phenethyl ester-induced apoptosis in C6 glioma cells. J. Biomed. Sci. 2014, 21, 61. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.C.; Kismali, G.; Aggarwal, B.B. Curcumin, a component of turmeric: From farm to pharmacy. BioFactors 2013, 39, 2–13. [Google Scholar] [CrossRef] [PubMed]
- Kasi, P.D.; Tamilselvam, R.; Skalicka-Woźniak, K.; Nabavi, S.F.; Daglia, M.; Bishayee, A.; Pazoki-Toroudi, H.; Nabavi, S.M. Molecular targets of curcumin for cancer therapy: An updated review. Tumour Biol. 2016, 37, 13017–13028. [Google Scholar] [CrossRef] [PubMed]
- Perry, M.C.; Demeule, M.; Régina, A.; Moumdjian, R.; Béliveau, R. Curcumin inhibits tumor growth and angiogenesis in glioblastoma xenografts. Mol. Nutr. Food Res. 2010, 54, 1192–1201. [Google Scholar] [CrossRef] [PubMed]
- Boudreau, L.H.; Maillet, J.; LeBlanc, L.M.; Jean-François, J.; Touaibia, M.; Flamand, N.; Surette, M.E. Caffeic acid phenethyl ester and its amide analogue are potent inhibitors of leukotriene biosynthesis in human polymorphonuclear leukocytes. PLoS ONE 2012, 7, e31833. [Google Scholar] [CrossRef] [PubMed]
- Peters-Golden, M.; Henderson, W.R., Jr. Leukotrienes. N. Engl. J. Med. 2007, 357, 1841–1854. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Li, N.; Wang, S.; Wu, N.; Hong, J.; Jiao, X.; Krasna, M.J.; Beer, D.G.; Yang, C.S. Leukotriene A4 hydrolase in rat and human esophageal adenocarcinomas and inhibitory effects of bestatin. J. Natl. Cancer Inst. 2003, 95, 1053–1061. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, J.; Myers, C.E. Inhibition of arachidonate 5-lipoxygenase triggers massive apoptosis in human prostate cancer cells. Proc. Natl. Acad. Sci. USA 1998, 95, 13182–13187. [Google Scholar] [CrossRef] [PubMed]
- Rioux, N.; Castonguay, A. Inhibitors of lipoxygenase: A new class of cancer chemopreventive agents. Carcinogenesis 1998, 19, 1393–1400. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Sood, S.; Li, N.; Ramji, D.; Yang, P.; Newman, R.A.; Yang, C.S.; Chen, X. Involvement of the 5-lipoxygenase/leukotriene A4 hydrolase pathway in 7,12-dimethylbenz[a]anthracene (DMBA)-induced oral carcinogenesis in hamster cheek pouch, and inhibition of carcinogenesis by its inhibitors. Carcinogenesis 2006, 27, 1902–1908. [Google Scholar] [CrossRef] [PubMed]
- Nathoo, N.; Prayson, R.A.; Bondar, J.; Vargo, L.; Arrigain, S.; Mascha, E.J.; Suh, J.H.; Barnett, G.H.; Golubic, M. Increased expression of 5-lipoxygenase in high-grade astrocytomas. Neurosurgery 2006, 58, 347–354. [Google Scholar] [CrossRef] [PubMed]
- Ishii, K.; Zaitsu, M.; Yonemitsu, N.; Kan, Y.; Hamasaki, Y.; Matsuo, M. 5-lipoxygenase pathway promotes cell proliferation in human glioma cell lines. Clin. Neuropathol. 2009, 28, 445–452. [Google Scholar] [CrossRef] [PubMed]
- Pergola, C.; Werz, O. 5-Lipoxygenase inhibitors: A review of recent developments and patents. Expert Opin. Ther. Pat. 2010, 20, 355–375. [Google Scholar] [CrossRef] [PubMed]
- Werz, O.; Steinhilbe, D. Pharmacological intervention with 5-lipoxygenase: New insights and novel compounds. Expert Opin. Ther. Pat. 2005, 15, 505–519. [Google Scholar] [CrossRef]
- Koeberle, A.; Muñoz, E.; Appendino, G.B.; Minassi, A.; Pace, S.; Rossi, A.; Weinigel, C.; Sautebin, L.; Caprioglio, D.; Collado, J.A.; et al. SAR studies on curcumin’s pro-inflammatory targets: Discovery of prenylated pyrazolocurcuminoids as potent and selective novel inhibitors of 5-lipoxygenase. J. Med. Chem. 2014, 57, 5638–5648. [Google Scholar] [CrossRef] [PubMed]
- Berger, W.; de Chandt, M.T.; Cairns, C.B. Zileuton: Clinical implications of 5-lipoxygenase inhibition in severe airway disease. Int. J. Clin. Pract. 2007, 61, 663–676. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Marcos, L.; Schuster, A.; Perez-Yarza, E.G. Benefit-risk assessment of antileukotrienes in the management of asthma. Drug Saf. 2003, 26, 483–518. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.C.; Dube, L.M.; Lancaster, J. Acute and chronic effects of a 5-lipoxygenase inhibitor in asthma: A 6-month randomized multicenter trial. Zileuton study group. J. Allergy Clin. Immunol. 1996, 98, 859–871. [Google Scholar] [CrossRef]
- Baughman, T.W.; Sworen, J.C.; Wagener, K.B. The facile preparation of alkenyl metathesis synthons. Tetrahedron 2004, 60, 10943–10948. [Google Scholar] [CrossRef]
- Morin, P., Jr.; Ferguson, D.; LeBlanc, L.M.; Hébert, M.J.; Paré, A.F.; Jean-François, J.; Surette, M.E.; Touaibia, M.; Cuperlovic-Culf, M. NMR metabolomics analysis of the effects of 5-lipoxygenase inhibitors on metabolism in glioblastomas. J. Proteome Res. 2013, 12, 2165–2176. [Google Scholar] [CrossRef] [PubMed]
- Boudreau, L.H.; Picot, N.; Doiron, J.; Villebonnet, B.; Surette, M.E.; Robichaud, G.A.; Touaibia, M. Caffeoyl and cinnamoyl clusters with anti-inflammatory and anticancer effects. Synthesis and structure-activity relationship. New J. Chem. 2009, 33, 1932–1940. [Google Scholar] [CrossRef]
- Touaibia, M.; Jean-François, J.; Doiron, J. Caffeic Acid, a versatile pharmacophore: An overview. Mini Rev. Med. Chem. 2011, 11, 695–713. [Google Scholar] [CrossRef] [PubMed]
- Doiron, J.A.; Leblanc, L.M.; Hébert, M.J.; Levesque, N.A.; Paré, A.F.; Jean-François, J.; Cormier, M.; Surette, M.E.; Touaibia, M. Structure-activity of caffeic acid phenthyl ester analogs as new 5-lipoxygenase inhibitors. Chem. Biol. Drug Des. 2017, 89, 514–528. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Yang, F.; Zhang, M.; Lam, C.; Qiao, Y.; Xiao, J.; Zhang, D.; Ge, Y.; Fu, L.; Xie, D. Antiproliferative activity and SARs of caffeic acid esters with mono-substituted phenylethanols moiety. Bioorg. Med. Chem. Lett. 2017, 27, 131–134. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Xie, D.; Yang, R.; Cheng, Y. Synthesis of Caffeic Acid Phenethyl Ester Derivatives, and Their Cytoprotective and Neuritogenic Activities in PC12 Cells. J. Agric. Food. Chem. 2014, 62, 5046–5063. [Google Scholar] [CrossRef] [PubMed]
- Doiron, J.; Boudreau, L.H.; Picot, N.; Villebonet, B.; Surette, M.E.; Touaibia, M. Synthesis and 5-lipoxygenase inhibitory activity of new cinnamoyl and caffeoyl clusters. Bioorg. Med. Chem. Lett. 2009, 19, 1118–1121. [Google Scholar] [CrossRef] [PubMed]
- Schneider, I.; Bucar, F. Lipoxygenase inhibitors from natural plant sources. Part 1: Medicinal plants with inhibitory activity on arachidonate 5-lipoxygenase and 5-lipoxygenase[sol] cyclooxygenase. Phytother. Res. 2005, 19, 81–102. [Google Scholar] [CrossRef] [PubMed]
- Son, S.; Lewis, B.A. Free radical scavenging and antioxidative activity of caffeic acid amide and ester analogues: Structure-activity relationship. J. Agric. Food Chem. 2002, 50, 468–472. [Google Scholar] [CrossRef] [PubMed]
- Kadoma, Y.; Fujisawa, S. A comparative study of the radical-scavenging activity of the phenolcarboxylic acids caffeic acid, p-coumaric acid, chlorogenic acid and ferulic acid, with or without 2-mercaptoethanol, a thiol, using the induction period method. Molecules 2008, 13, 2488–2499. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.J.; Kuo, H.C.; Chu, C.Y.; Wang, C.J.; Lin, W.C.; Tseng, T.C. Involvement of tumor suppressor protein p53 and p38 MAPK in caffeic acid phenethyl ester-induced apoptosis of C6 glioma cells. Biochem. Pharmacol. 2003, 66, 2281–2289. [Google Scholar] [CrossRef] [PubMed]
- Senft, C.; Polacin, M.; Priester, M.; Seifer, V.; Kögel, D.; Weissenberger, J. The nontoxic natural compound curcumin exerts antiproliferative, antimigratory, and anti-invasive properties against malignant gliomas. Cancer 2010, 10, 491–498. [Google Scholar] [PubMed]
- Cheng, C.; Jiao, J.T.; Qian, Y.; Guo, X.Y.; Huang, J.; Dai, M.C.; Zhang, L.; Ding, X.P.; Zong, D.; Shao, J.F. Curcumin induces G2/M arrest and triggers apoptosis via FoxO1 signaling in U87 human glioma cells. Mol. Med. Rep. 2016, 13, 3763–3770. [Google Scholar] [CrossRef] [PubMed]
- St-Coeur, P.D.; Poitras, J.J.; Cuperlovic-Culf, M.; Touaibia, M.; Morin, P., Jr. Investigagting a signature of temozolomide resistance in GBM cell lines using metabolomics. J. Neuro-Oncol. 2015, 125, 91–102. [Google Scholar] [CrossRef] [PubMed]
- Dittmann, L.M.; Danner, A.; Gronych, J.; Wolter, M.; Stühler, K.; Grzendowski, M.; Becker, N.; Bageritz, J.; Goidts, V.; Toedt, G.; et al. Downregulation of PRDX1 by promoter hypermethylation is frequent in 1p/19q-deleted oligodendroglial tumours and increases radio- and chemosensitivity of Hs683 glioma cells in vitro. Oncogene 2012, 31, 3409–3418. [Google Scholar] [CrossRef] [PubMed]
- Brassesco, M.S.; Roberto, G.M.; Morales, A.G.; Oliveira, J.C.; Delsin, L.E.; Pezuk, J.A.; Valera, E.T.; Carlotti, C.G., Jr.; Rego, E.M.; de Oliveira, H.F.; et al. Inhibition of NF-κ B by Dehydroxymethylepoxyquinomicin Suppresses Invasion and Synergistically Potentiates Temozolomide and γ-Radiation Cytotoxicity in Glioblastoma Cells. Chemother. Res. Pract. 2013, 2013. [Google Scholar] [CrossRef] [PubMed]
- Markiewicz-Żukowska, R.; Borawska, M.H.; Fiedorowicz, A.; Naliwajko, S.K.; Sawicka, D.; Car, H. Propolis changes the anticancer activity of temozolomide in U87MG human glioblastoma cell line. BMC Complement. Altern. Med. 2013, 13, 50. [Google Scholar] [CrossRef] [PubMed]
- Borges, K.S.; Brassesco, M.S.; Scrideli, C.A.; Soares, A.E.; Tone, L.G. Antiproliferative effects of Tubi-bee propolis in glioblastoma cell lines. Genet. Mol. Biol. 2011, 34, 310–314. [Google Scholar] [CrossRef] [PubMed]
- Blough, M.D.; Westgate, M.R.; Beauchamp, D.; Kelly, J.J.; Stechishin, O.; Ramirez, A.L.; Weiss, S.; Cairncross, J.G. Sensitivity to temozolomide in brain tumor initiating cells. Neuro-Oncology 2010, 12, 756–760. [Google Scholar] [CrossRef] [PubMed]
- Robichaud, P.P.; Poirier, S.J.; Boudreau, L.H.; Doiron, J.A.; Barnett, D.A.; Boilard, E.; Surette, M.E. On the cellular metabolism of the click chemistry probe 19-alkyne arachidonic acid. J. Lipid Res. 2016, 57, 1821–1830. [Google Scholar] [CrossRef] [PubMed]
- Morris, G.M.; Huey, R.; Lindstrom, W.; Sanner, M.F.; Belew, R.K.; Goodsell, D.S.; Olson, A.J. Autodock4 and AutoDockTools4: Automated docking with selective receptor flexibility. J. Comput. Chem. 2009, 30, 2785–2791. [Google Scholar] [CrossRef] [PubMed]
- Morris, G.M.; Goodsell, D.S.; Pique, M.E.; Lindstrom, W.; Huey, R.; Forli, S.; Hart, S.E.; Halliday, S.; Belew, R.K.; Olson, A.J. Autodock 4.2 User Guide; The Scripps Research Institute, Molecular Graphics Laboratory, Department of Molecular Biology: La Jolla, CA, USA, 2009. [Google Scholar]
- Gilbert, N.C.; Bartlett, S.G.; Waight, M.T.; Neau, D.B.; Boeglin, W.E.; Brash, A.R.; Newcomer, M.E. The structure of human 5-lipoxygenase. Science 2011, 331, 217–219. [Google Scholar] [CrossRef] [PubMed]
- Schrödinger Release; Schrödinger, LLC.: New York, NY, USA, 2016.
- Laskowski, R.A.; Swindells, M.B. LigPlot+: Mutiple lignad-protein interaction diagrams for drug discovery. J. Chem. Inf. Model. 2011, 51, 2778–2786. [Google Scholar] [CrossRef] [PubMed]
- Cuperlovic-Culf, M.; Ferguson, D.; Culf, A.; Morin, P., Jr.; Touaibia, M. 1H NMR metabolomics analysis of glioblastoma subtypes: Correlation between metabolomics and gene expression characteristics. J. Biol. Chem. 2012, 287, 20164–20175. [Google Scholar] [CrossRef] [PubMed]
- Cuperlovic-Culf, M.; Touaibia, M.; St-Coeur, P.D.; Poitras, J.; Morin, P., Jr.; Culf, A.S. Metabolic Effects of Known and Novel HDAC and SIRT Inhibitors in Glioblastomas Independently or Combined with Temozolomide. Metabolites 2014, 4, 807–830. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are not available from the authors. |
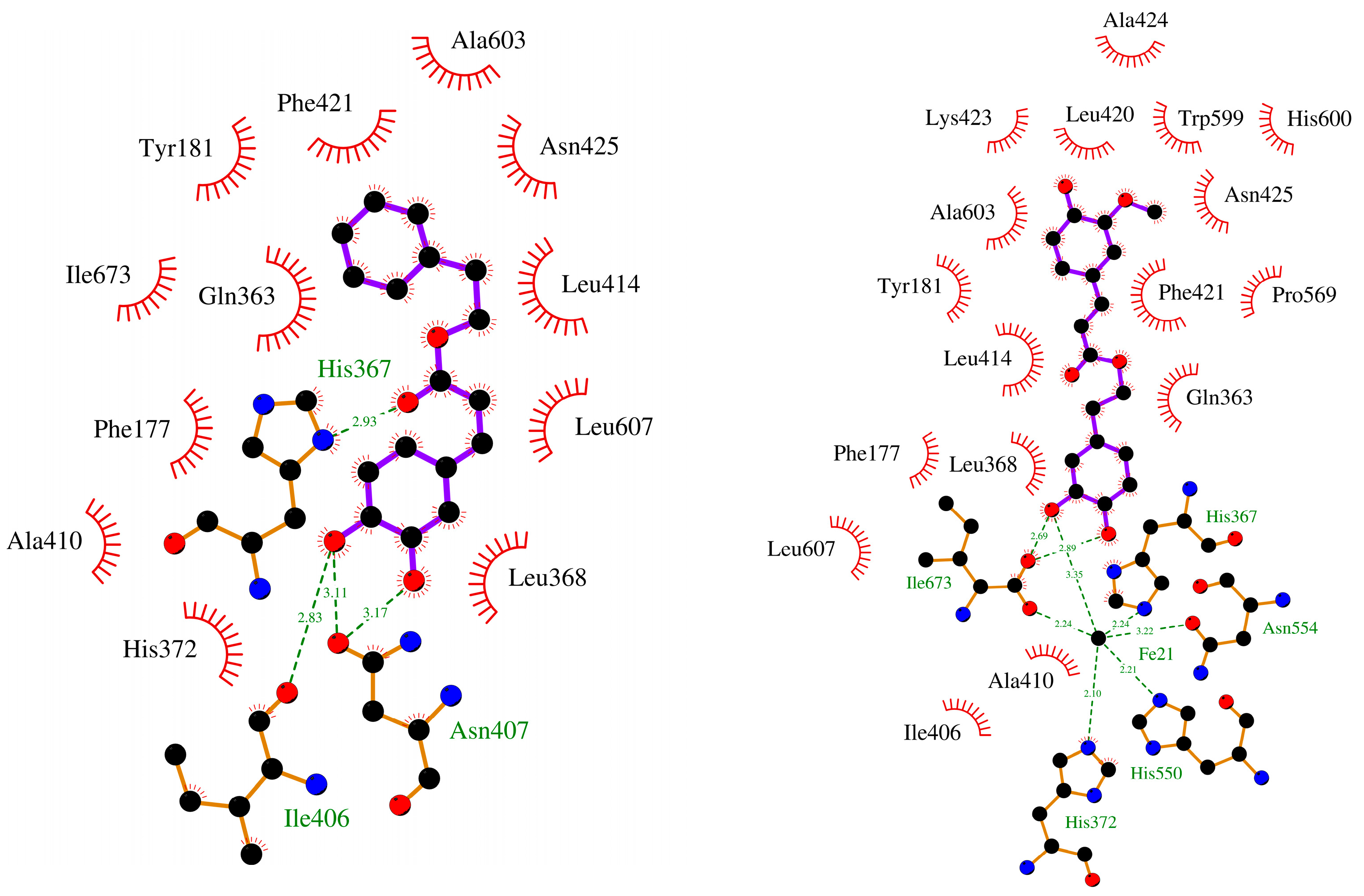


| Molecule | Binding Energy (kcal/mol) | Hydrogen Bond | π-π Interactions |
|---|---|---|---|
| CAPE (1a) | −8.19 | His367, Asn407 × 2 | Phe421 |
| FAPE (1b) | −8.13 | - | His372 |
| 7 | −7.84 | Asn407, Asn425 | Phe177, Phe421 |
| 8 | −8.59 | His367, Asn407 × 2 | Phe177, Phe421 |
| 27 | −8.30 | Ile673 × 2 | Tyr181, His367 |
| Zileuton (3, (R)) | −7.34 | Gln363, His367 | Phe177 |
| Zileuton (3, (S)) | −7.02 | Gln363, His367 | Phe1 77 |
| Molecule | Hs683 (μM) | LN319 (μM) |
|---|---|---|
| CAPE (1a) | 10.9 ± 1.2 | 4.6 ± 0.7 |
| FAPE (1b) | 17.4 ± 2.0 | 20.2 ± 1.5 |
| Curcumin (2) | 8.0 ± 2.6 | 2.7 ± 0.1 |
| CA (5a) | >400 | >400 |
| FA (5b) | >400 | >400 |
| 7 | 35.1 ± 2.2 | 17.9 ± 1.5 |
| 8 | 1.4 ± 1.3 | 24.9 ± 1.8 |
| 9 | 18.7 ± 1.4 | 43.5 ± 6.3 |
| 10 | 22.8 ± 1.7 | 38.0 ± 7.0 |
| 11 | 3.3 ± 1.2 | 24.3 ± 8.9 |
| 12 | 130.1 ± 2.8 | >400 |
| 22 | 22.7 ± 1.8 | 52.6 ± 13.5 |
| 23 | 18.4 ± 1.1 | 8.5 ± 1.6 |
| 24 | 54.1 ± 2.2 | 37.1 ± 7.6 |
| 25 | 13.1 ± 1.8 | 11.4 ± 3.2 |
| 26 | 8.7 ± 1.1 | 5.3 ± 2.1 |
| 27 | 22.9 ± 1.2 | 13.6 ± 2.2 |
| Propolis | 87.5 μg/mL ± 4.9 μg/mL | 87.6 μg/mL ± 5.8 μg/mL |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morin, P.; St-Coeur, P.-D.; Doiron, J.A.; Cormier, M.; Poitras, J.J.; Surette, M.E.; Touaibia, M. Substituted Caffeic and Ferulic Acid Phenethyl Esters: Synthesis, Leukotrienes Biosynthesis Inhibition, and Cytotoxic Activity. Molecules 2017, 22, 1124. https://doi.org/10.3390/molecules22071124
Morin P, St-Coeur P-D, Doiron JA, Cormier M, Poitras JJ, Surette ME, Touaibia M. Substituted Caffeic and Ferulic Acid Phenethyl Esters: Synthesis, Leukotrienes Biosynthesis Inhibition, and Cytotoxic Activity. Molecules. 2017; 22(7):1124. https://doi.org/10.3390/molecules22071124
Chicago/Turabian StyleMorin, Pier, Patrick-Denis St-Coeur, Jérémie A. Doiron, Marc Cormier, Julie J. Poitras, Marc E. Surette, and Mohamed Touaibia. 2017. "Substituted Caffeic and Ferulic Acid Phenethyl Esters: Synthesis, Leukotrienes Biosynthesis Inhibition, and Cytotoxic Activity" Molecules 22, no. 7: 1124. https://doi.org/10.3390/molecules22071124





