Characterization of the Fifth Putative Acetylcholinesterase in the Wolf Spider, Pardosa pseudoannulata
Abstract
:1. Introduction
2. Results
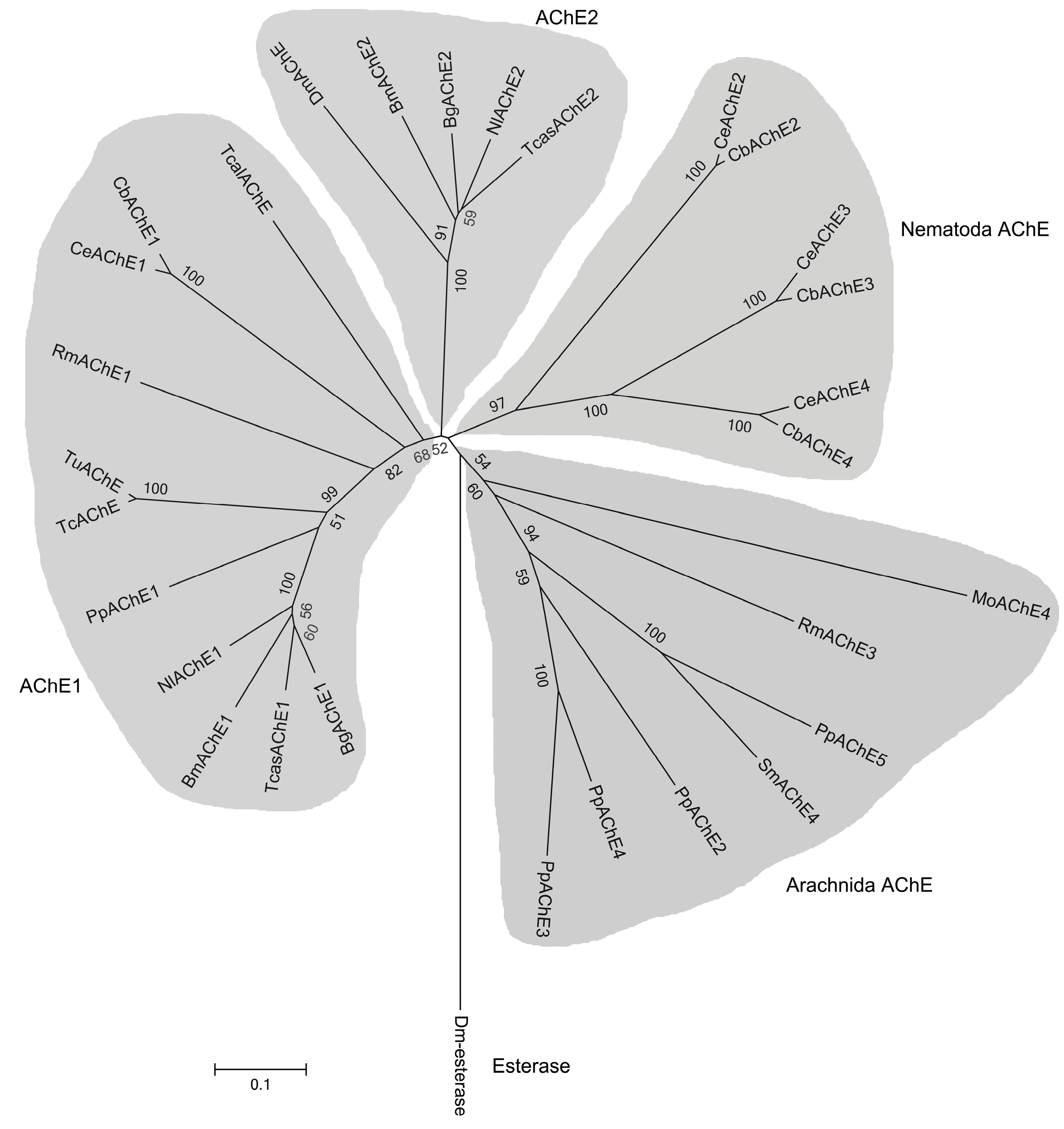
2.1. Cloning and Sequence Analysis of the Fifth Putative Ace Gene from P. pseudoannulata
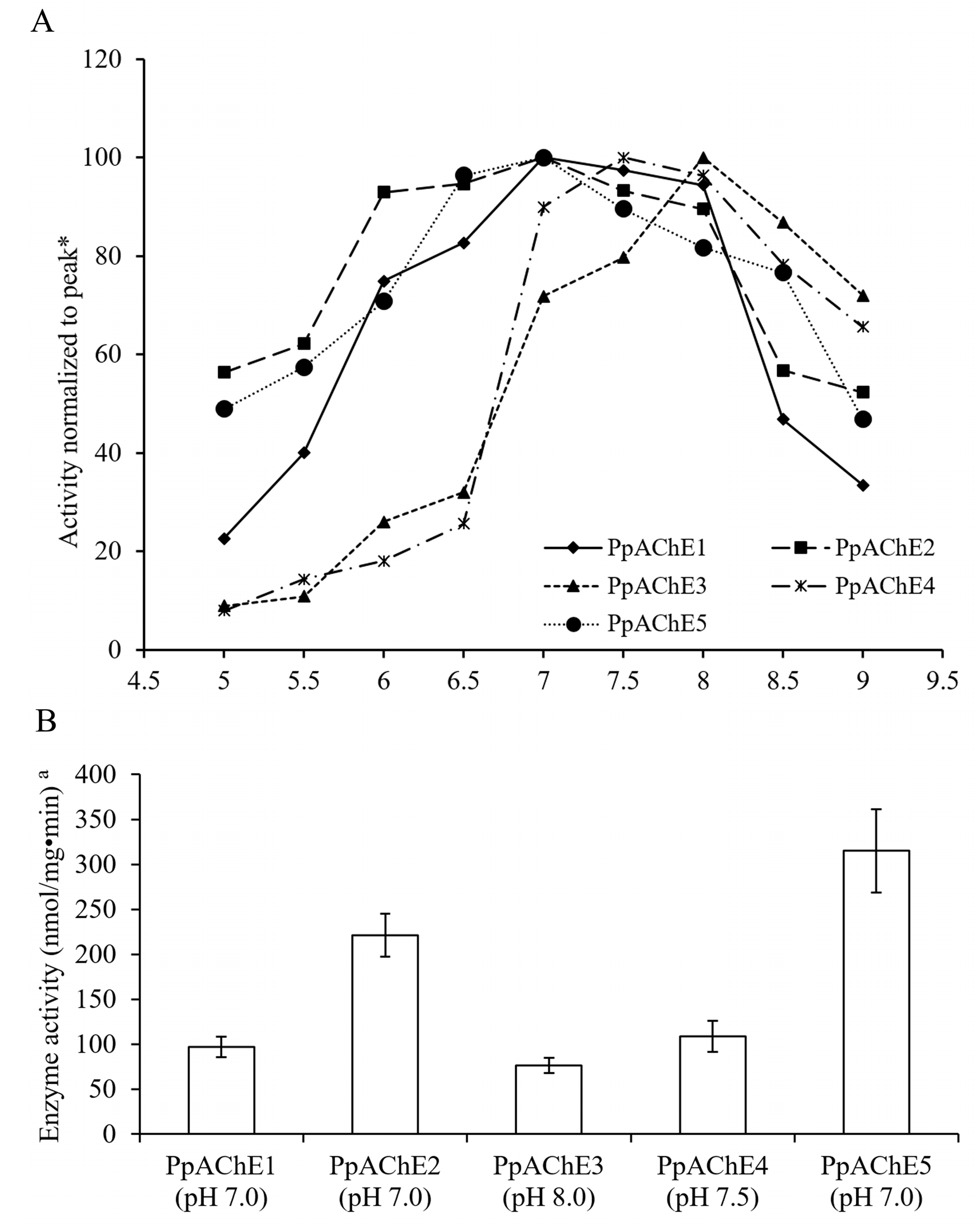
2.2. Recombinant Expression and Enzyme Activity Assay
2.3. Substrate Hydrolysis Kinetics of PpAChE5
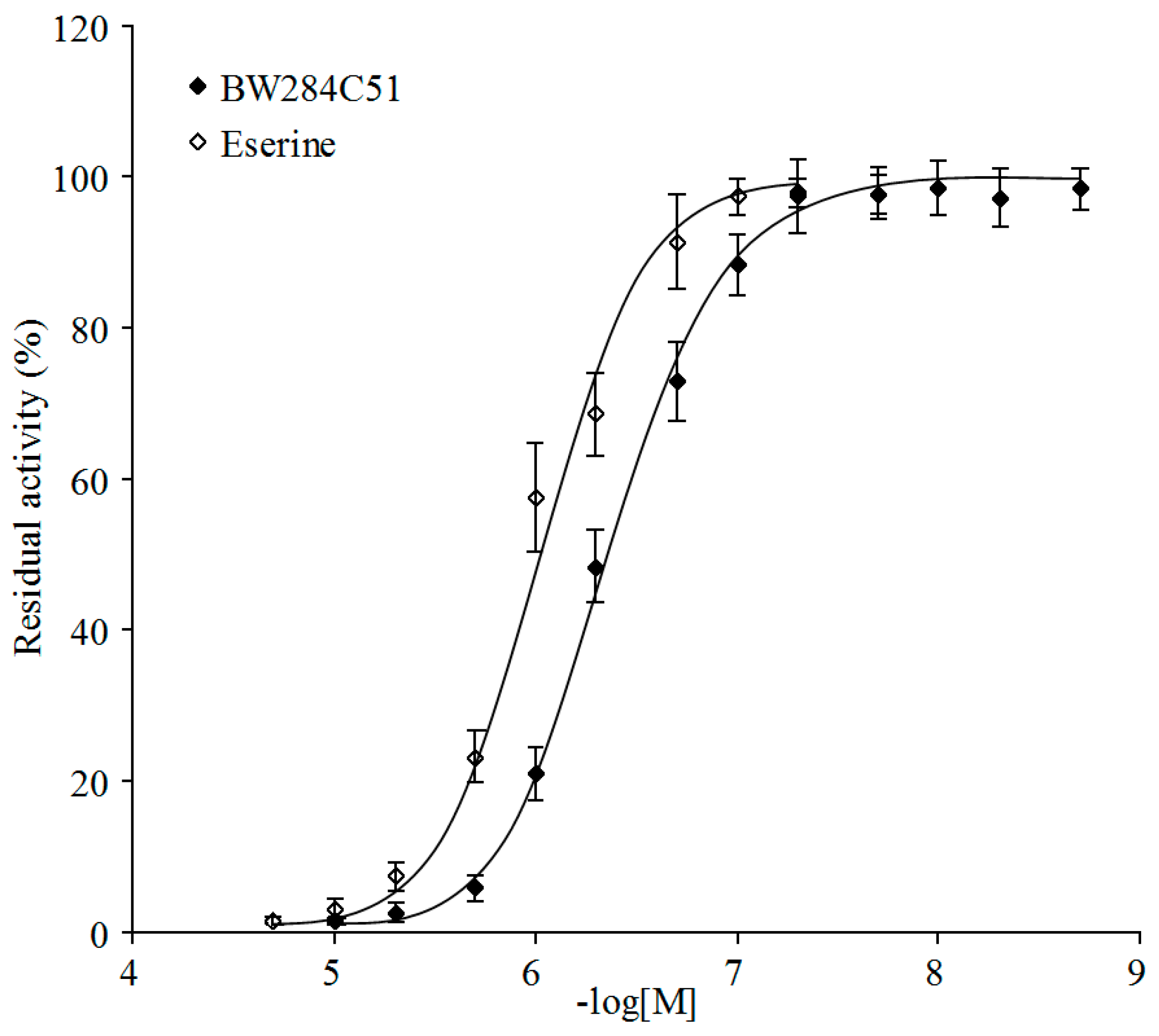
2.4. Inhibition Kinetics of PpAChE5
3. Discussion
4. Materials and Methods
4.1. Spiders, Expression Vector, Cell Lines and Chemicals
4.2. Cloning and Homology Analysis of the Putative Ace Gene
4.3. Expression and Biochemical Properties Assaying of the Putative AChE
4.4. Data Analysis
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Birks, J. Cholinesterase inhibitors for Alzheimer‘s disease. Cochrane Database Syst. Rev. 2006, 5, 1–91. [Google Scholar]
- Kuca, K.; Soukup, O.; Maresova, P.; Korabecny, J.; Nepovimova, E.; Klimova, B.; Honegr, J.; Ramalho, T.C.; Franca, T.C.C. Current approaches against Alzheimer’s Disease in clinical trials. J. Braz. Chem. Soc. 2016, 27, 641–649. [Google Scholar] [CrossRef]
- McHardy, S.F.; Wang, H.Y.L.; McCowen, S.V.; Valdez, M.C. Recent advances in acetylcholinesterase inhibitors and reactivators: An update on the patent literature (2012–2015). Expert Opin. Ther. Pat. 2017, 27, 455–476. [Google Scholar] [CrossRef] [PubMed]
- Malekmohammadi, M.; Galehdari, H. Target site insensitivity mutations in the AChE enzyme confer resistance to organophosphorous insecticides in Leptinotarsa decemlineata (Say). Pestic. Biochem. Physiol. 2016, 126, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Carlier, P.R.; Bloomquist, J.R.; Totrov, M.; Li, J. Discovery of species-selective and resistance-breaking anticholinesterase insecticides for the malaria mosquito. Curr. Med. Chem. 2017, 24, 42. [Google Scholar]
- Wessler, I.; Kilbinger, H.; Bittinger, F.; Unger, R.; Kirkpatrick, C.J. The non-neuronal cholinergic system in humans: Expression, function and pathophysiology. Life Sci. 2003, 72, 2055–2061. [Google Scholar] [CrossRef]
- Wessler, I.; Kilbinger, H.; Bittinger, F.; Kirkpatrick, C.J. The biological role of non-neuronal acetylcholine in plants and humans. Jpn. J. Pharmacol. 2001, 85, 2–10. [Google Scholar] [CrossRef] [PubMed]
- Silman, I.; Sussman, J.L. Acetylcholinesterase: ‘Classical’ and ‘non-classical’ functions and pharmacology. Curr. Opin. Pharmacol. 2005, 5, 293–302. [Google Scholar] [CrossRef] [PubMed]
- Soreq, H.; Seidman, S. Acetylcholinesterase-new roles for an old actor. Nat. Rev. Neurosci. 2001, 2, 294–302. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.H.; Lee, S.H. Which acetylcholinesterase functions as the main catalytic enzyme in the Class Insecta? Insect Biochem. Mol. Biol. 2013, 43, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Combes, D.; Fedon, Y.; Grauso, M.; Toutant, J.P.; Arpagaus, M. Four genes encode acetylcholinesterases in the nematodes Caenorhabditis elegans and Caenorhabditis briggsae. cDNA sequences, genomic structures, mutations and in vivo expression. J. Mol. Biol. 2000, 300, 727–742. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.; Li, C.; Xiu, C.; Zhang, J.; Li, J.; Huang, L.; Zhang, Y.; Liu, Z. Identification and biochemical properties of two new acetylcholinesterases in the pond wolf spider (Pardosa pseudoannulata). PLoS ONE 2016, 11, 6. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.; Zhang, Y.; Bao, H.; Liu, Z. Sequence analysis of insecticide action and detoxification-related genes in the insect pest natural enemy Pardosa pseudoannulata. PLoS ONE 2015, 10, 4. [Google Scholar] [CrossRef] [PubMed]
- De Almeida, J.S.F.D.; Cuya Guizado, T.R.; Guimarães, A.P.; Ramalho, T.C.; Gonçalves, A.S.; de Koning, M.C.; França, T.C.C. Docking and molecular dynamics studies of peripheral site ligand–oximes as reactivators of sarin-inhibited human acetylcholinesterase. J. Biomol. Struct. Dyn. 2016, 34, 2632–2642. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Shao, Y.; Jiang, F.; Li, J.; Liu, Z. Identification of two acetylcholinesterases in Pardosa pseudoannulata and the sensitivity to insecticides. Insect Biochem. Mol. Biol. 2014, 46, 25–30. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.; Li, C.; Bao, H.; Fang, J.; Liu, Z.; Zhang, Y. Validating the importance of two acetylcholinesterases in insecticide sensitivities by RNAi in Pardosa pseudoannulata, an important predatory enemy against several insect pests. Pestic. Biochem. Physiol. 2015, 125, 26–30. [Google Scholar] [CrossRef] [PubMed]
- Fournier, D.; Berge, J.B.; Cardoso de Almeida, M.L.; Bordier, C. Acetylcholinesterases from Musca domestica and Drosophila melanogaster brain are linked to membranes by a glycophospholipid anchor sensitive to an endogenous phospholipase. J. Neurochem. 1988, 50, 58–63. [Google Scholar] [CrossRef]
- Weill, M.; Malcolm, C.; Chandre, F.; Mogensen, K.; Berthomieu, A.; Marquine, M.; Raymond, M. The unique mutation in ace-1 giving high insecticide resistance is easily detectable in mosquito vectors. Insect Mol. Biol. 2004, 13, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Temeyer, K.B.; Pruett, J.H.; Olafson, P.U. Baculovirus expression, biochemical characterization and organophosphate sensitivity of rBmAChE1, rBmAChE2, and rBmAChE3 of Rhipicephalus (Boophilus) microplus. Vet. Parasitol. 2010, 172, 114–121. [Google Scholar] [CrossRef] [PubMed]
- Bigbee, J.W.; Sharma, K.V.; Chan, E.L.P.; Bogler, O. Evidence for the direct role of acetylcholinesterase in neurite outgrowth in primary dorsal root ganglion neurons. Brain Res. 2000, 861, 354–362. [Google Scholar] [CrossRef]
- Grifman, M.; Galyam, N.; Seidman, S.; Soreq, H. Functional redundancy of acetylcholinesterase and neuroligin in mammalian neuritogenesis. Proc. Natl. Acad. Sci. USA 1998, 95, 13935–13940. [Google Scholar] [CrossRef] [PubMed]
- Sternfeld, M.; Ming, G.; Song, H.; Sela, K.; Timberg, R.; Poo, M.; Soreq, H. Acetylcholinesterase enhances neurite growth and synapse development through alternative contributions of its hydrolytic capacity, core protein, and variable C termini. J. Neurosci. 1998, 18, 1240–1249. [Google Scholar] [PubMed]
- Darboux, I.; Barthalay, Y.; Piovant, M.; Hipeau-Jacquotte, R. The structure-function relationships in Drosophila neurotactin show that cholinesterasic domains may have adhesive properties. EMBO J. 1996, 15, 4835–4843. [Google Scholar] [PubMed]
- Jin, Q.H.; He, H.Y.; Shi, Y.F.; Lu, H.; Zhang, X.J. Overexpression of acetylcholinesterase inhibited cell proliferation and promoted apoptosis in NRK cells. Acta Pharmacol. Sin. 2004, 25, 1013–1021. [Google Scholar] [PubMed]
- Holmes, C.; Jones, S.A.; Budd, T.C.; Greenfield, S.A. Non-cholinergic, trophic action of recombinant acetylcholinesterase on mid-brain dopaminergic neurons. J. Neurosci. Res. 1997, 49, 207–218. [Google Scholar] [CrossRef]
- Rees, T.; Hammond, P.I.; Soreq, H.; Younkin, S.; Brimijoin, S. Acetylcholinesterase promotes beta-amyloid plaques in cerebral cortex. Neurobiol. Aging 2003, 24, 777–787. [Google Scholar] [CrossRef]
- Kawashima, K.; Fujii, T. Extraneuronal cholinergic system in lymphocytes. Pharmacol. Therapeu. 2000, 86, 29–48. [Google Scholar] [CrossRef]
- Paoletti, F.; Mocali, A.; Vannucchi, A.M. Acetylcholinesterase in murine erythroleukemia (Friend) cells: Evidence for megakaryocyte-like expression and potential growth-regulatory role of enzyme activity. Blood 1992, 79, 2873–2879. [Google Scholar] [PubMed]
- Dori, A.; Ifergane, G.; Saar-Levy, T.; Bersudsky, M.; Mor, I.; Soreq, H.; Wirguin, I. Readthrough acetylcholinesterase in inflammation-associated neuropathies. Life Sci. 2007, 80, 2369–2374. [Google Scholar] [CrossRef] [PubMed]
- Gilboa-Geffen, A.; Lacoste, P.P.; Soreq, L.; Cizeron-Clairac, G.; Le Panse, R.; Truffault, F.; Shaked, I.; Soreq, H.; Berrih-Aknin, S. The thymic theme of acetylcholinesterase splice variants in myasthenia gravis. Blood 2007, 109, 4383–4391. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Park, Y.; Gao, X.; Zhang, X.; Yao, J.; Pang, Y.; Jiang, H.; Zhu, K. Cholinergic and non-cholinergic functions of two acetylcholinesterase genes revealed by gene-silencing in Tribolium castaneum. Sci. Rep. 2012, 2, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Xiao, D.; Lu, Y.H.; Shang, Q.L.; Song, D.L.; Gao, X.W. Gene silencing of two acetylcholinesterases reveals their cholinergic and non-cholinergic functions in Rhopalosiphum padi and Sitobion avenae. Pest. Manag. Sci. 2015, 71, 523–530. [Google Scholar] [CrossRef] [PubMed]
- Hui, X.M.; Yang, L.W.; He, G.L.; Yang, Q.P.; Han, Z.J.; Li, F. RNA interference of ace1 and ace2 in Chilo suppressalis reveals their different contributions to motor ability and larval growth. Insect Mol. Biol. 2011, 20, 507–518. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.H.; Kim, J.H.; Kim, K.; Lee, S.H. Expression of acetylcholinesterase 1 is associated with brood rearing status in the honey bee, Apis mellifera. Sci. Rep. 2017, 7, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; Gupta, G.P.; Rajam, M.V. Silencing of acetylcholinesterase gene of Helicoverpa armigera by siRNA affects larval growth and its life cycle. J. Insect Physiol. 2009, 55, 273–278. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Not available. |

| Subsite | T. californica | T. urticae | PpAChE1 | PpAChE2 | PpAChE3 | PpAChE4 | PpAChE5 |
|---|---|---|---|---|---|---|---|
| Catalytic triad | S200 | S | S | S | S | S | S |
| E327 | E | E | E | D | D | E | |
| H440 | H | H | H | H | H | H | |
| Oxyanion hole | G118 | G | G | G | G | G | G |
| G119 | S | G | G | G | A | G | |
| A201 | A | A | A | A | A | A | |
| Choline binding site | W84 | W | W | W | W | W | W |
| Y130 | Y | Y | Y | F | Y | H | |
| F330 | Y | Y | F | T | L | F | |
| F331 | F | F | Q | L | I | Q | |
| Acyl pocket | F288 | F | F | R | R | R | R |
| F290 | F | F | F | Y | T | F | |
| V400 | F | F | L | R | R | L | |
| Peripheral anionic site | Y70 | V | I | Y | Y | F | Y |
| Y121 | W | Y | S | R | V | F | |
| W279 | E | W | N | S | S | S | |
| Wall of the gorge | W114 | W | W | W | F | W | W |
| W233 | W | W | L | D | D | L | |
| Y334 | Y | Y | T | K | N | T | |
| W432 | W | W | W | W | F | W | |
| Y442 | E | D | E | D | D | S |
| KM (µM) | Vmax (nmol/mg·min) | Vmax/KM (mL/mg·min) | Vmax Ratio | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ATC | BTC | PTC | ATC | BTC | PTC | ATC | BTC | PTC | ATC vs. BTC | ATC vs. PTC | BTC vs. BTC | |
| PpAChE1 | 536.8 ± 63.4 c | 858.8 ± 103.2 c | 1148.6 ± 133.5 c | 112.6 ± 8.8 c | 95.4 ± 12.8 b | 76.2 ± 9.2 c | 209.8 | 111.1 | 66.3 | 1.18 | 1.48 | 1.25 |
| PpAChE2 | 4413.5 ± 533.7 a | 4542.2 ± 326.0 a | 4496.3 ± 318.8 a | 253.9 ± 20.4 b | 233.9 ± 16.5 a | 156.8 ± 21.3 a | 57.5 | 51.5 | 34.9 | 1.09 | 1.62 | 1.49 |
| PpAChE3 | 42.9 ± 5.8 e | 198.6 ± 22.3 e | 503.6 ± 86.8 d | 124.7 ± 11.5 c | 40.3 ± 5.6 c | 106.2 ± 15.1 b | 2906.8 | 202.9 | 210.9 | 3.09 | 1.17 | 0.38 |
| PpAChE4 | 61.6 ± 7.5 d | 269.4 ± 35.5 d | 279.3 ± 35.2 e | 86.6 ± 9.7 d | 105.5 ± 8.9 b | 49.7 ± 8.4 d | 1405.8 | 391.6 | 177.9 | 0.82 | 1.74 | 2.12 |
| PpAChE5 | 1303.5 ± 162.9 b | 1528.9 ± 118.4 b | 1548.2 ± 129.1 b | 428.4 ± 29.2 a | 91.6 ± 11.3 b | 59.0 ± 7.2 cd | 328.7 | 59.9 | 38.1 | 4.68 | 7.26 | 1.55 |
| On ATC Hydrolysis | On BTC Hydrolysis | ||
|---|---|---|---|
| BW284C51 | Eserine | ISO-OMPA | |
| PpAChE1 | 5.12 ± 0.76 d | 11.67 ± 2.06 d | >10,000 |
| PpAChE2 | 254.17 ± 21.40 a | 186.42 ± 25.11 a | 2165.39 ± 325.67 |
| PpAChE3 | 3.68 ± 0.53 e | 6.52 ± 1.80 e | >10,000 |
| PpAChE4 | 7.36 ± 1.05 c | 24.50 ± 3.94 c | >10,000 |
| PpAChE5 | 47.10 ± 6.93 b | 116.28 ± 15.71 b | >10,000 |
| Compound | ki (×10−7M−1min−1) |
|---|---|
| Eserine | 6.24 ± 1.72 b |
| Fenobucarb | 18.46 ± 3.85 a |
| Carbaryl | 16.79 ± 2.90 a |
| Paraoxon | 15.50 ± 3.32 a |
| Diazoxon | 16.43 ± 3.58 a |
| Primers | ||
|---|---|---|
| RACE primers | 3′outer primer | TTACAACAAGCAACCCCGACC |
| 3′inner primer | CCATTTCAGCGAAGCGGCATT | |
| 5′outer primer | GGCTTCAGTCTCAACTCCAACGT | |
| 5′inner primer | GAAGCGCCATAGCATCAACACCT | |
| Expression primers | Sense primer: | TAGTGCGGCCGCTTTCGAATATGGCTTTTCTTTCCTTAGA |
| Anti-sense primer | CTCGAGACTGCAGGCTCTAGTCAGAATCCGAAATACGGGC |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meng, X.; Xu, X.; Bao, H.; Wang, J.; Liu, Z. Characterization of the Fifth Putative Acetylcholinesterase in the Wolf Spider, Pardosa pseudoannulata. Molecules 2017, 22, 1118. https://doi.org/10.3390/molecules22071118
Meng X, Xu X, Bao H, Wang J, Liu Z. Characterization of the Fifth Putative Acetylcholinesterase in the Wolf Spider, Pardosa pseudoannulata. Molecules. 2017; 22(7):1118. https://doi.org/10.3390/molecules22071118
Chicago/Turabian StyleMeng, Xiangkun, Xixia Xu, Haibo Bao, Jianjun Wang, and Zewen Liu. 2017. "Characterization of the Fifth Putative Acetylcholinesterase in the Wolf Spider, Pardosa pseudoannulata" Molecules 22, no. 7: 1118. https://doi.org/10.3390/molecules22071118