3. Experimental
3.1. General Information
Melting points were recorded on a Thermocouple digital melting point apparatus (Stuart, Staffordshire, UK) and are uncorrected. IR spectra were recorded as powders using a Bruker VERTEX 70 FT-IR Spectrometer (Bruker Optics, Billerica, MA, USA) with a diamond ATR (attenuated total reflectance) accessory by using the thin-film method. For column chromatography, Merck kieselgel 60 (0.063–0.200 mm) (Merck KGaA, Frankfurt, Germany) was used as stationary phase. NMR spectra were obtained as DMSO-
d6 solutions using Varian 300 MHz NMR spectrometer (Varian Inc., Palo Alto, CA, USA) and the chemical shifts are quoted relative to the TMS peak. Low- and high-resolution mass spectra were recorded using Waters Synapt G2 Quadrupole Time-of-flight mass spectrometer (Waters Corp., Milford, MA, USA) at the University of Stellenbosch Mass Spectrometry Unit. The synthesis and characterization of compounds
1a–
g have been described before [
11].
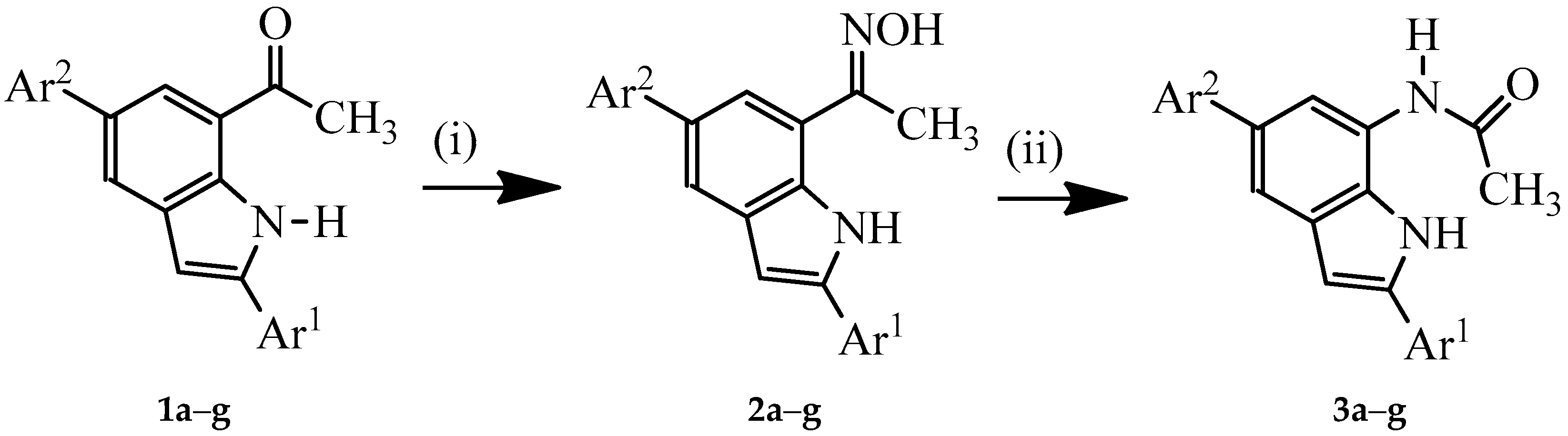
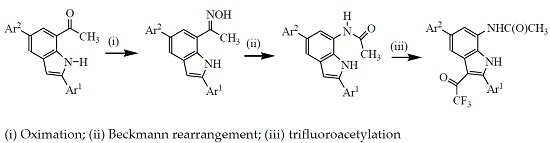
3.2. Typical Procedure for the Synthesis of Oxime Derivatives 2a–g
A stirred mixture of 1 (1 equivalent) and hydroxylamine hydrochloride (1.5 equivalent) and pyridine (1.5 equivalent) in ethanol (10 mL/mmol of 1) was heated at 80 °C overnight. The mixture was cooled to room temperature and quenched with an ice-cold water and the product was extracted into chloroform. The combined organic phases were washed with water and dried over anhyd. MgSO4. The salt was filtered off and the solvent was evaporated under reduced pressure. The residue was purified by column chromatography on silica gel (20% EtOAc–hexane as eluent) to afford the oxime derivative 2. The following compounds were prepared in this fashion:
1-(2,5-bis(4-Fluorophenyl)-1H-indol-7-yl) Ethanone Oxime (2a). A mixture of 1a (0.15 g, 0.43 mmol), hydroxylamine hydrochloride (0.05 g, 0.65 mmol) and pyridine (0.05 g, 0.65 mmol) in ethanol (10 mL) afforded 2a as a solid (0.15 g, 94%), Rf 0.67, mp. 238–241 °C; νmax (ATR) 462, 509, 539, 612, 663, 753, 785, 813, 826, 867, 921, 990, 1099, 1159, 1221, 1232, 1262, 1290, 1373, 1433, 1469, 1514, 3215, 3422 cm−1; 1H-NMR (DMSO-d6) 2.38 (3H, s, CH3), 6.99 (1H, s, 3-H), 7.26 (2H, t, J = 8.7 Hz, 3″,5″-H), 7.33 (2H, t, J = 9.0 Hz, 3′,5′-H), 7.58 (1H, d, J = 1.2 Hz, 4-H), 7.74 (2H, dd, J = 5.7 and 8.7 Hz, 2″,6″-H), 7.81–7.87 (3H, m, 6-H and 2′,6′-H), 10.85 (1H, s, NH), 11.43 (1H, s, OH); 13C-NMR (DMSO-d6) 11.3, 100.1, 116.0 (d, 2JCF = 21.8 Hz), 116.5 (d, 2JCF = 21.8 Hz), 119.8, 120.3, 120.6, 127.4 (d, 3JCF = 8.0 Hz), 128.6 (d, 4JCF = 3.5 Hz), 129.2 (d, 3JCF = 8.0 Hz), 130.2, 131.8, 133.4, 137.7, 138.3 (d, 4JCF = 3.5 Hz), 155.0, 161.9 (d, 1JCF = 241.7 Hz), 162.3 (d, 1JCF = 243.9 Hz); HRMS (ES): MH+, found 363.1309. C22H17N2F2O+ requires: 363.1309.
1-(5-(4-Fluorophenyl)-2-(4-methoxyphenyl)-1H-indol-7-yl) Ethanone Oxime (2b). A mixture of 1b (0.15 g, 0.42 mmol), hydroxylamine hydrochloride (0.04 g, 0.62 mmol) and pyridine (0.05 g, 0.62 mmol) in ethanol (10 mL) afforded 2b as a solid (0.14 g, 87%), Rf 0.47, mp. 212–214 °C; νmax (ATR) 433, 518, 541, 554, 615, 664, 692, 752, 784, 827, 921, 986, 1021, 1158, 1182, 1217, 1240, 1315, 1371, 1436, 1464, 1513, 3226, 3405 cm−1; 1H-NMR (DMSO-d6) 2.39 (3H, s, CH3), 3.80 (3H, s, CH3), 6.88 (1H, s, 3-H), 7.05 (2H, d, J = 8.7 Hz, 3′,5′-H), 7.27 (2H, t, J = 8.7 Hz, 3″,5″-H), 7.57 (1H, d, J = 1.2 Hz, 4-H), 7.72–7.89 (5H, m, 6-H, 2′,6′-H and 2″,6″-H), 10.76 (1H, s, NH), 11.43 (1H, s, OH); 13C-NMR (DMSO-d6) 11.3, 55.7, 98.8, 115.0, 116.0 (d, 2JCF = 20.6 Hz), 119.5, 119.9, 120.2, 124.6, 126.7, 129.1 (d, 3JCF = 8.0 Hz), 130.4, 131.6, 133.1, 138.4 (d, 4JCF = 3.4 Hz), 138.7, 155.1, 159.6, 161.8 (d, 1JCF = 241.7 Hz); HRMS (ES): MH+, found: 375.1507. C23H20N2FO2+ requires: 375.1509.
1-(2-(4-Fluorophenyl)-5-(4-methoxyphenyl)-1H-indol-7-yl)ethanone Oxime (2c). A mixture of 1c (0.15 g, 0.43 mmol), hydroxylamine hydrochloride (0.04 g, 0.62 mmol) and pyridine (0.05 g, 0.62 mmol) in ethanol (10 mL) afforded 2c as a solid (0.14 g, 88%), Rf 0.58, mp. 219–222 °C; νmax (ATR) 512, 561, 613, 664, 692, 753, 789, 826, 921, 989, 1023, 1099, 1156, 1182, 1240, 1272, 1371, 1427, 1466, 1520, 3369, 3406 cm−1; 1H-NMR (DMSO-d6) 2.37 (3H, s, CH3), 3.77 (3H, s, OCH3), 6.96 (1H, s, 3-H), 7.00 (2H, d, J = 8.7 Hz, 3″,5″-H), 7.33 (2H, t, J = 8.7 Hz, 3′,5′-H), 7.56 (1H, d, J = 1.2 Hz, 4-H), 7.63 (2H, d, J = 8.1 Hz, 2″,6″-H), 7.76 (1H, d, J = 1.2 Hz, 6-H), 7.84 (2H, d, J = 8.7 Hz, 2′,6′-H), 10.81 (1H, s, NH), 11.41 (1H, s, OH); 13C-NMR (DMSO-d6) 11.3, 55.6, 100.1, 114.7, 116.5 (d, 2JCF = 21.8 Hz), 119.2, 120.1, 120.5, 127.3 (d, 3JCF = 9.2 Hz), 128.3, 128.7 (d, 4JCF = 3.5 Hz), 130.2, 132.5, 133.1, 134.2, 137.5, 155.0, 158.7, 162.2 (d, 1JCF = 242.8 Hz); HRMS (ES): MH+, found: 375.1509. C23H20N2FO2+ requires: 375.1509.
1-(2,5-Bis(4-methoxyphenyl)-1H-indol-7-yl)ethanone Oxime (2d). A mixture of 1d (0.15 g, 0.40 mmol), hydroxylamine hydrochloride (0.04 g, 0.61 mmol) and pyridine (0.05 g, 0.61 mmol) in ethanol (10 mL) afforded 2d as a solid (0.14 g, 88%), Rf 0.38, mp. 209–212 °C; νmax (ATR) 545, 615, 663, 752, 784, 818, 828, 838, 921, 986, 1028, 1114, 1176, 1236, 1370, 1434, 1466, 1516, 3390, 3416 cm−1; 1H-NMR (DMSO-d6) 2.37 (3H, s, CH3), 3.78 (3H, s, OCH3), 3.80 (3H, s, OCH3), 6.87 (1H, d, s, 3-H), 7.00 (2H, d, J = 8.7 Hz, 3″,5″-H), 7.05 (2H, d, J = 8.7 Hz, 3′,5′-H), 7.53 (1H, d, J = 1.2 Hz, 4-H), 7.63 (2H, d, J = 8.7 Hz, 2″,6″-H), 7.72 (2H, d, J = 8.7 Hz, 2′,6′-H), 7.74 (1H, d, J = 1.2 Hz, 6-H), 10.71 (1H, s, NH), 11.40 (1H, s, OH); 13C-NMR (DMSO-d6) 11.2, 55.6, 55.7, 98.7, 114.7, 115.0, 119.0, 119.8, 120.0, 124.7, 126.6, 128.3, 130.4, 132.4, 134.4, 138.5, 155.1, 158.7, 159.5; HRMS (ES): MH+, found: 387.1714. C24H23N2O3+ requires: 387.1709.
1-(5-(4-Fluorophenyl)-2-phenyl)-1H-indol-7-yl)ethanone Oxime (2e). A mixture of 1e (0.15 g, 0.42 mmol), hydroxylamine hydrochloride (0.04 g, 0.63 mmol) and pyridine (0.05 g, 0.63 mmol) in ethanol (10 mL) afforded 2e as a solid (0.12 g, 76%), Rf 0.58, mp. 197–199 °C; νmax (ATR) 484, 508, 533, 605, 684, 735, 785, 841, 885, 952, 989, 1027, 1137, 1153, 1228, 1274, 1360, 1376, 1449, 1507, 3218, 3412 cm−1; 1H-NMR (DMSO-d6) 2.39 (3H, s, CH3), 6.98 (1H, d, s, 3-H), 7.19 (2H, t, J = 8.7 Hz, 3″,5″-H), 7.27–7.37 (3H, m, 2x CH=C and 4′-H), 7.48 (2H, t, J = 7.5 Hz, 3′,5′-H), 7.63 (2H, d, J = 8.7 Hz, 2″,6″-H), 7.69 (1H, d, J = 1.2 Hz, 4-H), 7.78–7.80 (3H, m, 6-H and 2′,6′-H), 10.89 (1H, s, NH), 11.46 (1H, s, OH); 13C-NMR (DMSO-d6) 11.2, 100.0, 116.0 (d, 2JCF = 21.8 Hz), 120.0, 120.1, 120.2, 125.2 (2 × C), 128.3, 128.4 (d, 3JCF = 8.0 Hz), 129.4, 129.5, 129.9, 130.0, 131.9, 133.7, 134.7 (d, 4JCF = 2.3 Hz), 138.4, 155.0, 161.8 (d, 1JCF = 242.8 Hz); HRMS (ES): MH+, found: 371.1568. C23H20N2FO2+ requires: 371.1560.
1-(2-(4-Fluorophenyl)-5-(4-fluorostyryl)-1H-indol-7-yl)ethanone Oxime (2f). A mixture of 1f (0.15 g, 0.40 mmol), hydroxylamine hydrochloride (0.04 g, 0.60 mmol) and pyridine (0.05g, 0.60 mmol) in ethanol (10 mL) afforded 2f as a solid (0.12 g, 75%), Rf 0.67, mp. 238‒240 °C; νmax (ATR) 480, 509, 540, 578, 667, 718, 745, 772, 784, 805, 832, 881, 949, 988, 1097, 1154, 1223, 1286, 1376, 1431, 1467, 1505, 3234, 3409 cm−1; 1H-NMR (DMSO-d6) 2.37 (3H, s, CH3), 6.95 (1H, d, s, 3-H), 7.19 (2H, t, J = 8.7 Hz, 3″,5″-H), 7.29 (2H, d, J = 17.0 Hz, CH=CH), 7.31 (2H, d, J = 16.2 Hz, CH=CH), 7.32 (2H, t, J = 8.7 Hz, 3′,5′-H), 7.63 (2H, d, J = 8.7 Hz, 2″,6″-H), 7.67 (1H, d, J = 1.2 Hz, 4-H), 7.77 (1H, d, J = 1.2 Hz, 6-H), 7.83 (2H, d, J = 8.7 Hz, 2′,6′-H), 10.85 (1H, s, NH), 11.42 (1H, s, OH); 13C-NMR (DMSO-d6) 11.3, 100.0, 116.0 (d, 2JCF 21.8 Hz), 116.5 (d, 2JCF = 21.8 Hz), 120.1 (2 × C), 120.2, 125.2, 127.3 (d, 3JCF = 8.0 Hz), 128.3 (d, 3JCF = 8.0 Hz), 128.6 (d, 4JCF = 3.4 Hz), 129.5, 129.9, 130.0, 133.7, 134.7 (d, 4JCF = 3.5 Hz), 137.6, 154.9, 161.8 (d, 1JCF = 242.8 Hz), 162.2 (d, 1JCF = 243.9 Hz); HRMS (ES): MH+, found: 389.1465. C24H19N2F2O+ requires: 389.1465.
1-(5-(4-Fluorostyryl)-2-(4-methoxyphenyl)-1H-indol-7-yl)ethanone Oxime (2g). A mixture of 1f (0.15 g, 0.39 mmol), hydroxylamine hydrochloride (0.04 g, 0.59 mmol) and pyridine (0.05 g, 0.59 mmol) in ethanol (10 mL) afforded 2f as a solid (0.11 g, 76%), Rf 0.47, mp. 220–222 °C; νmax (ATR) 462, 507, 584, 606, 666, 715, 745, 786, 830, 838, 881, 960, 989, 1020, 1179, 1224, 1249, 1355, 1435, 1467, 1499, 3423, 3461 cm−1; 1H-NMR (DMSO-d6) 2.37 (3H, s, CH3), 3.80 (3H, s, OCH3), 6.85 (1H, s, 3-H), 7.04 (2H, d, J = 8.7 Hz, 3′,5′-H), 7.19 (2H, t, J = 8.7 Hz, 3″,5″-H), 7.23 (2H, d, J = 16.5 Hz, CH=CH), 7.32 (2H, d, J = 16.5 Hz, CH=CH), 7.61‒7.66 (3H, m, 4-H and 2″,6″-H), 7.71 (2H, d, J = 8.7 Hz, 2′,6′-H), 7.75 (1H, d, J = 1.2 Hz, 6-H), 10.75 (1H, s, NH), 11.41 (1H, s, OH); 13C-NMR (DMSO-d6) 11.3, 55.7, 98.7, 115.0 (2 × C), 116.0 (d, 2JCF = 21.8 Hz), 119.8, 119.9, 124.6, 125.0, 126.6, 128.3 (d, 3JCF = 8.0 Hz), 129.3, 130.0, 130.2, 133.5, 134.7 (d, 4JCF = 3.4 Hz), 138.6, 155.0, 159.6, 161.8 (d, 1JCF = 241.6 Hz); HRMS (ES): MH+, found: 401.1664. C25H22N2FO2+ requires: 401.1665.
3.3. Typical Procedure for the Beckmann Rearrangement of 3a–g
A stirred mixture of 2 (1 equivalent) and TFA (1 equivalent) in acetonitrile (20 mL/mmol of 2) was heated at 80 °C for 2 h. The mixture was cooled to room temperature and quenched with ice-cold water. The product was extracted with chloroform (3 × 20 mL) and the combined organic layers were dried over anhydrous MgSO4 and the salt was filtered off. The solvent was evaporated under reduced pressure and the residue was purified by column chromatography on a silica gel (60% EtOAc–hexane as eluent) to afford 3 as a solid. Products 3a–f were prepared in this fashion:
N-(2,5-Bis(4-fluorophenyl)-1H-indol-7-yl) Acetamide (3a). A mixture of 2a (0.10 g, 0.28 mmol) and TFA (0.03 g, 0.28 mmol) in acetonitrile (5 mL) afforded 3a as solid (0.08 g, 80%); Rf 0.51, mp. 272–275 °C; νmax (ATR) 459, 509, 538, 595, 653, 753, 795, 810, 828, 1010, 1102, 1159, 1218, 1230, 1280, 1433, 1474, 1513, 1549, 1630, 1653, 3243, 3339 cm−1; 1H-NMR (DMSO-d6) 2.19 (3H, s, CH3), 6.92 (1H, s, 3-H), 7.25 (2H, t, J = 8.7 Hz, 3′,5′-H), 7.34 (2H, t, J = 8.7 Hz, 3″,5″-H), 7.53 (1H, d, J = 1.2 Hz, 4-H), 7.63 (2H, d, J = 8.7 Hz, 2″,6″-H), 7.85–7.90 (3H, m, 6-H and 2′,6′-H), 9.73 (1H, s, NH), 11.10 (1H, s, NH); 13C-NMR (DMSO-d6) 24.5, 100.3, 113.3, 114.6, 116.0 (d, 2JCF = 21.8 Hz), 116.1, 116.4 (d, 2JCF = 20.6 Hz), 124.4, 127.6 (d, 3JCF = 8.0 Hz), 128.8 (d, 4JCF = 3.4 Hz), 128.9 (d, 3JCF = 8.0 Hz), 130.9, 131.8, 137.6, 138.5 (d, 4JCF = 2.3 Hz), 161.8 (d, 1JCF = 241.6 Hz), 162.2 (d, 1JCF = 242.8 Hz), 169.0; HRMS (ES): MH+, found: 363.1311. C22H17N2F2O+ requires: 363.1231.
N-(5-(4-Fluorophenyl)-2-(4-methoxyphenyl)-1H-indol-7-yl) Acetamide (3b). A mixture of 2b (0.10 g, 0.27 mmol) and TFA (0.03 g, 0.27 mmol) in acetonitrile (5 mL) afforded 3b as a solid (0.08 g, 75%); Rf 0.37, mp. 213–215 °C; νmax (ATR) 518, 533, 595, 745, 774, 811, 831, 1025, 1101, 1177, 1226, 1253, 1367, 1390, 1426, 1472, 1516, 1553, 1609, 1654, 3316 cm−1; 1H-NMR (DMSO-d6) 2.20 (3H, s, CH3), 3.80 (3H, s, OCH3), 6.80 (1H, s, 3-H), 7.06 (2H, d, J = 8.7 Hz, 3′,5′-H), 7.25 (2H, t, J = 8.7 Hz, 3″,5″-H), 7.49 (1H, d, J = 1.2 Hz, 4-H), 7.62 (2H, t, = 8.7 Hz, 2″,6″-H), 7.76 (2H, d, J = 8.7 Hz 2′,6′-H), 7.87 (1H, d, J = 1.2 Hz, 6-H), 9.72 (1H, s, NH), 11.01 (1H, s, NH); 13C-NMR (DMSO-d6) 24.5, 55.7, 99.0, 112.7, 114.2, 114.9, 116.0 (d, 2JCF 20.6 Hz), 124.3, 124.9, 126.9, 128.4, 128.8 (d, 3JCF 8.0 Hz), 131.1, 131.6, 138.6 (d, 4JCF 3.5 Hz), 159.5, 161.7 (d, 1JCF 241.7 Hz), 169.0; HRMS (ES): MH+, found: 375.1510. C23H20N2FO2+ requires: 375.1509.
N-(2-(4-Fluorophenyl)-5-(4-methoxyphenyl)-1H-indol-7-yl) Acetamide (3c). A mixture of 2c (0.10 g, 0.27 mmol) and TFA (0.03 g, 0.27 mmol) in acetonitrile (5 mL) afforded 3c as a solid (0.07 g, 73%); Rf 0.47, mp. 216–218 °C; νmax (ATR) 505, 523, 550, 600, 719, 749, 786, 805, 826, 1032, 1159, 1178, 1231, 1371, 1427, 1480, 1516, 1566, 1631, 1654, 3258, 3320 cm−1; 1H-NMR (DMSO-d6) 2.19 (3H, s, CH3), 3.77 (3H, s, OCH3), 6.90 (1H, s, 3-H), 6.99 (2H, d, J = 8.4 Hz, 3″,5″-H), 7.34 (2H, t, J = 8.7 Hz, 3′,5′-H), 7.48 (1H, d, J = 1.2 Hz, 4-H), 7.53 (2H, t, J = 8.4 Hz, 2″,6″-H), 7.84–7.89 (3H, m, 6-H and 2′,6′-H), 9.71 (1H, s, NH), 11.04 (1H, s, NH); 13C-NMR (DMSO-d6) 24.5, 55.6, 100.2, 113.2, 114.0, 114.7, 116.4 (d, 2JCF = 20.6 Hz), 124.3, 127.5 (d, 3JCF = 8.0 Hz), 128.1, 128.5, 129.1 (d, 4JCF = 3.5 Hz), 130.9, 132.6, 134.5, 137.4, 158.6, 162.1 (d, 1JCF = 242.8 Hz), 169.0; HRMS (ES): MH+, found: 375.1508. C23H20N2FO2+ requires: 375.1509.
N-(2,5-Bis(4-methoxyphenyl)-1H-indol-7-yl) Acetamide (3d). A mixture of 2d (0.10 g, 0.26 mmol) and TFA (0.03 g, 0.26 mmol) in acetonitrile (5 mL) afforded 3d as a solid (0.07 g, 70%); Rf 0.31, mp. 236–239 °C; νmax (ATR) 529, 564, 600, 723, 775, 830, 1030, 1112, 1176, 1244, 1280, 1372, 1432, 1440, 1514, 1550, 1605, 1632, 1656, 3269, 3321 cm−1; 1H-NMR (DMSO-d6) 2.19 (3H, s, CH3), 3.77 (3H, s, OCH3), 3.80 (3H, s, OCH3), 6.79 (1H, s, 3-H), 6.99 (2H, d, J = 9.0 Hz, 3″,5″-H), 7.06 (2H, d, J = 8.1 Hz, 3′,5′-H), 7.45 (1H, d, J = 1.2 Hz, 4-H), 7.53 (2H, d, J = 8.4 Hz, 2″,6″-H), 7.76 (2H, d, J = 8.7 Hz 2′,6′-H), 7.83 (1H, d, J = 1.2 Hz, 6-H), 9.70 (1H, s, NH), 10.96 (1H, s, NH); 13C-NMR (DMSO-d6) 24.5, 55.6, 55.7, 98.9, 112.6, 113.7, 114.7, 114.9 (2 × C), 124.2, 125.0, 126.9, 128.0, 131.1, 132.4, 134.6, 138.4, 158.6, 159.4, 168.9; HRMS (ES): MH+, found: 387.1708. C24H23N2O3+ requires: 387.1709.
(E)-N-(5-(4-Fluorostyryl)-2-phenyl-1H-indol-7-yl) Acetamide (3e). A mixture of 2e (0.10 g, 0.27 mmol) and TFA (0.03 g, 0.27 mmol) in acetonitrile (5 mL) afforded 3e as a solid (0.08 g, 78%); Rf 0.51, mp. 262–264 °C; νmax (ATR) 481, 512, 564, 598, 689, 738, 756, 799, 814, 847, 957, 1158, 1204, 1223, 1286, 1353, 1455, 1507, 1646, 3267, 3299 cm−1; 1H-NMR (DMSO-d6) 2.18 (3H, s, CH3), 6.90 (1H, s, 3-H), 7.03 (1H, d, J = 16.5 Hz, CH=CH), 7.16 (2H, t, J = 8.4 Hz, 3″,5″-H), 7.22 (1H, d, J = 16.5 Hz, CH=CH), 7.33–7.35 (1H, m, 4′-H), 7.45–7.48 (3H, m, 4-H and 2″,6″-H), 7.46–7.59 (2H, m, 3′,5′-H), 7.82 (2H, d, J = 7.8 Hz, 2′,6′-H) 7.88 (1H, d, J = 1.2 Hz, 6-H), 9.70 (1H, s, NH), 11.12 (1H, s, NH); 13C-NMR (DMSO-d6) 24.5, 100.2, 112.4, 115.7, 115.9 (d, 2JCF = 20.6 Hz), 124.3, 124.7, 125.5, 128.3, 128.2 (d, 3JCF = 8.0 Hz), 128.4, 129.3, 129.4, 129.5, 130.4, 130.5, 132.3, 134.6 (d, 4JCF = 2.3 Hz), 138.4, 161.7 (d, 1JCF = 242.8 Hz), 170.0; HRMS (ES): MH+, found: 371.1554. C24H20N2FO+ requires: 371.1560.
(E)-N-(2-(4-Fluorophenyl)-5-(4-fluorostyryl)-1H-indol-7-yl) Acetamide (3f). A mixture of 2f (0.10 g, 0.28 mmol) and TFA (0.03 g, 0.28 mmol) in acetonitrile (5 mL) afforded 3f as a solid (0.08 g, 77%), Rf 0.51, mp. 278–280 °C; νmax (ATR) 467, 509, 552, 581, 609, 761, 789, 831, 848, 957, 1012, 1099, 1159, 1221, 1232, 1352, 1441, 1477, 1502, 1545, 1652, 3257, 3301 cm−1; 1H-NMR (DMSO-d6) 2.19 (3H, s, CH3), 6.88 (1H, s, 3-H), 7.04 (2H, d, J = 16.5 Hz, CH=CH), 7.18 (2H, t, J = 8.7 Hz, 3′,5′-H), 7.23 (2H, d, J = 16.5 Hz, CH=CH), 7.34 (2H, t, J = 8.7 Hz, 3″,5″-H), 7.49 (1H, d, J = 1.2 Hz, 4-H), 7.63 (2H, t, J = 8.7 Hz, 2″,6″-H), 7.84–7.89 (3H, m, 6-H and 2′,6′-H), 9.68 (1H, s, NH), 11.08 (1H, s, NH); 13C-NMR (DMSO-d6) 24.4, 100.2, 110.0, 112.7, 115.9 (d, 2JCF = 21.8 Hz), 116.4 (d, 2JCF = 20.6 Hz), 124.2, 124.7, 127.6 (d, 3JCF = 9.1 Hz), 128.3 (d, 3JCF = 8.0 Hz), 128.9 (d, 4JCF = 2.3 Hz), 129.5, 129.6, 130.3, 130.6, 134.6 (d, 4JCF = 2.3 Hz), 137.5, 161.7 (d, 1JCF = 241.6 Hz), 162.2 (d, 1JCF = 243.9 Hz), 169.0; HRMS (ES): MH+, found: 389.1465. C24H19N2F2O+ requires: 389.1465.
(E)-N-(5-(4-Fluorostyryl)-2-(4-methoxyphenyl)-1H-indol-7-yl) Acetamide (3g). A mixture of 2g (0.10 g, 0.25 mmol) and TFA (0.03 g, 0.25 mmol) in acetonitrile (5 mL) afforded 3g as a solid (0.07 g, 69%); Rf 0.33, mp. 233–235 °C; νmax (ATR) 488, 516, 549, 584, 614, 746, 786, 809, 830, 843, 954, 1025, 1095, 1159, 1181, 1223, 1253, 1276, 1346, 1371, 1460, 1502, 1554, 1609, 1646, 3298 cm−1; 1H-NMR (DMSO-d6) 2.18 (3H, s, CH3), 3.80 (3H, s, OCH3), 6.77 (1H, d, s, 3-H), 7.02 (1H, d, J = 15.9 Hz, CH=CH) 7.05 (2H, d, J = 8.1 Hz, 3′,5′-H), 7.17 (2H, t, J = 8.7 Hz, 3″,5″-H), 7.22 (2H, d, J = 15.9 Hz, CH=CH), 7.45 (1H, d, J = 1.2 Hz, 4-H), 7.62 (2H, d, J = 8.7 Hz, 2″,6″-H), 7.75 (2H, d, J = 8.7 Hz, 2′,6′-H), 7.82 (1H, d, J = 1.2 Hz, 6-H), 9.66 (1H, s, NH), 10.99 (1H, s, NH); 13C-NMR (DMSO-d6) 24.5, 55.7, 98.9, 112.1, 114.9, 115.4, 115.9 (d, 2JCF = 21.8 Hz), 124.1, 124.6, 124.9, 126.9, 128.3 (d, 3JCF = 8.0 Hz), 129.1, 129.3, 130.5 130.8, 134.6 (d, 4JCF = 3.4 Hz), 138.6, 159.5, 161.7 (d, 1JCF = 242.8 Hz), 170.0; HRMS (ES): MH+, found: 401.1658. C25H22N2FO2+ requires: 401.1665.
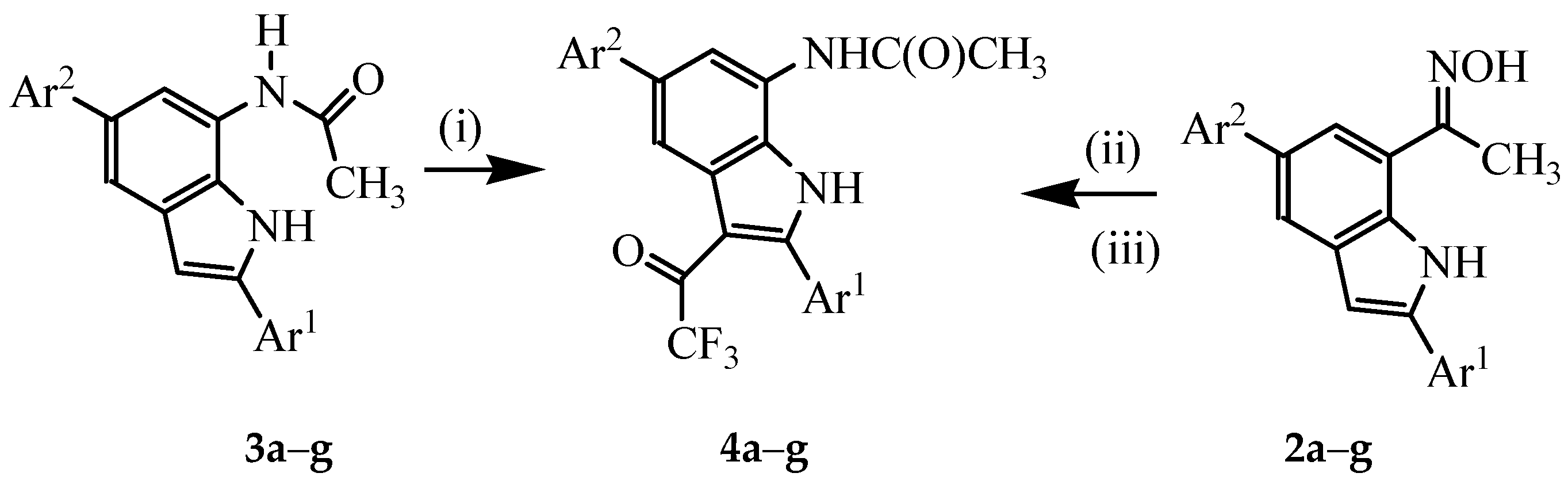
3.4. Typical Procedure for the Trifluoroacetylation of 3a–g
A mixture of 3 (1 equivalent) and TFAA (1.5 equivalent) in THF (25 mL/mmol of 3) was heated at 60 °C for 5 h. The mixture was cooled to room temperature quenched with saturated sodium hydrogen carbonate solution. The mixture was extracted with chloroform (3 × 20 mL) and the combined organic layers were dried with anhydrous MgSO4 and the salt was filtered off. The solvent was evaporated under reduced pressure and the residue was purified by column chromatography on a silica gel (60% EtOAc–hexane as eluent) to afford 4 as a solid. Compounds 4a–g were prepared in this fashion.
N-(2,5-Bis(4-fluorophenyl)-3-(2,2,2-trifluoroacetyl)-1H-indol-7-yl) Acetamide (4a). A mixture of 3a (0.15 g, 0.41 mmol) and TFAA (0.13 g, 0.62 mmol) in THF (10 mL) afforded 4a as a solid (0.15 g, 79%), Rf 0.51, mp. 215–218 °C; νmax (ATR) 436, 514, 565, 589, 644, 721, 744, 814, 832, 900, 927, 1013, 1041, 1098, 1143, 1186, 1202, 1225, 1374, 1436, 1513, 1605, 1634, 1653, 3251, 3312 cm−1; 1H-NMR (DMSO-d6) 2.13 (3H, s, CH3), 7.30 (2H, t, J = 8.7 Hz, 3″,5″-H), 7.40 (2H, t, J = 8.7 Hz, 3′,5′-H), 7.61–7.72 (4H, m, 2′,6′-H and 2″,6″-H), 7.91 (1H, d, J = 1.2 Hz, 4-H), 8.02 (1H, d, J = 1.2 Hz, 6-H), 9.83 (1H, s, NH), 12.47 (1H, s, NH); 13C-NMR (DMSO-d6) 24.2, 108 .1, 115.6 (d, 2JCF = 21.8 Hz), 116.2 (d, 2JCF = 21.8 Hz), 116.3, 116.7 (q, 1JCF = 288.6 Hz), 125.2, 127.9 (d, 4JCF = 3.5 Hz), 128.2, 128.7, 129.2 (d, 3JCF = 8.0 Hz), 132.8 (d, 3JCF = 8.0 Hz), 135.6, 137.9 (d, 4JCF = 2.3 Hz), 148.2, 162.2 (d, 1JCF = 242.8 Hz), 163.6 (d, 1JCF = 246.2 Hz), 169.2, 170.8, 175.7 (q, 2JCF = 34.4 Hz); 19F-NMR (DMSO-d6) -115.9 (1F, ddd, J = 5.9, 8.7 and 14.7 Hz, 4′′-CF), −110.5 (1F, ddd, J = 4.8, 8.7 and 13.8 Hz, 4′-CF), −71.2 (3F, s, COCF3); HRMS (ES): MH+, found 459.1131. C24H16 N2F5O2+ requires: 459.1132.
N-(5-(4-Fluorophenyl)-2-(4-methoxyphenyl)-3-(2,2,2-trifluoroacetyl)-1H-indol-7-yl) Acetamide (4b). A mixture of 3b (0.15 g, 0.40 mmol) and TFAA (0.13 g, 0.60 mmol) in THF (10 mL) afforded 4b as a solid (0.15 g, 86%), Rf 0.34, mp. 222–224 °C; νmax (ATR) 417, 517, 539, 569, 594, 713, 722, 834, 912, 963, 1014, 1038, 1144, 1180, 1206, 1249, 1291, 1435, 1473, 1513, 1612, 1634, 1654, 1676, 3209, 3276 cm−1; 1H-NMR (DMSO-d6) 2.13 (3H, s, CH3), 3.84 (3H, s, OCH3), 7.11 (2H, d, J = 8.4 Hz, 3′,5′-H), 7.30 (2H, t, J = 8.7 Hz, 3″,5″-H), 7.57 (2H, d, J = 8.4 Hz, 2′,6′-H), 7.63 (2H, t, J = 8.7 Hz, 2″,6″-H), 7.91 (1H, d, J = 1.2 Hz, 4-H), 7.99 (1H, d, J = 1.2 Hz, 6-H), 9.80 (1H, s, NH), 12.33 (1H, s, NH); 13C-NMR (DMSO-d6) 24.2, 55.8, 107.7, 114.1 (2 × C), 115.0, 115.9, 116.2 (d, 2JCF = 20.6 Hz), 116.8 (q, 1JCF J = 288.5 Hz), 123.7, 125.1, 127.9, 129.0, 129.2 (d, 3JCF = 8.0 Hz), 131.8, 135.4, 138.0 (d, 4JCF = 3.4 Hz), 149.4, 161.1, 162.1 (d, 1JCF = 242.8 Hz), 169.2, 176.0 (q, 2JCF = 35.5 Hz); 19F-NMR (DMSO-d6) −116.0 (1F, ddd, J = 5.9, 9.0 and 14.9 Hz, 4″-CF), −71.0 (3F, s, COCF3); HRMS (ES): MH+, found: 471.1333. C25H19N2F4O3+ requires: 471.1332.
N-(3-(2,2,2-Trifluoroacetyl)-2-(4-fluorophenyl)-5-(4-methoxyphenyl)-1H-indol-7-yl) Acetamide (4c). A mixture of 3c (0.15 g, 0.40 mmol) and TFAA (0.13 g, 0.60 mmol) in THF (10 mL) afforded 4c as a solid (0.17 g, 89%), Rf 0.42, mp. 255–257 °C; νmax (ATR) 448, 504, 517, 545, 592, 624, 643, 729, 783, 808, 832, 850, 901, 929, 1019, 1123, 1157, 1180, 1219, 1244, 1302, 1369, 1448, 1515, 1566, 1604, 1632, 1657, 3185, 3245 cm−1; 1H-NMR (DMSO-d6) 2.11 (3H, s, CH3), 3.78 (3H, s, OCH3), 7.04 (2H, d, J = 7.5 Hz, 3″,5″-H), 7.39 (2H, t, J = 7.5 Hz, 3′,5′-H), 7.55 (2H, d, J = 7.5 Hz, 2″,6″-H), 7.65–7.70 (2H, m, 2′,6′-H), 7.87 (1H, d, J = 1.2 Hz, 4-H), 7.99 (1H, d, J = 1.2 Hz, 6-H), 9.84 (1H, s, NH), 12.44 (1H, s, NH); 13C-NMR (DMSO-d6) 24.1, 55.6, 108.0, 114.5, 114.9, 115.6 (d, 2JCF = 21.8 Hz), 116.1, 116.8 (q, 1JCF = 288.5 Hz), 125.2, 127.9, 128.0 (d, 4JCF = 3.5 Hz), 128.3, 128.7, 132.8 (d, 3JCF = 9.1 Hz), 133.8, 136.4, 148.1, 159.2, 163.5 (d, 1JCF = 246.2 Hz), 169.3, 175.7 (q, 2JCF 35.5 Hz); 19F-NMR (282 MHz, DMSO-d6) −111.9 (1F, ddd, J 4.8, 8.7 and 14.7 Hz, 4′ -CF), −71.2 (3F, s, COCF3); HRMS (ES): MH+, found 471.1334. C25H19N2F4O3+ requires: 471.1332.
N-(2,5-Bis(4-methoxyphenyl)-3-(2,2,2-trifluoroacetyl)-1H-indol-7-yl) Acetamide (4d). A mixture of 3d (0.15 g, 0.39 mmol) and TFAA (0.12 g, 0.58 mmol) in THF (10 mL) afforded 4b as a solid (0.17 g, 90%), Rf 0.28, mp. 254–257 °C; νmax (ATR) 528, 545, 594, 643, 714, 729, 798, 852, 900, 928, 966, 1026, 1137, 1178, 1201, 1235, 1253, 1295, 1436, 1450, 1516, 1566, 1607, 1655, 1673, 3171, 3251 cm−1; 1H-NMR (DMSO-d6) 2.13 (3H, s, CH3), 3.79 (3H, s, OCH3), 3.85 (3H, s, OCH3), 7.05 (2H, d, J = 8.7 Hz, 3″,5″-H), 7.12 (2H, d, J = 8.7 Hz, 3′,5′-H), 7.54 (2H, d, J = 8.4 Hz, 2″,6″-H), 7.57 (2H, d, J = 8.4 Hz, 2′,6′-H), 7.88 (1H, d, J = 1.2 Hz, 4-H), 7.98 (1H, d, J = 1.2 Hz, 6-H), 9.78 (1H, s, NH), 12.28 (1H, s, NH); 13C-NMR (DMSO-d6) 24.2, 55.6, 55.8, 107.6, 114.1, 114.5, 114.9, 115.8, 116.8 (q, 1JCF = 288.6 Hz), 123.7, 125.0, 127.7, 128.1, 128.3, 129.0, 131.8, 133.9, 136.2, 149.2, 159.2, 161.1, 169.2, 175.9 (q, 2JCF = 34.4 Hz); 19F-NMR (DMSO-d6) −71.0 (3F, s, COCF3); HRMS (ES): MH+, found: 483.1533. C26H22N2F3O4+ requires: 483.1532.
(E)-N-[5,[5-(4-Fluorostyryl)-3-(2,2,2-trifluoroacetyl)-2-phenyl-1H-indol-7-yl] Acetamide (4e). A mixture of 3e (0.15 g, 0.41 mmol) and TFAA (0.13g, 0.61 mmol) in THF (10 mL) afforded 4e as a solid (0.15 g, 80%), Rf 0.35, mp. 230–233 °C; νmax (ATR) 475, 507, 527, 599, 674, 702, 731, 750, 773, 818, 852, 915, 962, 1012, 1141, 1184, 1224, 1260, 1285, 1370, 1434, 1456, 1508, 1558, 1628, 1657, 1667, 3198, 3331 cm−1; 1H-NMR (DMSO-d6) 2.12 (3H, s, CH3), 7.13 (1H, d, J = 16.5 Hz, CH=CH), 7.19 (2H, t, J = 8.1 Hz, 3″,5″-H), 7.37 (1H, d, J = 16.5 Hz, CH=CH), 7.57–8.00 (5H, m, 4′-H, 3′,5′-H, and 2″,6″-H), 7.68–7.72 (2H, m, 2′,6′-H), 7.92 (1H, d, J = 1.2 Hz, 4-H), 8.02 (1H, d, J = 1.2 Hz, 6-H) 9.74 (1H, s, NH), 12.43 (1H, s, NH); 13C-NMR (DMSO-d6) 24.1, 107.1, 115.4, 116.0 (d, 2JCF = 21.8 Hz), 116.2, 116.7 (q, 1JCF = 288.5 Hz), 124.8, 125.0, 126.6, 128.6, 128.8 (d, 3JCF = 8.0 Hz), 129.6, 129.7, 130.3, 130.4, 131.6, 133.3, 134.2 (d, 4JCF = 2.3 Hz), 149.0, 162.0 (d, 1JCF = 242.8 Hz), 169.2, 176.0 (q, 2JCF = 35.5 Hz); 19F-NMR (DMSO-d6) −114.6 (1F, ddd, J = 5.9, 8.7 and 14.7 Hz, 4″-CF), −71.02 (3F, s, COCF3); HRMS (ES): MH+, found: 467.1384. C26H19N2F4O2+ requires: 467.1383.
N-[5,[5-(4-Fluorostyryl)-3-(2,2,2-trifluoroacetyl)-2-(4-fluorophenyl)-1H-indol-7-yl] Acetamide (4f). A mixture of 3f (0.15 g, 0.39 mmol) and TFAA (0.12 g, 0.58 mmol) in THF (10 mL) afforded 4f as a solid (0.15 g, 81%), Rf 0.34, mp. 222–225 °C; νmax (ATR) 478, 514, 524, 578, 624, 733, 785, 816, 845, 920, 964, 1012, 1097, 1156, 1197, 1226, 1301, 1372, 1446, 1507, 1598, 1641, 1657, 3181, 3251 cm−1; 1H-NMR (DMSO-d6) 2.12 (3H, s, CH3), 7.14 (1H, d, J = 16.5 Hz, CH=CH), 7.20 (2H, t, J = 9.3 Hz, 3″,5″-H), 7.37 (1H, d, J = 16.5 Hz, CH=CH), 7.41 (2H, t, J = 8.7 Hz, 3′,5′-H), 7.66–7.73, (4H, m, 2′,6′-H and 2″,6″-H), 7.88 (1H, d, J = 1.2 Hz, 4-H), 8.01 (1H, d, J = 1.2 Hz, 6-H), 9.77 (1H, s, NH), 12.43 (1H, s, NH); 13C-NMR (DMSO-d6) 24.1, 107.1, 114.8, 115.6 (d, 2JCF = 21.8 Hz), 116.0 (d, 2JCF = 20.6 Hz), 116.2, 116.3, 117.0 (q, 1JCF = 230.2 Hz), 125.0, 126.6, 128.0 (d, 4JCF 3.5 Hz), 128.6, 128.8 (d, 3JCF 8.0 Hz), 129.0, 129.6, 132.8 (d, 3J J 8.0 Hz), 133.3, 134.2 (d, 4JCF = 3.5 Hz), 147.9, 162.0 (d, 1JCF = 243.9 Hz), 163.5 (d, 1JCF = 246.2 Hz), 175.8 (q, 2JCF = 34.4 Hz); 19F-NMR (DMSO-d6) −114.6 (1F, ddd, J = 5.9, 9.0 and 14.9 Hz 4″-CF), −110.9 (1F, ddd, J = 5.9, 9.0 and 14.9 Hz, 4′-CF), −71.2 (3F, s, COCF3); HRMS (ES): MH+, found: 485.1285. C26H18N2F5O2+ requires: 485.1288.
((E)-N-(5-(4-Fluorostyryl)-2-(4-methoxyphenyl)-3-(2,2,2-trifluoroacetyl)-1H-indol-7-yl) Acetamide (4g). A mixture of 3g (0.15 g, 0.38 mmol) and TFAA (0.12 g, 0.56 mmol) in THF (10 mL) afforded 4g as a solid (0.14 g, 74%), Rf 0.20, mp. 264–267 °C; νmax (ATR) 466, 527, 613, 633, 730, 749, 749, 817, 817, 846, 893, 922, 964, 1007, 1030, 1046, 1118, 1133, 1177, 1200, 1212, 1229, 1251, 1292, 1369, 1391, 1442, 1506, 1560, 1606, 1653, 1668, 3179, 3240 cm−1; 1H-NMR (DMSO-d6) 2.12 (3H, s, CH3), 3.84 (3H, s, OCH3), 7.11 (2H, d, J = 8.7 Hz, 3′,5′-H), 7.14‒7.22 (3H, m, CH=CH and 3″,5″-H), 7.36 (1H, d, J = 16.5 Hz, CH=CH), 7.55 (2H, d, J = 8.7 Hz, 2′,6′-H), 7.70 (2H, d, J = 8.7 Hz, 2″,6″-H), 7.89 (1H, d, J = 1.2 Hz, 4-H), 7.99 (1H, d, J = 1.2 Hz, 6-H) 9.74 (1H, s, NH), 12.30 (1H, s, NH); 13C-NMR (DMSO-d6) 24.2, 55.8, 107.7, 114.1 (2 × C), 115.2, 116.0 (d, 2JCF = 20.6 Hz), 116.7 (q, 1JCF J = 289.7 Hz), 123.7, 124.9, 126.5, 128.7, 128.8 (d, 3JCF = 8.0 Hz), 129.6, 129.7, 131.8, 133.1, 134.2 (d, 4JCF = 2.3 Hz), 149.1, 161.1, 162.0 (d, 1JCF = 242.8 Hz), 169.2, 176.1 (q, 2JCF = 35.6 Hz); 19F-NMR (DMSO-d6) -114.6 (1F, ddd, J = 5.9, 9.0 and 14.9 Hz, 4″-CF), -71.0 (3F, s, COCF3); HRMS (ES): MH+, found: 497.1488. C27H21F4N2O3+ requires: 497.1488.
3.5. Typical Procedure for the One-pot Sequential Beckmann Rearrangement of 2a–g and Subsequent Trifluoroacetylation
A mixture of 2 (1 equivalent) and TFA (1.2 equivalent) in acetonitrile (25 mL/mmol of 2) was refluxed at 80 °C for 2 h. TFAA (2 equiv.) was added to the mixture and heating was continued for 5 h at this temperature. The solvent was evaporated under reduced pressure and the residue was quenched with an ice-cold water. The product was extracted with chloroform and the combined organic layers were dried over anhydrous MgSO4. The salt was filtered off and then the solvent was evaporated under reduced pressure on a rotary evaporator. The residue was purified by column chromatography on a silica gel to afford 4 as a solid. Products 4a–f were prepared in this fashion.
N-(2,5-Bis(4-fluorophenyl)-3-(2,2,2-trifluoroacetyl)-1H-indol-7-yl) Acetamide (4a). A mixture of 2a (0.15 g, 0.41 mmol) and TFA (0.06 g, 0.50 mmol) in CH3CN (10 mL) followed by treatment with TFAA (0.17 g, 0.83 mmol) afforded 4a as a solid (0.14 g, 74%).
N-(5-(4-Fluorophenyl)-2-(4-methoxyphenyl)-3-(2,2,2-trifluoroacetyl)-1H-indol-7-yl) Acetamide (4b). A mixture of 2b (0.15 g, 0.40 mmol) and TFA (0.05 g, 0.48 mmol) in CH3CN (10 mL) followed by treatment with TFAA (0.17 g, 0.80 mmol) afforded 4b as a solid (0.15 g, 80%).
N-(3-(2,2,2-Trifluoroacetyl)-2-(4-fluorophenyl)-5-(4-methoxyphenyl)-1H-indol-7-yl) Acetamide (4c). A mixture of 2c (0.15 g, 0.40 mmol) and TFA (0.05 g, 0.48 mmol) in CH3CN (10 mL) followed by treatment with TFAA (0.17 g, 0.80 mmol) afforded 4c as a solid (0.16 g, 82%).
N-(3-(2,2,2-Trifluoroacetyl)-2,5-bis(4-methoxyphenyl)-1H-indol-7-yl) Acetamide (4d). A mixture of 2d (0.15 g, 0.39 mmol) and TFA (0.05 g, 0.47 mmol) in CH3CN (10 mL) followed by treatment with TFAA (0.16 g, 0.78 mmol) afforded 4d as a solid (0.16 g, 84%).
(E)-N-[5,[5-(4-Fluorostyryl)-3-(2,2,2-trifluoroacetyl)-2-phenyl-1H-indol-7-yl] Acetamide (4e). A mixture of 2e (0.15 g, 0.41 mmol) and TFA (0.6 g, 0.48 mmol) in CH3CN (10 mL) followed by treatment with TFAA (0.17 g, 0.81 mmol) afforded 4e as a solid (0.14 g, 76%).
(E)-N-[5,[5-(4-Fluorostyryl)-3-(2,2,2-trifluoroacetyl)-2-(4-fluorophenyl)-1H-indol-7-yl] Acetamide (4f). A mixture of 2f (0.15 g, 0.39 mmol) and TFA (0.05 g, 0.46 mmol) in CH3CN (10 mL) followed by treatment with TFAA (0.16 g, 0.77 mmol) afforded 4f as a solid (0.15 g, 79%).
((E)-N-(5-(4-Fluorostyryl)-2-(4-methoxyphenyl)-3-(2,2,2-trifluoroacetyl)-1H-indol-7-yl) Acetamide (4g). A mixture of 2g (0.15 g, 0.38 mmol) and TFA (0.05 g, 0.46 mmol) in CH3CN (10 mL) followed by treatment with TFAA (0.16 g, 0.75 mmol) afforded 4g as a solid (0.13 g, 72%).
3.6. pLDH Asssay
Three-fold serial dilutions of the test compounds were incubated in triplicates with 3D7 strain P. falciparum parasites in a transparent 96-well flat bottom plate. DMSO and chloroquine were used as negative and positive controls, respectively. The plate was put in an airtight box, gassed and incubated with complete RPMI 1640 medium for 48 h. At the end of incubation, Malstat reagent was added to the 96-well plate followed by developing with NBT/PES (nitro blue tetrazolium + phenazine ethosulphate) reagent. Parasite growth was determined spectrophotometrically at 620 nm, by measuring the activity of the pLDH in control and drug-treated cultures using an Infinite F500 multiwell plate reader (Tecan Group Ltd., Zürich, Switzerland). The OD values from control wells devoid of drug were referred to as having 100% pLDH activity. The IC50 are expressed as the % parasite survival relative to the control, calculated from fitted sigmoidal dose response curves. The dose response curves were obtained by plotting percentage parasite survival against the logarithm of the concentration using the GraphPad Prism software package (GraphPad software Inc., La Jolla, CA, USA). IC50 values were calculated graphically by interpolation from these curves.
3.7. In Vitro Cytotoxicity Assay
Monkey kidney cells (Vero) used in this experiment were obtained from Cellonex (Johannesburg, South Africa). The cells were maintained in Dulbecco’s Modified Eagle’s (DMEM, HyClone, Thermo Scientific, Aalst, Belgium) supplemented with 0.4 mML-glutamine and sodium pyruvate and 10% foetal bovine serum (FBS, HyClone, Thermo Scientific). The cells of a sub-confluent culture were harvested using trypsin-EDTA (HyClone, Thermo Scientific) and centrifuged at 200×
g for 5 min and re-suspended in growth medium to 5 × 10
4 cells/mL. A total of 200 µL of the cell suspension was pipetted into each well of columns 2 to 11 of a 96 well culture plate. The same amount of the growth medium was added to wells of column 1 and 12 to maintain humidity and minimize the edge effect. The plates were incubated at 37 °C in a 5% CO
2 incubator overnight until the cells were in the exponential phase of growth. After incubation, the DMEM was aspirated from the cells and replaced with 200 µL of different concentrations of the test samples (0.1–100 µM). Each dilution of the test sample was tested in quadruplicate in each experiment and the experiments were repeated three times. The plates were again incubated for 2 days at 37 °C in a 5% incubator. A negative control (untreated cells) and positive control (cells treated with different concentrations of doxorubicin hydrochloride, Sigma, Schnelldorf, Germany) were included. After incubation, 30 µL of 5 mg/mL MTT, (Sigma-Aldrich, GmBH, Schnelldorf, Germany) in phosphate buffered saline PBS was added to each well and the plates were incubated for a further 4 h at 37 °C. After incubation with MTT, the medium in each well was removed and the formazan crystals formed were dissolved by adding 50 µL of DMSO to each well of the plates. The plates were gently shaken until the crystals were dissolved. The amount of MTT reduction was measured immediately by detecting the absorbance using a microplate reader at a wavelength of 570 nm (BioTek Synergy, Analytical and Diagnostic Products, Johannesburg, South Africa). The wells in column 1 and 12, containing medium and MTT but no cells was used to blank the microplate reader. The percentage of cell viability was calculated using the formula below:
The LC50 values (lethal concentration at which 50% of the cells are killed) were calculated as the concentration of the test sample that resulted in 50% reduction of absorbance compared to untreated cells. The intensity of the MTT formazan produced by living metabolically active cells is directly proportional to the number of live cells present.
3.8. Methodology-Docking Simulation
3.8.1. Protein Structure
The starting structure of parasite lactate dehydrogenase (pLDH) was obtained from PDB (PDB id: 1T24) with 1.7 Å resolution. All heteroatoms and water molecules were removed pLDH. Polar hydrogen atoms, Kollman-Amber united atom partial charges and solvation parameters were added by utilizing AutoDockTools [
29].
3.8.2. Ligand Structure
The initial structure of positive control 4-hydroxy-1,2,5-oxazole-3-carboxylic acid (OXD) was obtained from the ligand of pLDH (PDB id: 1T24) while the coordinates for test compound
3a,
3f,
4a and
4g were generated using Hyperchem 7.0 (Hypercube Inc., Gainesville, FL, USA). All ligands were retained with polar hydrogen atoms. Gasteiger charges and torsional angles were added by utilizing AutoDockTools [
29].
3.8.3. Molecular Docking Simulation
Grid maps of 40 × 40 × 40 points with 0.375 Å spacing were centered at OXD binding site in the crystal structure (PDB id: 1T24) for positive control docking and the docking of lactate (the substrate of pLDH). While for compound
3(
a,
f) and
4(
a,
g), the grid maps of 50 × 50 × 50 points with 0.375 Å spacing were centered at NAD+ of the crystal structure (PDB id: 1T24). A total of 200 docking runs by AutoDock4.2.6 [
30] were performed by employing Lamarckian genetic algorithm with 2,500,000 energy evaluations per run and maximum number of 27,000 generation. The number of individuals in population was 150 and the crossover rate was 0.8. The results were processed by conformational cluster analysis with 1.0 Å cut-off for positive control and 2.0 Å cut-off for lactate,
3(
a,
f) and
4(
a,
g). The ligand conformation with lowest free energy of binding in the most populated cluster was selected for comparison.