The C5 Variant of the Butyrylcholinesterase Tetramer Includes a Noncovalently Bound 60 kDa Lamellipodin Fragment
Abstract
:1. Introduction
2. Results
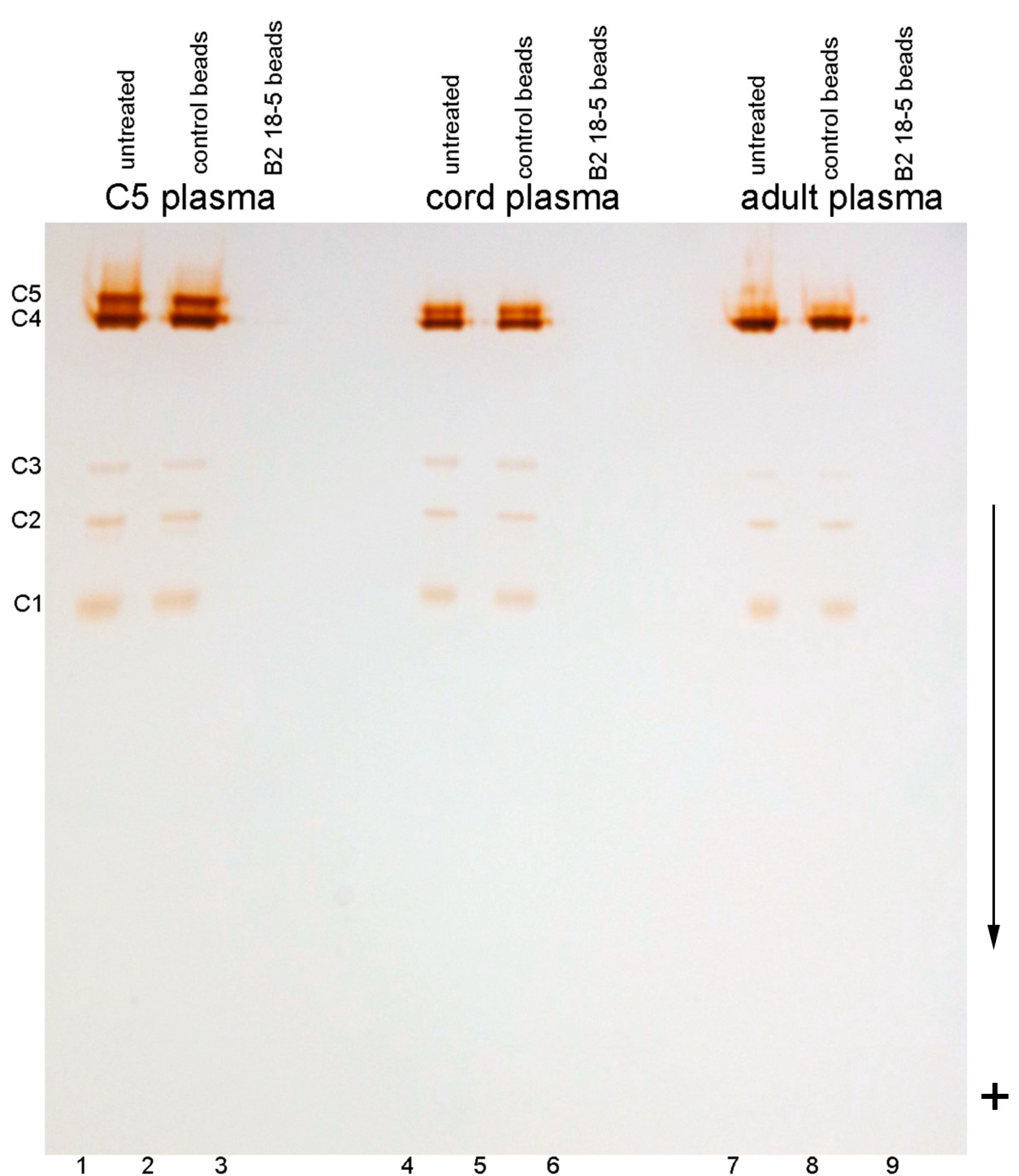
2.1. C5 Visualized on A Polyacrylamide Gel
2.2. C5 and C5-Like Bands In Umbilical Cord Plasma
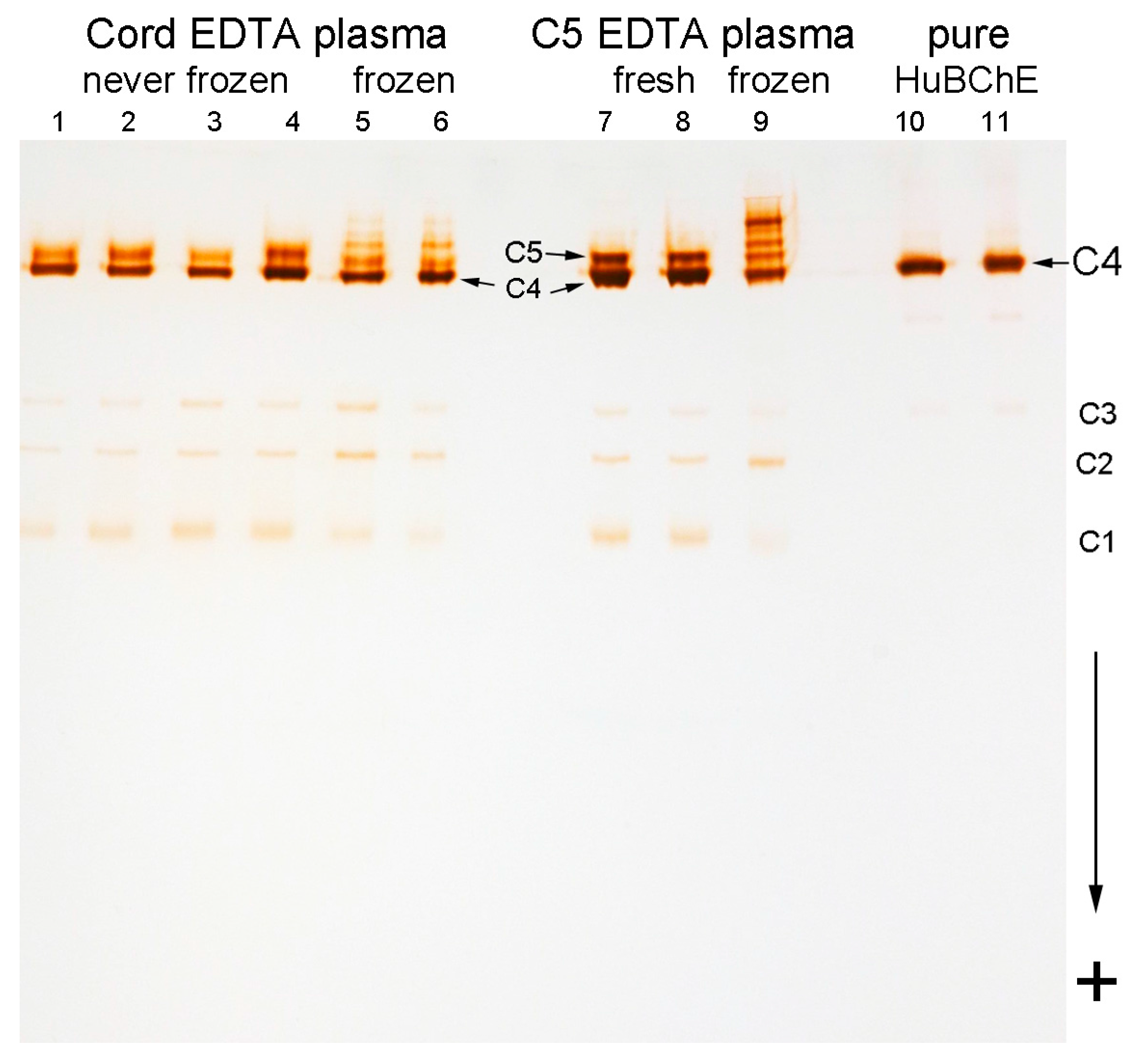
2.3. Storage Bands
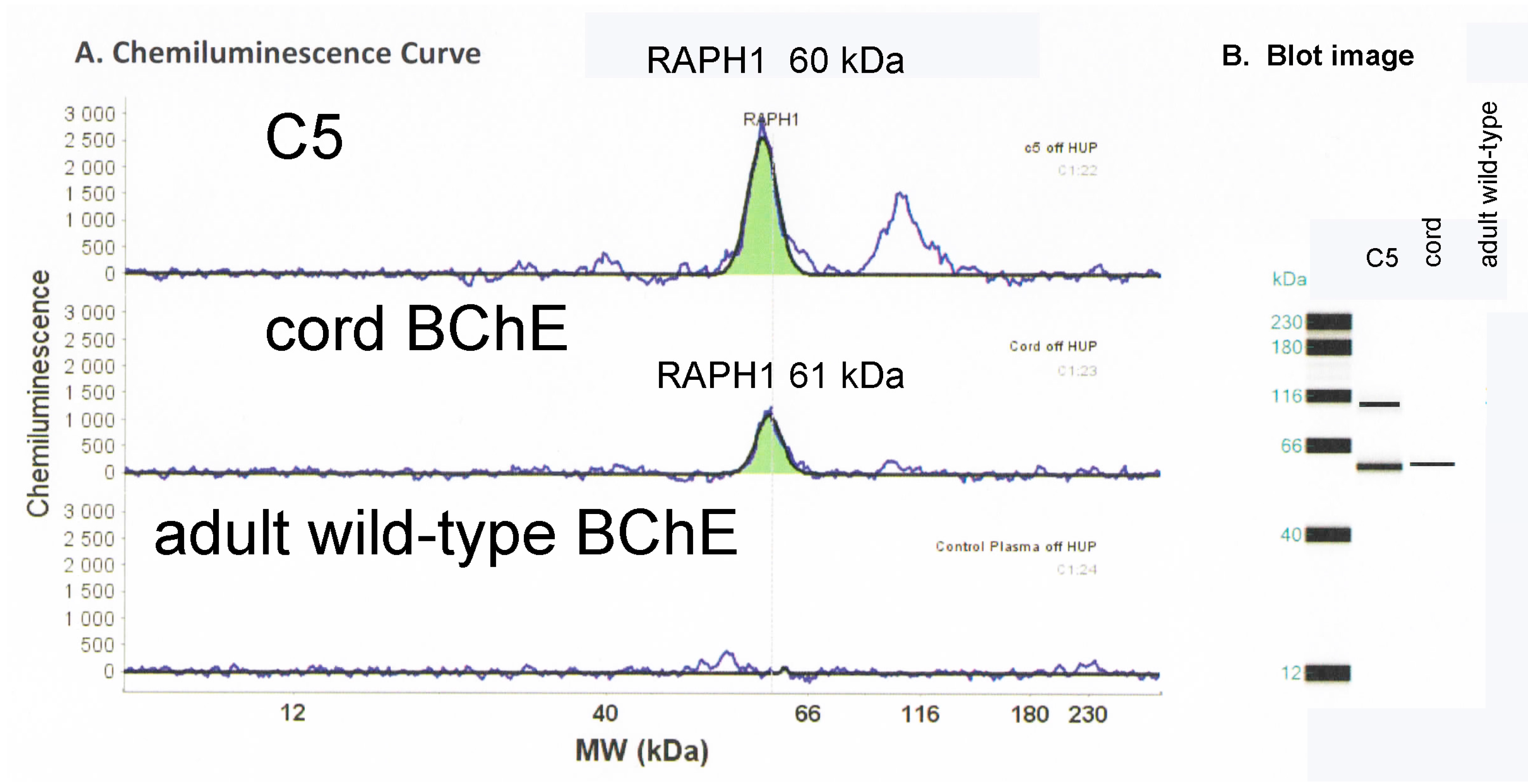
2.4. Mass Spectrometry Analysis Identifies Lamellipodin, a Proline Rich Protein Encoded by the Last Exon of the RAPH1 Gene In C5 and Cord BChE
2.5. Capillary Electrophoresis Western Blot
2.6. Genotyping Results
2.7. Partially Purified C5 Is Unstable
3. Discussion
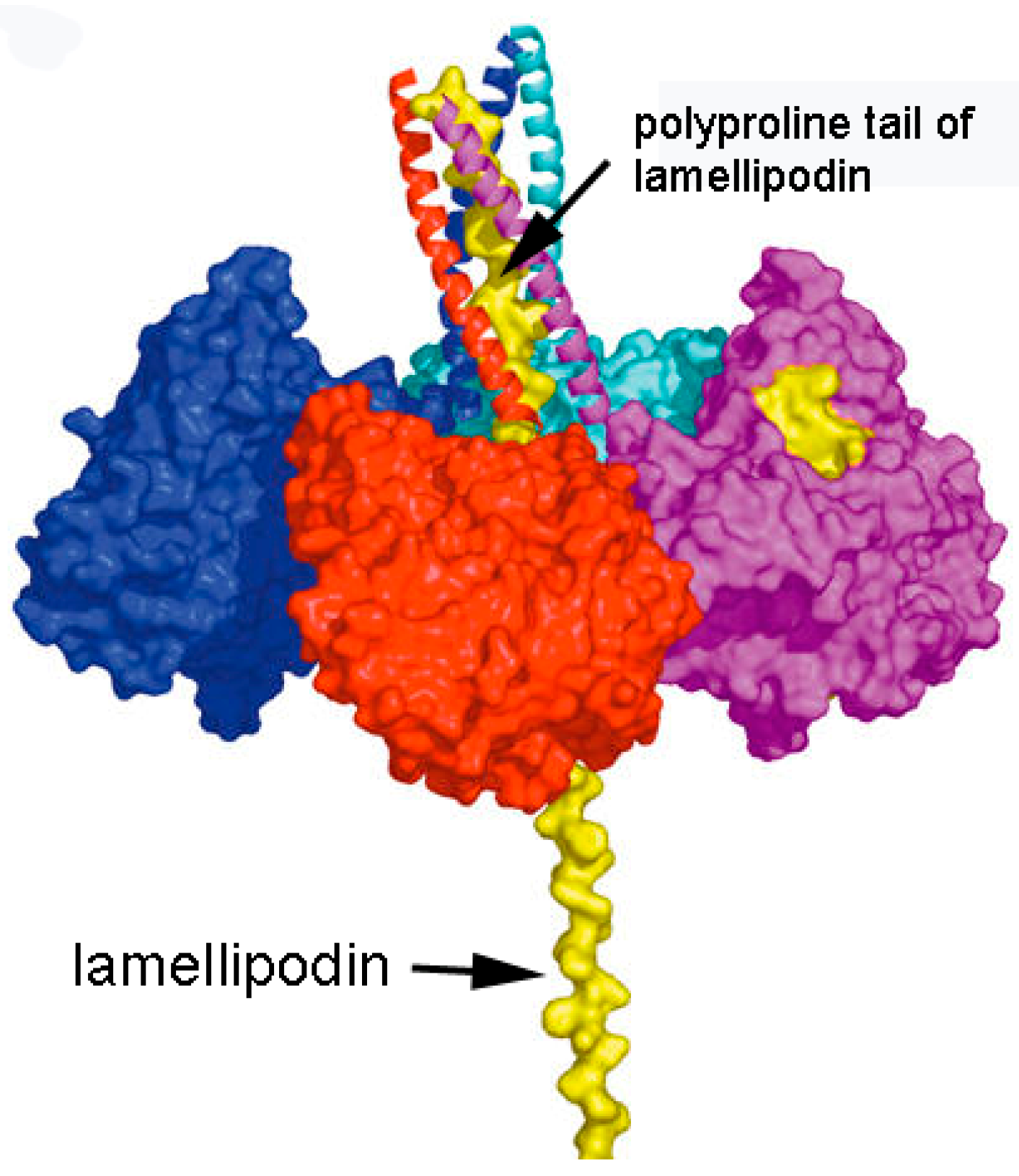
3.1. Model of C5 BChE
3.2. C5 Expression Is Non-Mendelian
3.3. Cord Blood
3.4. Motif for Associating Subunits Into Tetramers
3.5. Potential Clinical Application
4. Materials and Methods
4.1. Human Plasma
4.2. C5 Phenotyping
4.3. BChE Activity
4.4. BChE Purified from Cohn Fraction IV-4
4.5. Purification of C5 from Plasma on Hupresin-Sepharose
4.6. Immunopurification of BChE
4.7. Trypsin Digestion
4.8. Liquid Chromatography-Tandem Mass Spectrometry (LC-MS/MS)
4.9. Capillary Electrophoresis Simple Western Blot
4.10. Genotyping Analysis
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Sussman, J.L.; Harel, M.; Frolow, F.; Oefner, C.; Goldman, A.; Toker, L.; Silman, I. Atomic structure of acetylcholinesterase from Torpedo Californica: A prototypic acetylcholine-binding protein. Science 1991, 253, 872–879. [Google Scholar] [CrossRef] [PubMed]
- Dvir, H.; Harel, M.; Bon, S.; Liu, W.Q.; Vidal, M.; Garbay, C.; Sussman, J.L.; Massoulie, J.; Silman, I. The synaptic acetylcholinesterase tetramer assembles around a polyproline II helix. EMBO J. 2004, 23, 4394–4405. [Google Scholar] [CrossRef] [PubMed]
- Nicolet, Y.; Lockridge, O.; Masson, P.; Fontecilla-Camps, J.C.; Nachon, F. Crystal structure of human butyrylcholinesterase and of its complexes with substrate and products. J. Biol. Chem. 2003, 278, 41141–41147. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Schopfer, L.M.; Masson, P.; Lockridge, O. Lamellipodin proline rich peptides associated with native plasma butyrylcholinesterase tetramers. Biochem. J. 2008, 411, 425–432. [Google Scholar] [CrossRef] [PubMed]
- Peng, H.; Schopfer, L.M.; Lockridge, O. Origin of polyproline-rich peptides in human butyrylcholinesterase tetramers. Chem. Biol. Interact. 2016, 259, 63–69. [Google Scholar] [CrossRef] [PubMed]
- Larson, M.A.; Lockridge, O.; Hinrichs, S.H. Polyproline promotes tetramerization of recombinant human butyrylcholinesterase. Biochem. J. 2014, 462, 329–335. [Google Scholar] [CrossRef] [PubMed]
- Bon, S.; Coussen, F.; Massoulie, J. Quaternary associations of acetylcholinesterase. II. The polyproline attachment domain of the collagen tail. J. Biol. Chem. 1997, 272, 3016–3021. [Google Scholar] [CrossRef] [PubMed]
- Chitlaru, T.; Kronman, C.; Velan, B.; Shafferman, A. Overloading and removal of N-glycosylation targets on human acetylcholinesterase: Effects on glycan composition and circulatory residence time. Biochem. J. 2002, 363, 619–631. [Google Scholar] [CrossRef] [PubMed]
- Ruiz, C.A.; Rossi, S.G.; Rotundo, R.L. Rescue and Stabilization of Acetylcholinesterase in Skeletal Muscle by N-terminal Peptides Derived from the Noncatalytic Subunits. J. Biol. Chem. 2015, 290, 20774–20781. [Google Scholar] [CrossRef] [PubMed]
- Harris, H.; Hopkinson, D.A.; Robson, E.B.; Whittaker, M. Genetical studies on a new variant of serum cholinesterase detected by electrophoresis. Ann. Hum. Genet. 1963, 26, 359–382. [Google Scholar] [CrossRef] [PubMed]
- Simpson, N.E. Polyacrylamide electrophoresis used for the detection of C5+ cholinesterase in Canadian caucasians, Indians, and Eskimos. Am. J. Hum. Genet. 1972, 24, 317–320. [Google Scholar] [PubMed]
- Souza, R.L.; Furtado, L.; Diniz, A.C.; Silva, A.C.; Kaiss, J.; Petzl-Erler, M.L.; Chautard-Freire-Maia, E.A. Studies on a heterologous complex formed by human butyrylcholinesterase. Biochem. Genet. 2003, 41, 141–150. [Google Scholar] [CrossRef] [PubMed]
- Masson, P. A naturally occurring molecular form of human plasma cholinesterase is an albumin conjugate. Biochim. Biophys. Acta 1989, 998, 258–266. [Google Scholar] [CrossRef]
- Sugimori, T. Shortened action of succinylcholine in individuals with cholinesterase C5 isozyme. Can. Anaesth. Soc. J. 1986, 33, 321–327. [Google Scholar] [CrossRef] [PubMed]
- Chautard-Freire-Maia, E.A.; Primo-Parmo, S.L.; Picheth, G.; Lourenco, M.A.; Vieira, M.M. The C5 isozyme of serum cholinesterase and adult weight. Hum. Hered. 1991, 41, 330–339. [Google Scholar] [CrossRef] [PubMed]
- Eiberg, H.; Nielsen, L.S.; Klausen, J.; Dahlen, M.; Kristensen, M.; Bisgaard, M.L.; Moller, N.; Mohr, J. Linkage between serum cholinesterase 2 (CHE2) and gamma-crystallin gene cluster (CRYG): Assignment to chromosome 2. Clin. Genet. 1989, 35, 313–321. [Google Scholar] [CrossRef] [PubMed]
- Masson, P.; Chatonnet, A.; Lockridge, O. Evidence for a single butyrylcholinesterase gene in individuals carrying the C5 plasma cholinesterase variant (CHE2). FEBS Lett. 1990, 262, 115–118. [Google Scholar] [CrossRef]
- Arpagaus, M.; Kott, M.; Vatsis, K.P.; Bartels, C.F.; La Du, B.N.; Lockridge, O. Structure of the gene for human butyrylcholinesterase. Evidence for a single copy. Biochemistry 1990, 29, 124–131. [Google Scholar] [CrossRef] [PubMed]
- Allderdice, P.W.; Gardner, H.A.; Galutira, D.; Lockridge, O.; LaDu, B.N.; McAlpine, P.J. The cloned butyrylcholinesterase (BChE) gene maps to a single chromosome site, 3q26. Genomics 1991, 11, 452–454. [Google Scholar] [CrossRef]
- Gaughan, G.; Park, H.; Priddle, J.; Craig, I.; Craig, S. Refinement of the localization of human butyrylcholinesterase to chromosome 3q26.1-q26.2 using a PCR-derived probe. Genomics 1991, 11, 455–458. [Google Scholar] [CrossRef]
- Akizuki, S.; Ohnishi, A.; Kotani, K.; Sudo, K. Genetic and immunological analyses of patients with increased serum butyrylcholinesterase activity and its C5 variant form. Clin. Chem. Lab. Med. 2004, 42, 991–996. [Google Scholar] [CrossRef] [PubMed]
- Scott, E.M.; Powers, R.F. Properties of the C5 variant form of human serum cholinesterase. Am. J. Hum. Genet. 1974, 26, 189–194. [Google Scholar] [PubMed]
- Masson, P.; Froment, M.T.; Audras, J.C.; Renault, F. Molecular characterization of the C5 human plasma cholinesterase variant. In Cholinesterases: Structure, Function, Mechanism, Genetics, and Cell, Biology, Proceedings of the Third International Meeting on Cholinesterases, La Grande-Motte, France, 12–16 May 1990; Massoulie, J., Bacou, F., Barnard, E., Chatonnet, A., Doctor, B.P., Quinn, D.M., Eds.; American Chemical Society: Washington, DC, USA, 1991; pp. 43–44, 60. [Google Scholar]
- Benyamin, B.; Middelberg, R.P.; Lind, P.A.; Valle, A.M.; Gordon, S.; Nyholt, D.R.; Medland, S.E.; Henders, A.K.; Heath, A.C.; Madden, P.A.; et al. GWAS of butyrylcholinesterase activity identifies four novel loci, independent effects within BChE and secondary associations with metabolic risk factors. Hum. Mol. Genet. 2011, 20, 4504–4514. [Google Scholar] [CrossRef] [PubMed]
- De Andrade, F.A.; Batistela, M.S.; Amaral Sad, C.; Dos Santos, W.; Mikami, L.R.; Chautard-Freire-Maia, E.A.; Furtado-Alle, L.; Souza, R.L. Association between RAPH1 Gene Haplotypes and CHE2 Locus Phenotypes. Ann. Hum. Genet. 2016, 80, 203–209. [Google Scholar] [CrossRef] [PubMed]
- Harris, H.; Hopkinson, D.A.; Robson, E.B. Two-dimensional electrophoresis of pseudocholinesterase components in normal human serum. Nature 1962, 196, 1296–1298. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, K.; Morito, F.; Setoguchi, Y.; Fujii, S.; Kariya, T.; Sakai, T. Characterization of serum cholinesterase in familial hyper-cholinesterasemia associated with an isozyme variant band. Gastroenterol. Jpn. 1987, 22, 187–193. [Google Scholar] [PubMed]
- Deutsch, D.G.; Mertz, E.T. Plasminogen: Purification from human plasma by affinity chromatography. Science 1970, 170, 1095–1096. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Muzyka, J.L.; Zhan, C.G. Model of human butyrylcholinesterase tetramer by homology modeling and dynamics simulation. J. Phys. Chem. B 2009, 113, 6543–6552. [Google Scholar] [CrossRef] [PubMed]
- Ashton, G.C.; Simpson, N.E. C5 types of serum cholinesterase in a Brazilian population. Am. J. Hum. Genet. 1966, 18, 438–447. [Google Scholar] [PubMed]
- Whittaker, M. Cholinesterase. Monographs in Human Genetics 11: 14–15; Karger: Basel, Switzerland, 1986; Volume 11. [Google Scholar]
- Simon, S.; Krejci, E.; Massoulie, J. A four-to-one association between peptide motifs: Four C-terminal domains from cholinesterase assemble with one proline-rich attachment domain (PRAD) in the secretory pathway. EMBO J. 1998, 17, 6178–6187. [Google Scholar] [CrossRef] [PubMed]
- Perrier, A.L.; Massoulie, J.; Krejci, E. PRiMA: The membrane anchor of acetylcholinesterase in the brain. Neuron 2002, 33, 275–285. [Google Scholar] [CrossRef]
- Chen, V.P.; Xie, H.Q.; Chan, W.K.; Leung, K.W.; Chan, G.K.; Choi, R.C.; Bon, S.; Massoulie, J.; Tsim, K.W. The PRiMA-linked cholinesterase tetramers are assembled from homodimers: Hybrid molecules composed of acetylcholinesterase and butyrylcholinesterase dimers are up-regulated during development of chicken brain. J. Biol. Chem. 2010, 285, 27265–27278. [Google Scholar] [CrossRef] [PubMed]
- Massoulie, J. The origin of the molecular diversity and functional anchoring of cholinesterases. Neuro-Signals 2002, 11, 130–143. [Google Scholar] [CrossRef] [PubMed]
- Ostergaard, D.; Viby-Mogensen, J.; Hanel, H.K.; Skovgaard, L.T. Half-life of plasma cholinesterase. Acta Anaesthesiol. Scand. 1988, 32, 266–269. [Google Scholar] [CrossRef] [PubMed]
- Karnovsky, M.J.; Roots, L. A direct-coloring thiocholine method for cholinesterases. J. Histochem. Cytochem. 1964, 12, 219–221. [Google Scholar] [CrossRef] [PubMed]
- Ellman, G.L.; Courtney, K.D.; Andres, V., Jr.; Feather-Stone, R.M. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol. 1961, 7, 88–95. [Google Scholar] [CrossRef]
- Saxena, A.; Tipparaju, P.; Luo, C.; Doctor, B.P. Pilot-scale production of human serum butyrylcholinesterase suitable for use as a bioscavenger against nerve agent toxicity. Process Biochem. 2010, 45, 1313–1318. [Google Scholar] [CrossRef]
- Peng, H.; Brimijoin, S.; Hrabovska, A.; Targosova, K.; Krejci, E.; Blake, T.A.; Johnson, R.C.; Masson, P.; Lockridge, O. Comparison of 5 monoclonal antibodies for immunopurification of human butyrylcholinesterase on Dynabeads: Kd values, binding pairs, and amino acid sequences. Chem. Biol. Interact. 2015, 240, 336–345. [Google Scholar] [CrossRef] [PubMed]
- Krause, M.; Leslie, J.D.; Stewart, M.; Lafuente, E.M.; Valderrama, F.; Jagannathan, R.; Strasser, G.A.; Rubinson, D.A.; Liu, H.; Way, M.; et al. Lamellipodin, an Ena/VASP ligand, is implicated in the regulation of lamellipodial dynamics. Dev. Cell 2004, 7, 571–583. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of pure BChE and hupresin are available from the authors. |



© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schopfer, L.M.; Delacour, H.; Masson, P.; Leroy, J.; Krejci, E.; Lockridge, O. The C5 Variant of the Butyrylcholinesterase Tetramer Includes a Noncovalently Bound 60 kDa Lamellipodin Fragment. Molecules 2017, 22, 1083. https://doi.org/10.3390/molecules22071083
Schopfer LM, Delacour H, Masson P, Leroy J, Krejci E, Lockridge O. The C5 Variant of the Butyrylcholinesterase Tetramer Includes a Noncovalently Bound 60 kDa Lamellipodin Fragment. Molecules. 2017; 22(7):1083. https://doi.org/10.3390/molecules22071083
Chicago/Turabian StyleSchopfer, Lawrence M., Hervé Delacour, Patrick Masson, Jacqueline Leroy, Eric Krejci, and Oksana Lockridge. 2017. "The C5 Variant of the Butyrylcholinesterase Tetramer Includes a Noncovalently Bound 60 kDa Lamellipodin Fragment" Molecules 22, no. 7: 1083. https://doi.org/10.3390/molecules22071083





