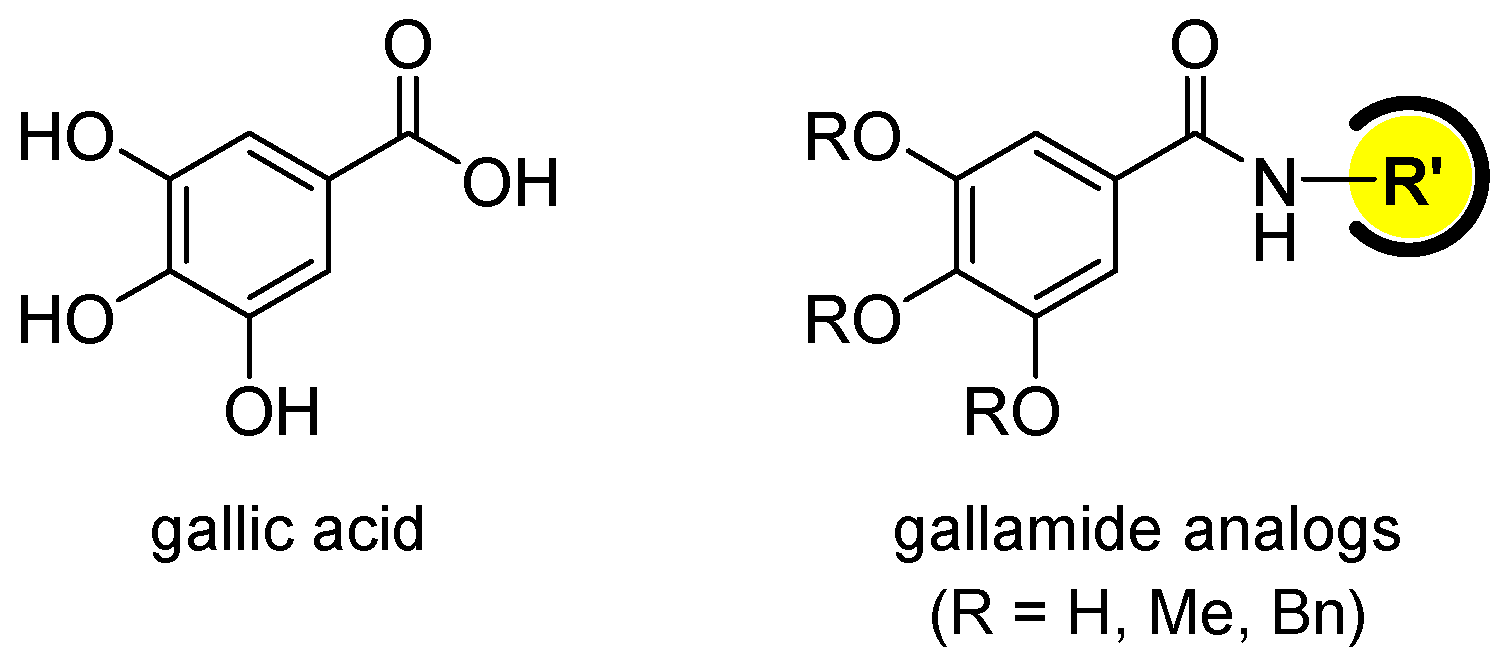
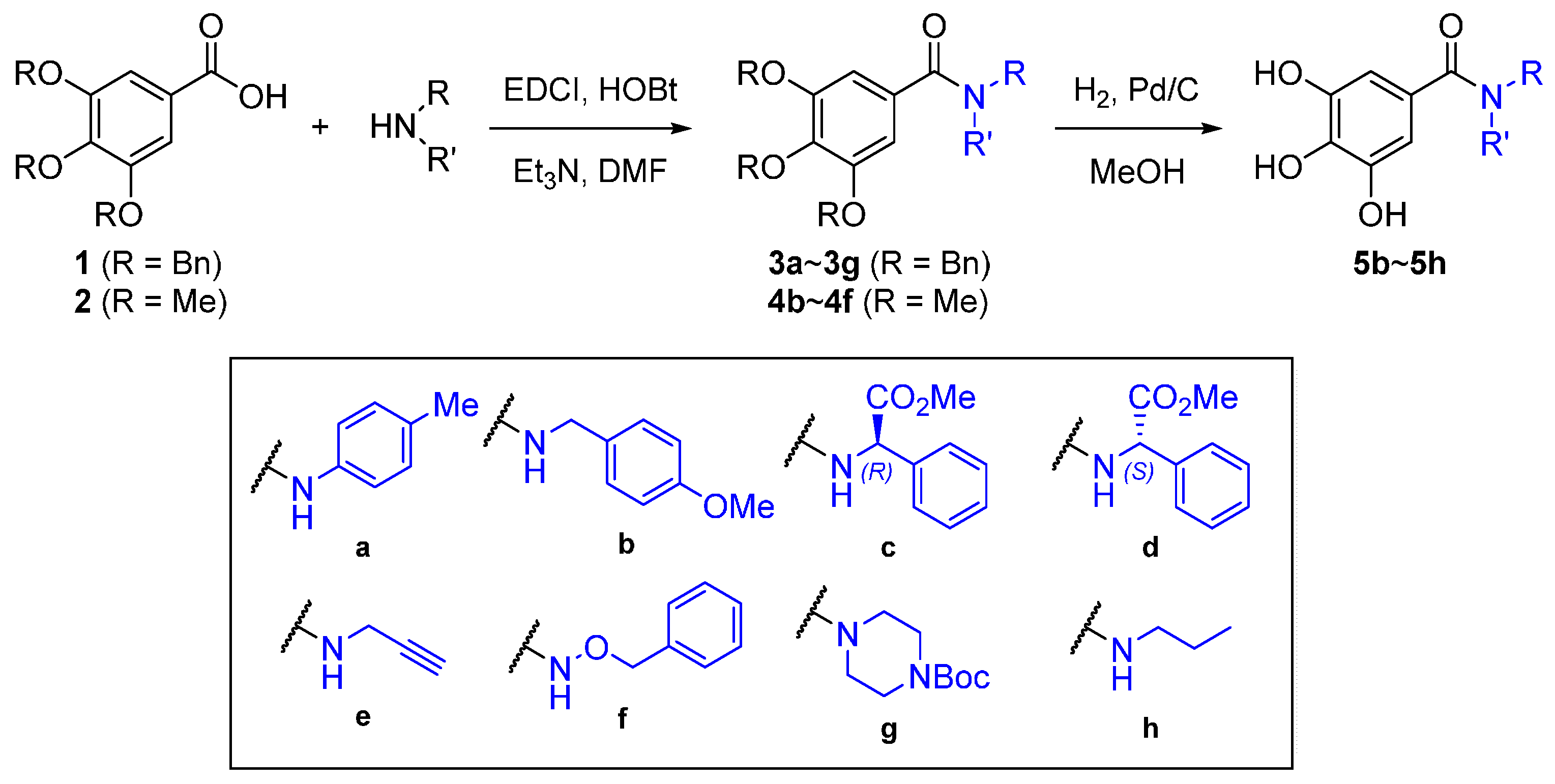
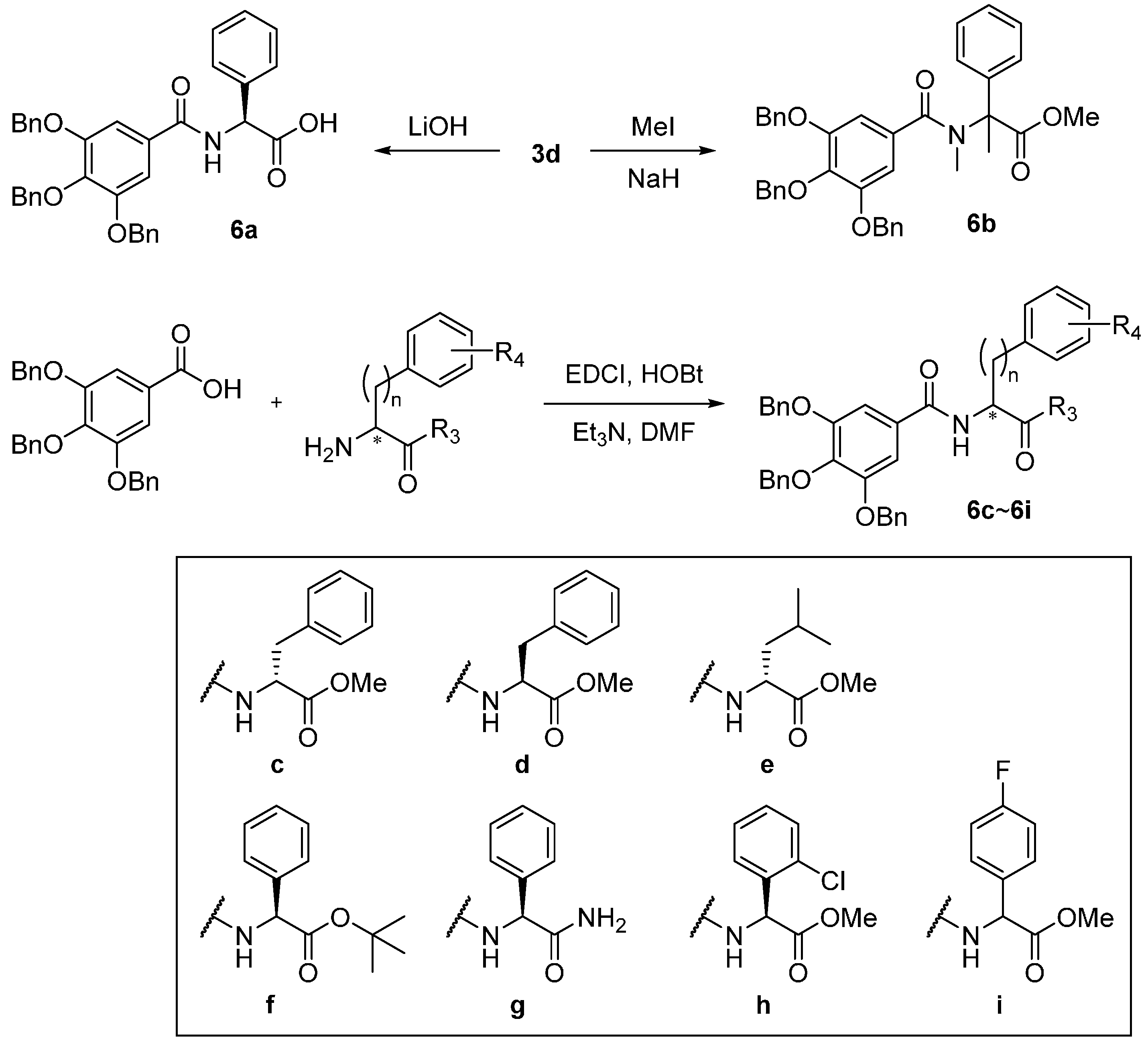
4.2. Synthesis of Gallamide Analogs
3,4,5-Tris(benzyloxy)-N-p-tolylbenzamide (3a). To a DMF solution (2 mL) of 3,4,5-tris(benzyloxy)benzoic acid (110 mg, 0.25 mmol), p-toluidine (32 mg, 0.3 mmol), EDCI (60 mg, 0.4 mmol), HOBt (4 mg, 30 μmol) and TEA (87 μL, 0.50 mmol) were added. After stirring overnight, the reaction mixture was diluted with ethyl acetate and washed with water and brine, dried over MgSO4 and concentrated under reduced pressure. The residue was purified by flash column chromatography on silica gel (ethyl acetate/n-hexane = 1:2) to generate pure 3a (111 mg, 80%). 1H-NMR (CDCl3) δ 7.46 (d, J = 8.3 Hz, 2H), 7.44–7.32 (m, 12H), 7.29–7.25 (m, 5H), 7.17 (d, J = 8.1 Hz, 3H), 7.12 (s, 2H), 5.15 (s, 4H), 5.12 (s, 2H), 2.34 (s, 3H); 13C-NMR (CDCl3) δ 165.41, 152.95, 141.52, 137.50, 136.74, 135.48, 134.32, 130.59, 129.71, 128.72, 128.70, 128.35, 128.20, 128.12, 127.69, 120.29, 107.13, 75.32, 71.57, 21.05; HRMS (EI) m/z calcd. for 529.2253; found 529.2252.
3,4,5-Tris(benzyloxy)-N-(4-methoxybenzyl)benzamide (3b). To a DMF solution (2 mL) of 3,4,5-tris(benzyloxy)benzoic acid (110 mg, 0.25 mmol), 4-methoxybenzylamine (56 mg, 0.28 mmol), EDCI (115 mg, 0.60 mmol), HOBt (4.0 mg, 30 μmol) and TEA (90 μL, 0.50 mmol) were added. After stirring overnight, the reaction mixture was diluted with ethyl acetate and washed with water and brine, dried over MgSO4 and concentrated under reduced pressure. The residue was purified by flash column chromatography on silica gel (ethyl acetate/n-hexane = 1:3) to generate pure 3b (98 mg, 70%). 1H-NMR (CDCl3) δ 7.21~7.41(m, 15H), 7.05 (s, 2H), 6.90 (m, 2H), 5.11 (s, 4H), 5.08 (s,2H), 4.53 (d, 2H), 3.81 (s, 3H); 13C-NMR (CDCl3) δ 136.6, 129.3, 128.5, 128.5, 128.2, 128.0, 127.5, 114.1,107.0, 71.5, 29.7; HRMS (EI) m/z calcd. for 559.2359; found 559.2361.
(R)-Methyl 2-phenyl-2-(3,4,5-tris(benzyloxy)benzamido)acetate (3c). To a DMF solution (2 mL) of 3,4,5-tris(benzyloxy)benzoic acid (110 mg, 0.25 mmol), (R)-2-Phenylglycine methyl ester hydrochloride (61 mg, 0.3 mmol), EDCI (59 mg, 0.4 mmol), HOBt (3.8 mg, 30 μmol) and TEA (90 μL, 0.50 mmol) were added. After stirring overnight, the reaction mixture was diluted with ethyl acetate and washed with water and brine, dried over MgSO4 and concentrated under reduced pressure. The residue was purified by flash column chromatography on silica gel (ethyl acetate/n-hexane = 1:3) to yield pure 3c (115 mg, 78%). 1H-NMR (CDCl3) δ 7.42–7.23 (m, 23H), 7.11 (s, 2H), 6.98 (d, J = 6.9 Hz, 1H), 5.70 (d, J = 6.9 Hz, 1H), 5.11 (s, 4H), 5.09 (s, 2H), 3.77 (s, 3H); 13C-NMR (CDCl3) δ 171.83, 166.43, 153.10, 141.92, 137.71, 136.97, 136.78, 129.37, 129.20, 128.98, 128.92, 128.89, 128.54, 128.37, 128.31, 127.92, 127.69, 107.50, 75.49, 71.79, 57.25, 53.28; HRMS (EI) m/z calcd. for 587.2308; found 587.2307.
(S)-Methyl 2-phenyl-2-(3,4,5-tris(benzyloxy)benzamido)acetate (3d). To a DMF solution (2 mL) of 3,4,5-tris(benzyloxy)benzoic acid (110 mg, 0.25 mmol), (S)-2-phenylglycine methyl ester hydrochloride (56 mg, 0.28 mmol), EDCI (115 mg, 0.60 mmol), HOBt (4.0 mg, 30 μmol) and TEA (90 μL, 0.50 mmol) were added. After stirring for 3 h, the reaction mixture was diluted with ethyl acetate and washed with water and brine, dried over MgSO4 and concentrated under reduced pressure. The residue was purified by flash column chromatography on silica gel (ethyl acetate/n-hexane = 1:3) to yield pure 3d (127 mg, 86%). 1H-NMR (CDCl3) δ 7.41–7.32 (m, 17H), 7.27–7.22 (m, 3H), 7.12 (s, 2H), 7.06 (d, J = 6.8 Hz, 1H), 5.69 (d, J = 6.9 Hz, 1H), 5.08 (s, 2H), 5.08 (s, 4H), 3.75 (s, 3H); 13C-NMR (CDCl3) δ 171.59, 166.21, 152.83, 141.64, 137.48, 136.73, 136.52, 129.10, 128.91, 128.71, 128.66, 128.63, 128.28, 128.11, 128.05, 127.67, 127.48, 107.23, 75.23, 71.50, 57.03, 53.01; HRMS (EI) m/z calcd. for 587.2308; found 587.2307.
3,4,5-Tris(benzyloxy)-N-(prop-2-yn-1-yl)benzamide (3e). To a DMF solution (4 mL) of 3,4,5-tris-(benzyloxy)benzoic acid (220 mg, 0.50 mmol), propargylamine (33 mg, 0.60 mmol), EDCI (115 mg, 0.60 mmol), HOBt (7.0 mg, 50 μmol) and TEA (90 μL, 0.50 mmol) were added. After stirring for 24 h, the reaction mixture was diluted with ethyl acetate and washed with water and brine, dried over MgSO4 and concentrated under reduced pressure. The residue was purified by flash column chromatography on silica gel (ethyl acetate/n-hexane = 1:3) to produce the amide 3e (212 mg, 89%). 1H-NMR (CDCl3) δ 7.41–7.21 (m, 15H), 7.08 (s, 2H), 6.40 (s, 1H), 5.07 (s, 2H), 5.06 (s, 4H), 4.17 (dd, J = 5.2, 2.5 Hz, 2H), 2.24 (t, J = 2.5 Hz, 1H); 13C-NMR (CDCl3) δ 166.90, 152.87, 141.48, 137.46, 136.68, 129.22, 128.65, 128.29, 128.15, 128.07, 127.64, 107.05, 79.63, 75.24, 71.92, 71.46, 29.94; HRMS (EI) m/z calcd. for 477.1940; found 477.1942.
N,3,4,5-Tetrakis(benzyloxy)benzamide (3f). To a DMF solution (2 mL) of 3,4,5-tris(benzyloxy)benzoic acid (1) (110 mg, 0.25 mmol), O-benzylhydroxylamine hydrochloride (48 mg, 0.3 mmol), EDCI (58 mg, 0.3 mmol), HOBt (4 mg, 30 μmol) and TEA (90 μL, 0.50 mmol) were added. After stirring overnight, the reaction mixture was diluted with ethyl acetate and washed with water and brine, dried over MgSO4 and concentrated under reduced pressure. The residue was purified by flash column chromatography on silica gel (ethyl acetate/n-hexane = 1:2) to generate pure 3f (102 mg, 74%). 1H-NMR (CDCl3) δ 7.42–7.20 (m, 21H), 6.95 (s, 2H), 5.06 (s, 2H), 5.03 (s, 4H), 4.97 (s, 2H); 13C-NMR (CDCl3) δ 166.20, 152.80, 141.56, 137.35, 136.51, 135.30, 129.39, 128.84, 128.66, 128.56, 128.22, 128.07, 128.00, 127.54, 127.11, 106.83, 78.35, 75.17, 71.27; HRMS (EI) m/z calcd. for 545.2202; found 545.2205.
tert-Butyl 4-(3,4,5-tris(benzyloxy)benzoyl)piperazine-1-carboxylate (3g). To a DMF solution (2 mL) of 3,4,5-tris(benzyloxy)benzoic acid (110 mg, 0.25 mmol), 1-Boc-piperazine (56 mg, 0.3 mmol), EDCI (115 mg, 0.60 mmol), HOBt (5.0 mg, 40 μmol) and TEA (90 μL, 0.50 mmol) were added. After stirring overnight, the reaction mixture was diluted with ethyl acetate and washed with water and brine, dried over MgSO4 and concentrated under reduced pressure. The residue was purified by flash column chromatography on silica gel (ethyl acetate/n-hexane = 1:3) to generate pure 3g (103 mg, 68%). 1H-NMR (CDCl3) δ 7.44–7.25 (m, 15H), 6.65 (s, 2H), 5.11 (s, 4H), 5.10 (s, 2H), 3.91–2.81 (m, 8H), 1.47 (s, 9H); 13C-NMR (CDCl3) δ 170.07, 154.52, 152.68, 139.79, 137.55, 136.70, 130.42, 128.60, 128.26, 128.23, 128.03, 127.98, 127.38, 107.24, 80.29, 75.23, 71.18, 28.43; HRMS (EI) m/z calcd. for 608.2886; found 608.2888.
3,4,5-Trihydroxy-N-(4-methoxybenzyl)benzamide (4b). To a CH2Cl2 solution (3 mL) of 3,4,5-trimethoxybenzoic acid (100 mg, 0.471 mmol), 4-Methoxybenzylamine (78 mg, 0.566 mmol), EDCI (117 mg, 0.613 mmol), HOBt (70 mg, 0.46 mmol) and TEA (0.31 mL, 1.78 mmol) were added. After stirring overnight, the reaction mixture was diluted with ethyl acetate and washed with water and brine, dried over MgSO4 and concentrated under reduced pressure. The residue was purified by flash column chromatography on silica gel (ethyl acetate/n-hexane = 2:1) to generate 85 mg of 4b (yield 54%).1H-NMR (CDCl3) δ 7.23 (d, J = 8.6 Hz, 2H), 7.01 (s, 2H), 6.83 (d, J = 8.6 Hz, 2H), 6.67 (t, J = 5.1 Hz, 1H), 4.51 (d, J = 5.7 Hz, 2H), 3.84 (s, 3H), 3.82 (s, 6H), 3.77 (s, 3H); 13C-NMR (CDCl3) δ 167.26, 159.31, 153.39, 141.09, 130.61, 130.11, 129.54, 114.34, 104.66, 61.16, 56.52, 55.57, 43.94.; HRMS (EI) m/z calcd. for 289.0950; found 289.0948.
(R)-Methyl 2-phenyl-2-(3,4,5-trimethoxybenzamido)acetate (4c). To a CH2Cl2 solution (3 mL) of 3,4,5-trimethoxybenzoic acid (100 mg, 0.471 mmol), (R)-(−)-2-phenylglycine methyl ester hydrochloride (114 mg, 0.56 mmol), EDCI (117 mg, 0.613 mmol), HOBt (70 mg, 0.46 mmol) and TEA (0.24 mL, 1.41 mmol) were added. After stirring overnight, the reaction mixture was diluted with CH2Cl2 and washed with water and brine, dried over MgSO4 and concentrated under reduced pressure. The residue was purified by flash column chromatography on silica gel (ethyl acetate/n-hexane = 1:1) to generate 85 mg of 4c (yield 50%). 1H-NMR (CDCl3) δ 7.46–7.33 (m, 5H), 7.13 (d, J = 6.8 Hz, 1H), 7.05 (s, 2H), 5.74 (d, J = 6.9 Hz, 1H), 3.89 (s, 6H), 3.88 (s, 3H), 3.78 (s, 3H); 13C-NMR (CDCl3) δ 171.69, 166.37, 153.30, 141.40, 136.57, 129.15, 129.02, 128.75, 127.51, 104.72, 61.02, 57.10, 56.47, 53.06; HRMS (EI) m/z calcd. for 359.1369; found 359.1370.
(S)-Methyl 2-phenyl-2-(3,4,5-trimethoxybenzamido)acetate (4d). To a CH2Cl2 solution (3 mL) of 3,4,5-trimethoxybenzoic acid (100 mg, 0.471 mmol), (S)-(−)-2-phenylglycine methyl ester hydrochloride (110 mg, 0.66 mmol), EDCI (210 mg, 1.09 mmol), HOBt (70 mg, 0.46 mmol) and TEA (0.24 mL, 1.41 mmol) were added. After stirring overnight, the reaction mixture was diluted with CH2Cl2 and washed with water and brine, dried over MgSO4 and concentrated under reduced pressure. The residue was purified by flash column chromatography on silica gel (ethyl acetate/n-hexane = 1:1) to produce 90 mg of 4d (yield 53%). 1H-NMR (CDCl3) δ 7.43–7.40 (m, 2H), 7.37–7.30 (m, 3H), 7.19 (d, J = 6.9 Hz, 1H), 7.04 (s, 2H), 5.72 (d, J = 6.9 Hz, 1H), 3.86 (s, 9H), 3.76 (s, 3H); 13C-NMR (CDCl3) δ 171.65, 166.35, 153.24, 141.33, 136.51, 129.08, 128.95, 128.69, 127.50, 104.69, 60.96, 57.07, 56.40, 52.99; HRMS (EI) m/z calcd. for 359.1369; found 359.1370.
3,4,5-Trimethoxy-N-(prop-2-yn-1-yl)benzamide (4e). To a CH2Cl2 solution (3 mL) of 3,4,5-trimethoxybenzoic acid (100 mg, 0.471 mmol), propargylamine (31 mg, 0.56 mmol), EDCI (250 mg, 1.32 mmol), HOBt (30 mg, 0.20 mmol) and TEA (0.3 mL, 1.71 mmol) were added. After stirring overnight, the reaction mixture was diluted with CH2Cl2 and washed with water and brine, dried over MgSO4 and concentrated under reduced pressure. The residue was purified by flash column chromatography on silica gel (ethyl acetate/n-hexane = 1:1) to generate 88 mg of 4e (yield 52%). 1H- NMR (CDCl3) δ 7.01 (s, 2H), 6.40 (s, 1H), 4.23 (dd, J = 5.3, 2.6 Hz, 2H), 3.88 (s, 6H), 3.87 (s, 3H), 2.28 (s, 1H); 13C-NMR (CDCl3) δ 166.81, 153.11, 141.02, 129.04, 104.35, 79.40, 71.77, 60.83, 56.23, 56.11, 29.78; HRMS (EI) m/z calcd. for 317.1263; found 317.1265.
N-(benzyloxy)-3,4,5-trimethoxybenzamide (4f). To a CH2Cl2 solution (3 mL) of 3,4,5-trimethoxybenzoic acid (100 mg, 0.471 mmol), O-benzylhydroxylamine hydrochloride (83 mg, 0.52 mmol), EDCI (253 mg, 1.32 mmol), HOBt (24 mg, 0.15 mmol) and TEA (0.3 mL, 1.71 mmol) were added. After stirring overnight, the reaction mixture was diluted with CH2Cl2 and washed with water and brine, dried over MgSO4 and concentrated under reduced pressure. The residue was purified by flash column chromatography on silica gel (ethyl acetate/n-hexane = 1:1) to generate 115 mg of 4f (yield 77%). 1H- NMR (CDCl3) δ 9.01 (s, 1H), 7.46–7.29 (m, 5H), 6.90 (s, 2H), 5.00 (s, 2H), 3.84 (s, 3H), 3.81 (s, 6H); 13C-NMR (CDCl3) δ 166.33, 153.31, 141.31, 135.32, 129.46, 128.91, 128.70, 127.26, 104.54, 78.45, 60.99, 56.32; HRMS (EI) m/z calcd. for 317.1263; found 317.1265.
3,4,5-Trihydroxy-N-(4-methoxybenzyl)benzamide (5b). A 10 mL round-bottom flask was charged with 3b (30 mg, 0.05 mmol), MeOH (3 mL), and a Teflon-coated magnetic stirring bar, 10% Pd/C (10 mg) were added, producing a black slurry. The flask was equipped with a hydrogen balloon attached to a stainless steel needle. The slurry was left to stir under hydrogen atmosphere. After 24 h, the hydrogen atmosphere of the flask was purged with nitrogen. The mixture was then filtered through a pad of Celite to give a dark green solution. This solution was concentrated to give 5b (14 mg, 90%). 1H-NMR (CD3OD) δ 7.24 (d, J = 8.7 Hz, 2H), 6.86 (d, J = 8.8 Hz, 4H), 4.44 (s, 2H), 3.76 (s, 3H); 13C- NMR (CD3OD) δ 170.20, 160.14, 146.50, 137.93, 132.29, 129.58, 126.05, 114.69, 107.69, 55.51, 43.73; HRMS (EI) m/z calcd. for 289.0950; found 289.0948.
(R)-Methyl 2-phenyl-2-(3,4,5-trihydroxybenzamido)acetate (5c). A 10 mL round-bottom flask was charged with 3a (89 mg, 0.15 mmol), MeOH (3 mL), and a Teflon-coated magnetic stirring bar. 10% Pd/C (12 mg) was added to the resulting solution, producing a black slurry. The flask was equipped with a hydrogen balloon attached to a stainless steel needle. The slurry was left to stir under hydrogen atmosphere. After 24 h, the hydrogen atmosphere of the flask was purged with nitrogen. The mixture was then filtered through a pad of Celite to give a dark green solution. This solution was concentrated to give 5c (43 mg, 88%). 1H-NMR (CD3OD) δ 7.34~7.43 (m, 5H), 6.89 (s, 2H), 5.63 (s, 1H), 3.71 (s, 3H); 13C-NMR (CD3OD) δ171.49, 168.75, 145.21, 137.04, 136.23, 128.48, 128.13, 127.51, 123.96, 106.76, 57.30, 51.75; HRMS (EI) m/z calcd. for 317.0899; found 317.0900.
(S)-Methyl 2-phenyl-2-(3,4,5-trihydroxybenzamido)acetate (5d). A 10 mL round-bottom flask was charged with 4a (60 mg, 0.1 mmol), MeOH (3 mL), and a Teflon-coated magnetic stirring bar. 10% Pd/C (5 mg) was added to the resulting solution, producing a black slurry. The flask was equipped with a hydrogen balloon attached to a stainless steel needle. The slurry was left to stir under hydrogen atmosphere. After 24 h, the hydrogen atmosphere of the flask was purged with nitrogen. The mixture was then filtered through a pad of Celite to give a dark green solution. This solution was concentrated to give 5d (30 mg, 92%) 1H-NMR (CD3OD) δ 7.28~7.41 (m, 5H), 6.86 (s, 2H), 5.60 (s, 1H), 3.68 (s, 3H); 13C-NMR (CD3OD) δ 171.50, 168.75, 145.24, 137.07, 136.25, 128.47, 128.12, 127.51, 123.96, 106.76, 57.30, 51.70; HRMS (EI) m/z calcd. for 317.0899; found 317.0900.
tert-Butyl 4-(3,4,5-trihydroxybenzoyl)piperazine-1-carboxylate (5g). A 10 mL round-bottom flask was charged with 7a (40 mg, 0.06 mmol), MeOH (3 mL), and a Teflon-coated magnetic stirring bar. 10% Pd/C (10 mg) was added to the resulting solution, producing a black slurry. The flask was equipped with a hydrogen balloon attached to a stainless steel needle. The slurry was left to stir under hydrogen atmosphere. After 24 h, the hydrogen atmosphere of the flask was purged with nitrogen. The mixture was then filtered through a pad of Celite to give a dark green solution. This solution was concentrated to give 5g (20 mg, 91%). 1H-NMR (CD3OD) δ 6.36 (s, 2H), 3.64–3.29 (m, 8H), 1.38 (s, 9H); 13C-NMR (CD3OD) δ 173.19, 156.18, 146.93, 136.53, 126.39, 107.80, 107.60, 81.60, 28.58; HRMS (EI) m/z calcd. for 338.1478; found 338.1477.
3,4,5-Trihydroxy-N-(prop-2-ynyl)benzamide (5h). A 10 mL round-bottom flask was charged with 5a (20 mg, 0.04 mmol), MeOH (3 mL), and a Teflon-coated magnetic stirring bar. 10% Pd/C (5 mg) was added to the resulting solution, producing a black slurry. The flask was equipped with a hydrogen balloon attached to a stainless steel needle. The slurry was left to stir under hydrogen atmosphere. After 24 h, the hydrogen atmosphere of the flask was purged with nitrogen. The mixture was then filtered through a pad of Celite to give a dark green solution. This solution was concentrated and purified by Biotage® SNAP Ultra C18 reversed phase cartridge ( from 10 to 90% ACN with 0.1% TFA) to give 5h (6 mg, 67%). 1H-NMR (CD3OD) δ 6.83 (s, 2H), 3.26 (t, J = 7.2 Hz, 2H), 1.59 (dd, J = 14.5, 7.3 Hz, 2H), 0.95 (t, J = 7.4 Hz, 3H); 13C-NMR (CD3OD) δ 170.59, 146.62, 137.95, 126.41, 107.73, 58.32, 42.67, 23.79, 18.36, 11.75; HRMS (EI) m/z calcd. for 211.0845; found 211.0842.
(S)-2-Phenyl-2-(3,4,5-tris(benzyloxy)benzamido)acetic acid (6a). The 3c (150 mg, 0.26 mmol) was suspended in THF (3 mL) in a 25-mL round-bottomed flask. In a separate flask, lithium hydroxide (16 mg) was dissolved in deionized water (1 mL). Both mixtures were chilled to 4 °C and combined to form a turbid white mixture. After 1 h of stirring, the mixture had become homogeneous. After 24 h, 1 mL of 1 M HCl was added, and the mixture was allowed to warm to room temperature. Following the addition of brine, the mixture was extracted four times with EtOAc, and the combined organic layers were evaporated. This yielded 6a as white powder (80 mg, 55%). 1H-NMR (CD3OD) δ 7.90 (s, 1H), 7.51 (dd, J = 8.1, 0.9 Hz, 2H), 7.46–7.44 (m, 3H), 7.40–7.29 (m, 13H), 7.25–7.14 (m, 5H), 5.46 (s, 1H), 5.13 (s, 4H), 5.01 (s, 2H); 13C-NMR (CD3OD) δ 174.62, 168.22, 154.02, 141.95, 141.24, 138.78, 138.32, 131.00, 129.74, 129.54, 129.33, 129.16, 129.06, 129.02, 128.92, 128.60, 128.36, 107.94, 76.19, 72.28, 61.10; HRMS (EI) m/z calcd. for 573.2151; found 573.2150.
Methyl 2-phenyl-2-(3,4,5-tris(benzyloxy)-N-methylbenzamido) propanoate (6b). Under a N2 atmosphere, to a solution of (S)-methyl 2-phenyl-2-(3,4,5-tris(benzyloxy)benzamido)acetate (200 mg, 0.34 mmol) in DMF (2.5 mL), NaH (20 mg, 60% purity, 0.51 mmol) was added slowly at 0 °C. After 30 min, iodomethane (0.042 mL, 0.48 mmol) was added slowly. The solution was stirred for 10 min at room temperature, after which the reaction was quenched by adding an excess amount of saturated NH4Cl aqueous solution, followed by extraction with ethyl acetate. The organic phase was washed with brine and then dried over anhydrous MgSO4. After the solution was filtered and the solvent was evaporated under vacuum, the residue was subjected to silica gel column chromatography using 25% EtOAc in hexane to give 6b (30 mg, 15%). H-NMR (CDCl3) δ 7.62–7.51 (m, 2H), 7.46–7.18 (m, 19H), 6.87–6.79 (m, 2H), 5.18–5.07 (m, 6H), 3.66 (s, 3H), 2.38 (s, 3H), 1.96 (s, 3H); 13C-NMR (CDCl3) δ 172.9, 172.1, 152.6, 140.3, 137.8, 137.5, 136.8, 131.0, 128.6, 128.5, 128.4, 128.3, 128.2, 127.9 (2), 127.4, 108.0, 75.2, 66.9, 52.6, 35.9, 18.6. HRMS (EI) m/z calcd. for 615.2621; found 615.2619.
Methyl (3,4,5-tris(benzyloxy)benzoyl)-d-phenylalaninate (6c). To a DMF solution (10 mL) of 3,4,5-tris(benzyloxy)benzoic acid (408 mg, 0.92 mmol), d-phenylalanine methyl ester hydrochloride (200 mg, 0.92 mmol), EDCI (196 mg, 1.02 mmol), HOBt (71 mg, 0.46 mmol) and TEA (0.30 mL, 2.04 mmol) were added. After stirring overnight, the reaction mixture was diluted with ethyl acetate and washed with water and brine, dried over MgSO4 and concentrated under reduced pressure. The residue was purified by flash column chromatography on silica gel (ethyl acetate/n-hexane = 1:3) to generate pure 6c (220 mg, 40%). 1H-NMR (CDCl3) δ 7.25–7.41 (m, 18H), 7.11 (dd, 2H, J = 1.8, 7.8 Hz), 7.00 (s, 2H), 6.37 (d, 1H), 5.09 (s, 4H), 5.08 (s, 2H), 5.02–5.05 (m, 1H), 3.78 (s, 3H), 3.23 (qd, 2H, J = 7.8, 24.6 Hz); 13C- NMR (CDCl3) δ 172.0, 166.4, 152.7, 141.4, 137.4, 136.6, 135.8, 129.3, 129.2, 128.6, 128.5, 128.0, 127.9, 127.5, 127.2, 106.8, 75.1, 71.3, 53.5, 52.4, 37.8; HRMS (EI) m/z calcd. for 601.2464; found 601.2463.
Methyl (3,4,5-tris(benzyloxy)benzoyl)-l-phenylalaninate (6d). To a DMF solution (10 mL) of 3,4,5-tris(benzyloxy)benzoic acid (517 mg, 1.17 mmol), l-phenylalanine methyl ester hydrochloride (229 mg, 1.06 mmol), EDCI (230 mg, 1.20 mmol), HOBt (80 mg, 0.52 mmol) and TEA (0.37 mL, 2.09 mmol) were added. After stirring overnight, the reaction mixture was diluted with ethyl acetate and washed with water and brine, dried over MgSO4 and concentrated under reduced pressure. The residue was purified by flash column chromatography on silica gel (ethyl acetate/n-hexane = 1:3) to generate pure 6d (420 mg, 66%). 1H-NMR (CDCl3) δ 7.25–7.41 (m, 18H), 7.11 (dd, 2H, J = 1.8, 7.8 Hz), 7.00 (s, 2H), 6.37 (d, 1H), 5.09 (s, 4H), 5.08 (s, 2H), 5.02–5.05 (m, 1H), 3.78 (s, 3H), 3.23 (qd, 2H, J = 7.8, 24.6 Hz); 13C- NMR (CDCl3) δ 172.0, 166.4, 152.7, 141.4, 137.4, 136.6, 135.8, 129.3, 129.2, 128.6, 128.5, 128.0, 127.9, 127.5, 127.2, 106.8, 75.1, 71.3, 53.5, 52.4, 37.8; HRMS (EI) m/z calcd. for 601.2464; found 601.2466.
Methyl (3,4,5-tris(benzyloxy)benzoyl)leucinate (6e). To a CH2Cl2 solution (3 mL) of 3,4,5-tris(benzyloxy)benzoic acid (240 mg, 0.54 mmol), l-leucine methyl ester hydrochloride (180 mg, 1.06 mmol), EDCI (190 mg, 1.00 mmol), HOBt (30 mg, 0.19 mmol) and TEA (0.14 mL, 0.85 mmol) were added. After stirring overnight, the reaction mixture was diluted with ethyl acetate and washed with water and brine, dried over MgSO4 and concentrated under reduced pressure. The residue was purified by flash column chromatography on silica gel (ethyl acetate/n-hexane = 1:5) to generate 120 mg of 6e (yield 47%). 1H-NMR (CDCl3) δ 7.44–7.17 (m, 15H), 7.10 (s, 2H), 6.93 (d, J = 8.1 Hz, 1H), 4.85–4.79 (m, 1H), 3.74 (s, 3H), 1.77–1.56 (m, 3H), 0.96 (dd, J = 6.3, 2.7 Hz, 6H); 3C-NMR (CDCl3) δ 174.61, 166.67, 152.75, 141.35, 137.56, 136.85, 128.91, 128.62, 128.52, 128.21, 127.98, 127.92, 127.77, 106.89, 75.18, 71.32, 52.48, 51.36, 41.37, 25.06, 23.03, 21.85; HRMS (EI) m/z calcd. for 567.2421; found 567.2466.
tert-Butyl (S)-2-phenyl-2-(3,4,5-tris(benzyloxy)benzamido)acetate (6f). To a CH2Cl2 solution (3 mL) of 3,4,5-tris(benzyloxy)benzoic acid (180 mg, 0.40 mmol), l-Phenylalanine tert-butyl ester hydrochloride (100 mg, 0.41 mmol), EDCI (90 mg, 0.47 mmol), HOBt (30 mg, 0.19 mmol) and TEA (0.14 mL, 0.85 mmol) were added. After stirring overnight, the reaction mixture was diluted with ethyl acetate and washed with water and brine, dried over MgSO4 and concentrated under reduced pressure. The residue was purified by flash column chromatography on silica gel (ethyl acetate/n-hexane = 1:3) to generate 110 mg of 6f (yield 45%). 1H-NMR (CDCl3) δ 7.46–7.27 (m, 19H), 7.21 (t, J = 6.1 Hz, 1H), 7.19 (s, 2H), 5.65 (d, J = 7.0 Hz, 1H), 5.12 (s, 6H), 1.46 (s, 9H); 13C-NMR (CDCl3) δ 170.22, 165.96, 152.79, 141.48, 137.49, 137.32, 136.74, 129.18, 128.82, 128.63, 128.58, 128.27, 128.24, 128.05, 127.99, 127.66, 127.26, 107.13, 82.84, 75.18, 71.42, 57.40, 27.90; HRMS (EI) m/z calcd. for 629.2777; found 629.2774.
(S)-N-(2-Amino-2-oxo-1-phenylethyl)-3,4,5-tris(benzyloxy)benzamide (6g). To a CH2Cl2 solution (3 mL) of 3,4,5-tris(benzyloxy)benzoic acid (390 mg, 0.88 mmol), (S)-2-amino-2-phenylacetamide (180 mg, 1.06 mmol), EDCI (190 mg, 1.00 mmol), HOBt (70 mg, 0.46 mmol) and TEA (0.31 mL, 1.78 mmol) were added. After stirring overnight, the reaction mixture was diluted with ethyl acetate and washed with water and brine, dried over MgSO4 and concentrated under reduced pressure. The residue was purified by flash column chromatography on silica gel (ethyl acetate/n-hexane = 1:1) to generate 302 mg of 6g (yield 57%). 1H-NMR (DMSO-d6) δ 8.75 (d, 1H, J = 7.8 Hz), 7.72 (s, 1H), 7.51 (d, 2H, J = 7.2 Hz), 7.46 (d, 4H, J = 7.2 Hz), 7.23–7.40 (m, 17H), 5.66 (d, 1H, J = 7.8 Hz), 5.17 (s, 4H), 4.99 (s, 2H); 13C- NMR (DMSO-d6) δ 172.26, 165.79, 152.37, 139.21, 137.36, 129.60, 128.84, 128.69, 128.63, 128.47, 128.33, 128.26, 128.12, 127.99, 127.95, 107.35, 74.65, 70.83, 60.18; HRMS (EI) m/z calcd. for 572.2311; found 572.2312.
Methyl 2-(2-chlorophenyl)-2-(3,4,5-tris(benzyloxy)benzamido)acetate (6h). To a CH2Cl2 solution (3 mL) of 3,4,5-tris(benzyloxy)benzoic acid (286 mg, 0.65 mmol), (S)-(+)-2-chlorophenylglycine methyl ester hydrochloride (130 mg, 0.65 mmol), EDCI (137 mg, 0.71 mmol), HOBt (50 mg, 0.33 mmol) and TEA (0.34 mL, 1.9 mmol) were added. After stirring overnight, the reaction mixture was diluted with ethyl acetate and washed with water and brine, dried over MgSO4 and concentrated under reduced pressure. The residue was purified by flash column chromatography on silica gel (ethyl acetate/n-hexane = 1:3) to generate 30 mg of 6h (yield 8%). 1H-NMR (CDCl3) δ 7.46–7.26 (m, 18H), 7.13 (d, J = 6.9 Hz, 1H), 7.10 (s, 2H), 6.01 (d, J = 7.0 Hz, 1H), 5.10 (d, J = 6.6 Hz, 4H), 5.09 (s, 2H), 3.77 (d, J = 8.4 Hz, 3H); 13C-NMR (CDCl3) δ 142.80, 138.02, 124.72, 113.56, 109.35, 108.60, 106.68, 105.53, 102.65, 102.19, 101.79, 100.75, 100.56, 100.54, 100.18, 100.00, 99.94, 99.53, 99.30, 79.13, 47.12, 43.37, 27.29, 25.14; HRMS (EI) m/z calcd. for 621.1918; found 621.1917.
Methyl 2-(4-fluorophenyl)-2-(3,4,5-tris(benzyloxy)benzamido)acetate (6i). To a CH2Cl2 solution (3 mL) of 3,4,5-tris(benzyloxy)benzoic acid (390 mg, 0.88 mmol), methyl amino(4-fluorophenyl)acetate hydrochloride (240 mg, 1.09 mmol), EDCI (310 mg, 1.62 mmol), HOBt (70 mg, 0.52 mmol) and TEA (0.57 mL, 3.2 mmol) were added. After stirring overnight, the reaction mixture was diluted with ethyl acetate and washed with water and brine, dried over MgSO4 and concentrated under reduced pressure. The residue was purified by flash column chromatography on silica gel (ethyl acetate/n-hexane = 1:3) to generate 320 mg of 6i (yield 48%). 1H-NMR (CDCl3) δ 7.46–7.29 (m, 15H), 7.29–7.27 (m, 2H), 7.13–7.08 (m, 2H), 7.08–6.99 (m, 2H), 6.99–6.91 (m, 1H), 5.69 (d, J = 6.7 Hz, 1H), 5.16–5.12 (m, 4H), 5.10 (s, 2H), 3.79 (s, 3H); 13C-NMR (CDCl3) δ 171.37, 166.04, 163.59, 161.95, 152.82, 141.73, 137.35, 136.62, 132.46, 129.12, 129.06, 128.73, 128.59, 128.23, 128.08, 128.01, 127.60, 116.06, 115.92, 107.20, 75.18, 71.52, 56.20, 53.07; HRMS (EI) m/z calcd. for 605.2214; found 605.2217.