Synthesis and Free Radical Scavenging Activity of New Hydroxybenzylidene Hydrazines
Abstract
:1. Introduction
2. Results and Discussion
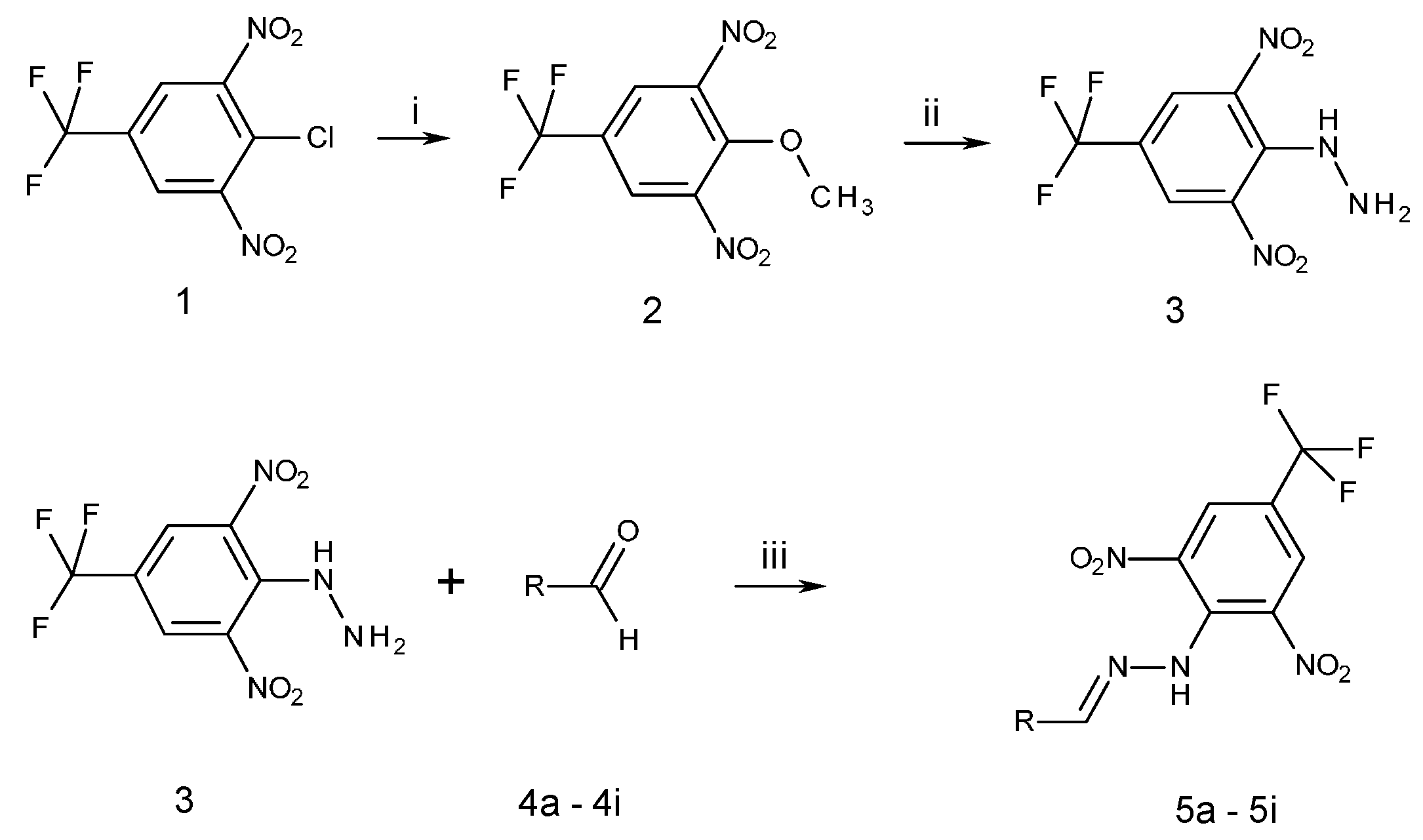
2.1. Chemistry
2.2. Free Radical Scavenging Activity Assay
2.3. Inhibition of Photosynthetic Electron Transport (PET) in Spinach Chloroplasts
3. Materials and Methods
3.1. General Information
3.2. Synthesis
3.2.1. Synthesis of 2,6-Dinitro-4-(Trifluoromethyl)Phenylhydrazine (3)

3.2.2. Synthesis of N-Hydroxybenzylidene-N′-[2,6-Dinitro-4-(Trifluoromethyl)]Phenylhydrazines (5a–5h) and N-(Benzylidene)-N′-[2,6-Dinitro-4-(Trifluoromethyl)]Phenylhydrazine (5i)









3.3. Free Radical Scavenging Activity Assay
3.4. Photosynthetic Electron Transport (PET) Study
3.5. Molecular Calculations
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Backes, G.L.; Neumann, D.M.; Jursic, B.S. Synthesis and antifungal activity of substituted salicylaldehyde hydrazones, hydrazides and sulfohydrazides. Bioorg. Med. Chem. 2014, 22, 4629–4636. [Google Scholar] [CrossRef] [PubMed]
- Dabideen, D.R.; Cheng, K.F.; Aljabari, B.; Miller, E.J.; Pavlov, V.A.; Al-Abed, Y. Phenolic hydrazones are potent inhibitors of macrophage migration inhibitory factor proinflammatory activity and survival improving agents in sepsis. J. Med. Chem. 2007, 50, 1993–1997. [Google Scholar] [CrossRef] [PubMed]
- Gadilohar, B.; Shankarling, G. Choline based ionic liquids and their applications in organic transformation. J. Mol. Liq. 2007, 227, 234–261. [Google Scholar] [CrossRef]
- Sersen, F.; Gregan, F.; Pesko, M.; Dvoranova, D.; Kralova, K.; Matkovicova, Z.; Gregan, J.; Donovalova, J. Synthesis and herbicidal activity of new hydrazide and hydrazonoyl derivatives. Molecules 2015, 20, 14139–14154. [Google Scholar] [CrossRef] [PubMed]
- Rollas, S.; Kucukguzel, S.G. Biological activities of hydrazone derivatives. Molecules 2007, 12, 1910–1939. [Google Scholar] [CrossRef] [PubMed]
- Narang, R.; Narasimhan, B.; Sharma, S. A review on biological activities and chemical synthesis of hydrazide derivatives. Curr. Med. Chem. 2012, 19, 569–612. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.X.; Tang, J.J.; Luo, H.; Jin, X.L.; Dai, F.; Yang, J.; Qian, Y.P.; Li, X.Z.; Zhou, B. Antioxidant and antiproliferative activities of hydroxyl-substituted Schiff bases. Bioorg. Med. Chem. Lett. 2010, 20, 2417–2420. [Google Scholar] [CrossRef] [PubMed]
- Torok, B.; Sood, A.; Bag, S.; Tulsan, R.; Ghosh, S.; Borkin, D.; Kennedy, A.R.; Melanson, M.; Madden, R.; Zhou, W.; et al. Diaryl hydrazones as multifunctional inhibitors of amyloid self-assembly. Biochemistry 2013, 52, 1137–1148. [Google Scholar] [CrossRef] [PubMed]
- El-Sayed, N.N.; Alafeefy, A.M.; Bakht, M.A.; Masand, V.H.; Aldalbahi, A.; Chen, N.; Fan, C.; Ben Bacha, A. Synthesis, antiphospholipase A(2), antiprotease, antibacterial evaluation and molecular docking analysis of certain novel hydrazones. Molecules 2016, 21, 1664. [Google Scholar] [CrossRef] [PubMed]
- El-Faham, A.; Farooq, M.; Khattab, S.N.; Abutaha, N.; Wadaan, M.A.; Ghabbour, H.A.; Fun, H.K. Synthesis, characterization, and anti-cancer activity of some new N′-(2-Oxoindolin-3-ylidene)-2-propylpentane hydrazide-hydrazones derivatives. Molecules 2015, 20, 14638–14655. [Google Scholar] [CrossRef] [PubMed]
- Casanova, B.B.; Muniz, M.N.; de Oliveira, T.; de Oliveira, L.F.; Machado, M.M.; Fuentefria, A.M.; Gosmann, G.; Gnoatto, S.C. Synthesis and biological evaluation of hydrazone derivatives as antifungal agents. Molecules 2015, 20, 9229–9241. [Google Scholar] [CrossRef] [PubMed]
- Toledano-Magana, Y.; Garcia-Ramos, J.C.; Navarro-Olivarria, M.; Flores-Alamo, M.; Manzanera-Estrada, M.; Ortiz-Frade, L.; Galindo-Murillo, R.; Ruiz-Azuara, L.; Melendrez-Luevano, R.M.; Cabrera-Vivas, B.M. Potential amoebicidal activity of hydrazone derivatives: synthesis, characterization, electrochemical behavior, theoretical study and evaluation of the biological activity. Molecules 2015, 20, 9929–9948. [Google Scholar] [CrossRef] [PubMed]
- La Regina, G.; Sarkar, T.; Bai, R.; Edler, M.C.; Saletti, R.; Coluccia, A.; Piscitelli, F.; Minelli, L.; Gatti, V.; Mazzoccoli, C.; et al. New arylthioindoles and related bioisosteres at the sulfur bridging group. 4. Synthesis, tubulin polymerization, cell growth inhibition, and molecular modeling studies. J. Med. Chem. 2009, 52, 7512–7527. [Google Scholar] [CrossRef] [PubMed]
- Silvestri, R.; Cascio, M.G.; La Regina, G.; Piscitelli, F.; Lavecchia, A.; Brizzi, A.; Pasquini, S.; Botta, M.; Novellino, E.; Di Marzo, V.; et al. Synthesis, cannabinoid receptor affinity, and molecular modeling studies of substituted 1-aryl-5-(1H-pyrrol-1-yl)-1H-pyrazole-3-carboxamides. J. Med. Chem. 2008, 51, 1560–1576. [Google Scholar] [CrossRef] [PubMed]
- Baratto, L.C.; Porsani, M.V.; Pimentel, I.C.; Pereira Netto, A.B.; Paschke, R.; Oliveira, B.H. Preparation of betulinic acid derivatives by chemical and biotransformation methods and determination of cytotoxicity against selected cancer cell lines. Eur. J. Med. Chem. 2013, 68, 121–131. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.K.; Singh, A.K. DFT calculations on molecular structure, spectral analysis, multiple interactions, reactivity, NLO property and molecular docking study of flavanol-2,4-dinitrophenylhydrazone. J. Mol. Struct. 2017, 1129, 128–141. [Google Scholar] [CrossRef]
- Parodi, S.; de Flora, S.; Cavanna, M.; Pino, A.; Robbiano, L.; Bennicelli, C.; Brambilla, G. DNA-damaging activity in vivo and bacterial mutagenicity of sixteen hydrazine derivatives as related quantitatively to their carcinogenicity. Cancer Res. 1981, 41, 1469–1482. [Google Scholar] [PubMed]
- Heath, R.L. Hydrazine as an electron donor to the water-oxidation site in photosynthesis. Biochim. Biophys. Acta 1971, 245, 160–164. [Google Scholar] [CrossRef]
- Messinger, J.; Renger, G. Generation, oxidation by the oxidized form of the tyrosine of polypeptide D2, and possible electronic configuration of the redox states S0, S-1, and S-2 of the water oxidase in isolated spinach thylakoids. Biochemistry 1993, 32, 9379–9386. [Google Scholar] [CrossRef] [PubMed]
- Forster, V.; Junge, W. On the action of hydroxylamine, hydrazine and their derivatives on the water-oxidizing complex. Photosynth. Res. 1986, 9, 197–210. [Google Scholar] [CrossRef] [PubMed]
- Messinger, J.; Renger, G. The reactivity of hydrazine with photosystem II strongly depends on the redox state of the water oxidizing system. FEBS Lett. 1990, 277, 141–146. [Google Scholar] [CrossRef]
- Crampton, M.R.; Khan, H.A. The stabilities of meisenheimer complexes. J. Chem. Soc. Perkin Trans. 1972, 2, 1173–1177. [Google Scholar] [CrossRef]
- Sakamoto, H.; Goto, H.; Yokoshima, M.; Dobashi, M.; Ishikawa, J.; Doi, K.; Otomo, M. Benzocrown ether hydrazones as extractants for alkali metal ions. Bull. Chem. Soc. Jpn. 1993, 66, 2907–2914. [Google Scholar] [CrossRef]
- Sersen, F.; Mucaji, P.; Spilkova, J.; Valko, V.; Haladova, M.; Eisenreichova, E.; Grancai, D. Antioxidative effectivness of various solvent extracts from leaves of Cynara cardunculus, Philadelphus coronarius, Lilium candidum, Holodiscus discolor and Ligustrum vulgare. Acta Fac. Pharm. Univ. Comen. 2006, 53, 262–267. [Google Scholar]
- Kotora, P.; Sersen, F.; Filo, J.; Loos, D.; Gregan, J.; Gregan, F. The scavenging of DPPH, galvinoxyl and ABTS radicals by imine analogs of resveratrol. Molecules 2016, 21, 127. [Google Scholar] [CrossRef] [PubMed]
- Sersen, F.; Lacova, M. Antioxidant activity of some coumarins. Acta Fac. Pharm. Univ. Comen. 2015, 62, 41–45. [Google Scholar]
- Dolezal, M.; Miletin, M.; Kunes, J.; Kralova, K. Substituted amides of pyrazine-2-carboxylic acids: synthesis and biological activity. Molecules 2002, 7, 363–373. [Google Scholar] [CrossRef]
- Stewart, J.J. Optimization of parameters for semiempirical methods V: Modification of NDDO approximations and application to 70 elements. J. Mol. Model. 2007, 13, 1173–1213. [Google Scholar] [CrossRef] [PubMed]
- Stewart, J.J.P. MOPAC, version 2012; Stewart Computational Chemistry: Colorado Springs, CO, USA, 2012; Available online: http://openmopac.net/MOPAC2012brochure.pdf.
- Klamt, A.; Schüürmann, G. COSMO: A new approach to dielectric screening in solvents with explicit expressions for the screening energy and its gradient. J. Chem. Soc. Perkin Trans. 2 1993, 5, 799–805. [Google Scholar] [CrossRef]
- MOPAC Manual. Available online: http://OpenMOPAC.net/Manual (accessed on 10 January 2015).
Sample Availability: Samples of the compounds are not available from the authors. |

| Compound | SC50 μM DPPH | SC50 μM GOR | SC50 μM ABTS | IC50 μM PET Inhibition | BDE | PA + ETE | BDE | PA + ETE |
|---|---|---|---|---|---|---|---|---|
| (kJ/mol) in CH3OH | (kJ/mol) in CH3OH | (kJ/mol) in Water | (kJ/mol) in Water | |||||
| 5a | 144.4 | 168.1 | 17.3 | 1003 | 408.8 | 638.9 | 408.7 | 654.1 |
| 5b | 16.2 | 25.4 | 2.6 | 42.8 | 387.2 | 540.2 | 387.0 | 632.2 |
| 5c | 48.9 | 25.8 | 9.4 | 14.7 | 339.1 | 569.2 | 339.3 | 584.7 |
| 5d | 13.7 | 15.4 | 7.8 | 93 | 309.8 | 539.9 | 309.8 | 555.2 |
| 5e | NDA 1 | NDA 1 | 3.5 | NDA 1 | 421.3 | 651.7 | 421.1 | 666.5 |
| 5f | 10.8 | 5.1 | 5.4 | NDA 1 | 305.3 | 535.5 | 305.5 | 550.8 |
| 5g | 38.5 | 16.7 | 7.4 | 263 | 351.6 | 590.8 | 364.4 | 612.3 |
| 5h | 8.2 | 10.8 | 5.4 | NDA 1 | 299.5 | 529.7 | 299.9 | 545.2 |
| 5i | NDA 1 | NDA 1 | 182.9 | 344 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sersen, F.; Gregan, F.; Kotora, P.; Kmetova, J.; Filo, J.; Loos, D.; Gregan, J. Synthesis and Free Radical Scavenging Activity of New Hydroxybenzylidene Hydrazines. Molecules 2017, 22, 894. https://doi.org/10.3390/molecules22060894
Sersen F, Gregan F, Kotora P, Kmetova J, Filo J, Loos D, Gregan J. Synthesis and Free Radical Scavenging Activity of New Hydroxybenzylidene Hydrazines. Molecules. 2017; 22(6):894. https://doi.org/10.3390/molecules22060894
Chicago/Turabian StyleSersen, Frantisek, Fridrich Gregan, Peter Kotora, Jarmila Kmetova, Juraj Filo, Dusan Loos, and Juraj Gregan. 2017. "Synthesis and Free Radical Scavenging Activity of New Hydroxybenzylidene Hydrazines" Molecules 22, no. 6: 894. https://doi.org/10.3390/molecules22060894
APA StyleSersen, F., Gregan, F., Kotora, P., Kmetova, J., Filo, J., Loos, D., & Gregan, J. (2017). Synthesis and Free Radical Scavenging Activity of New Hydroxybenzylidene Hydrazines. Molecules, 22(6), 894. https://doi.org/10.3390/molecules22060894




