Effect of Selected Mercapto Flavor Compounds on Acrylamide Elimination in a Model System
Abstract
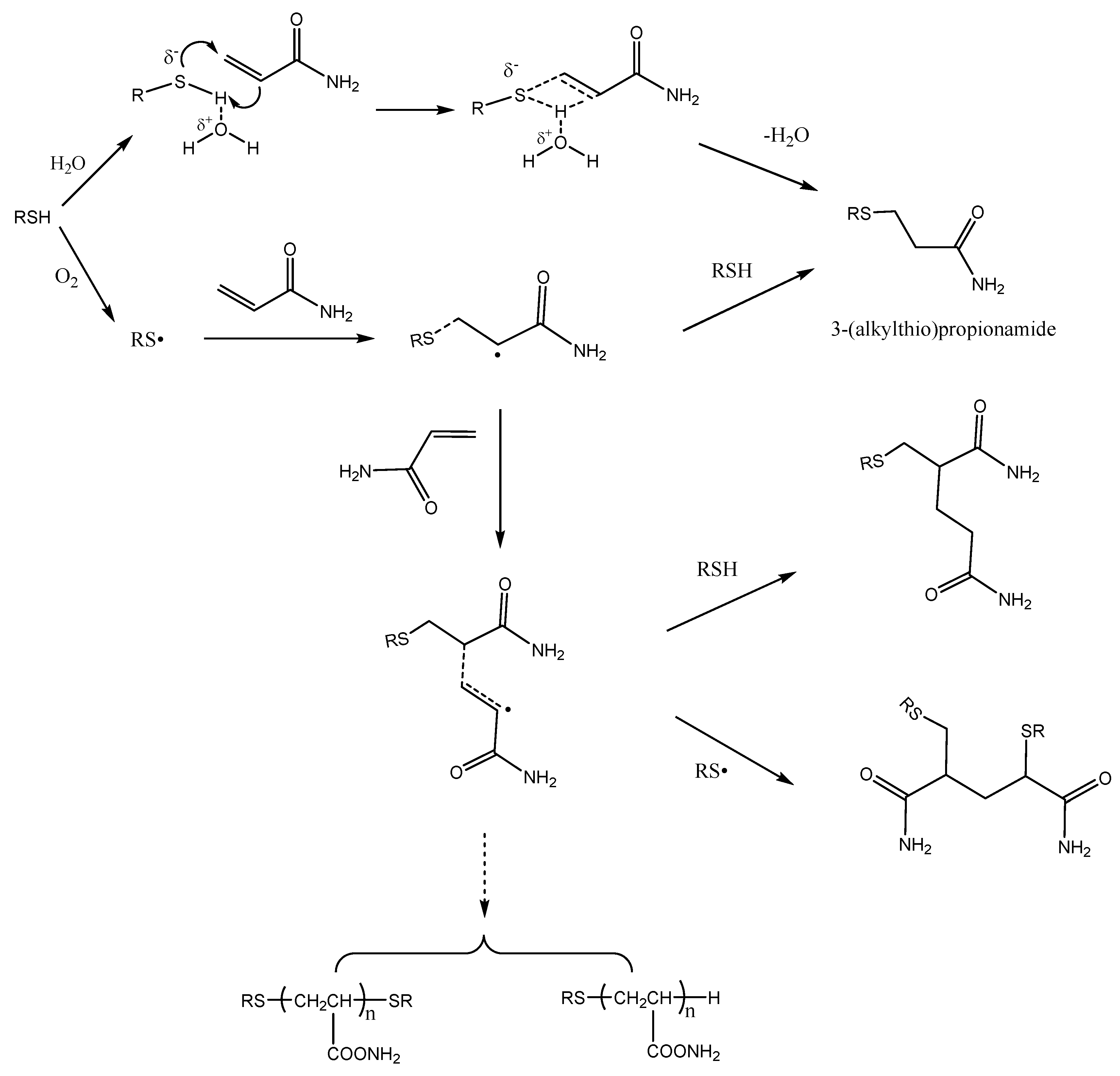
:1. Introduction
2. Results
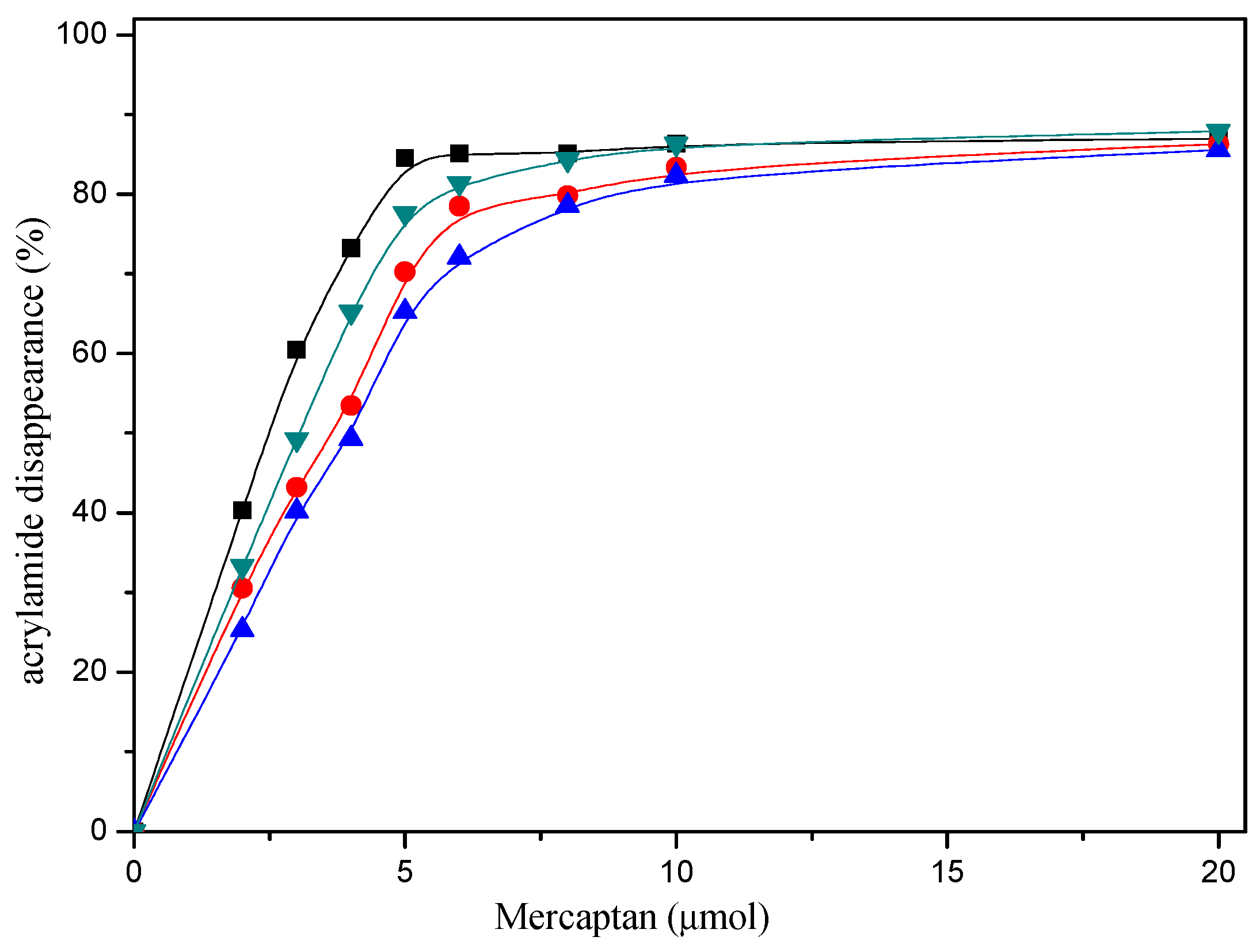
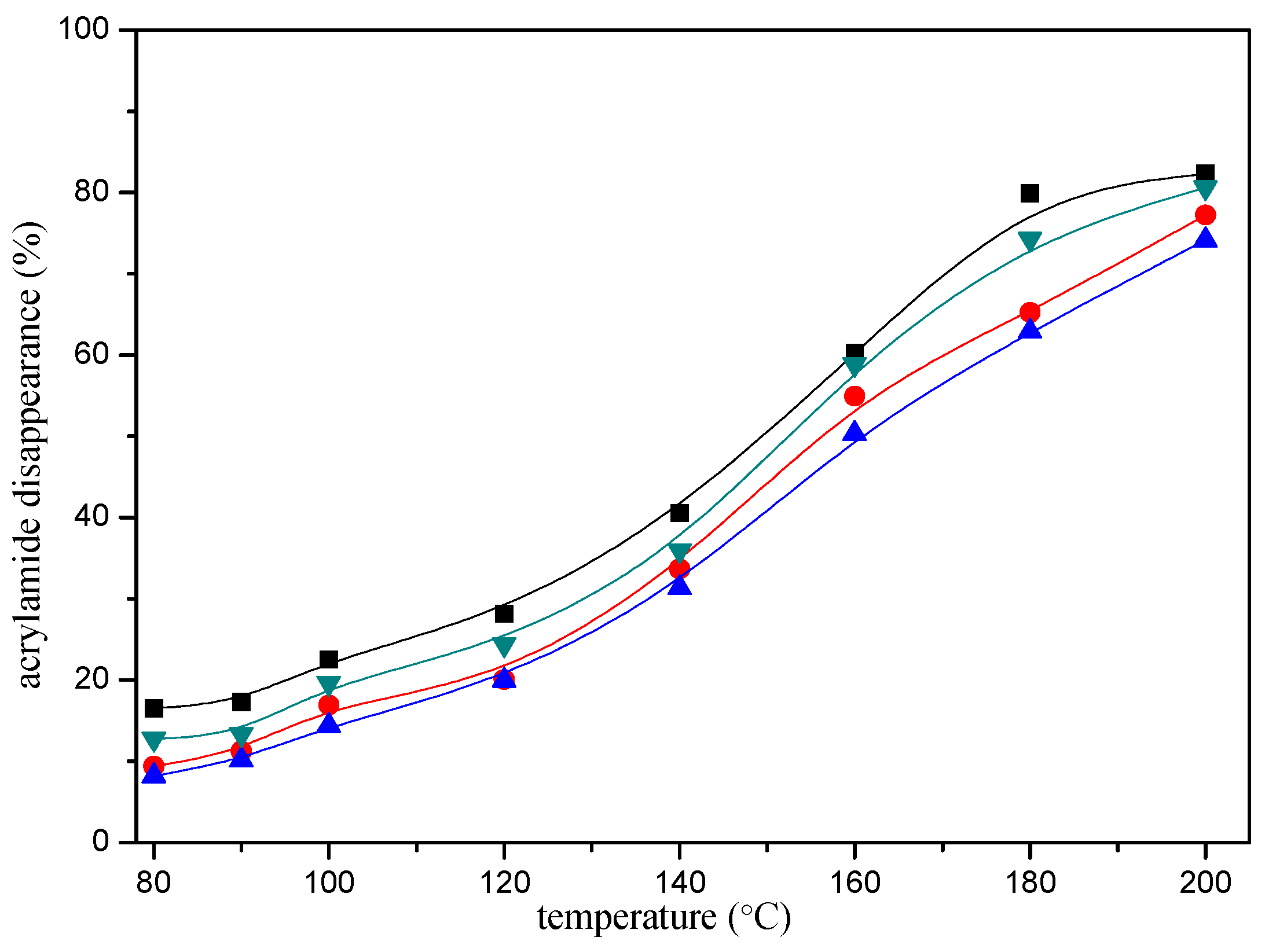
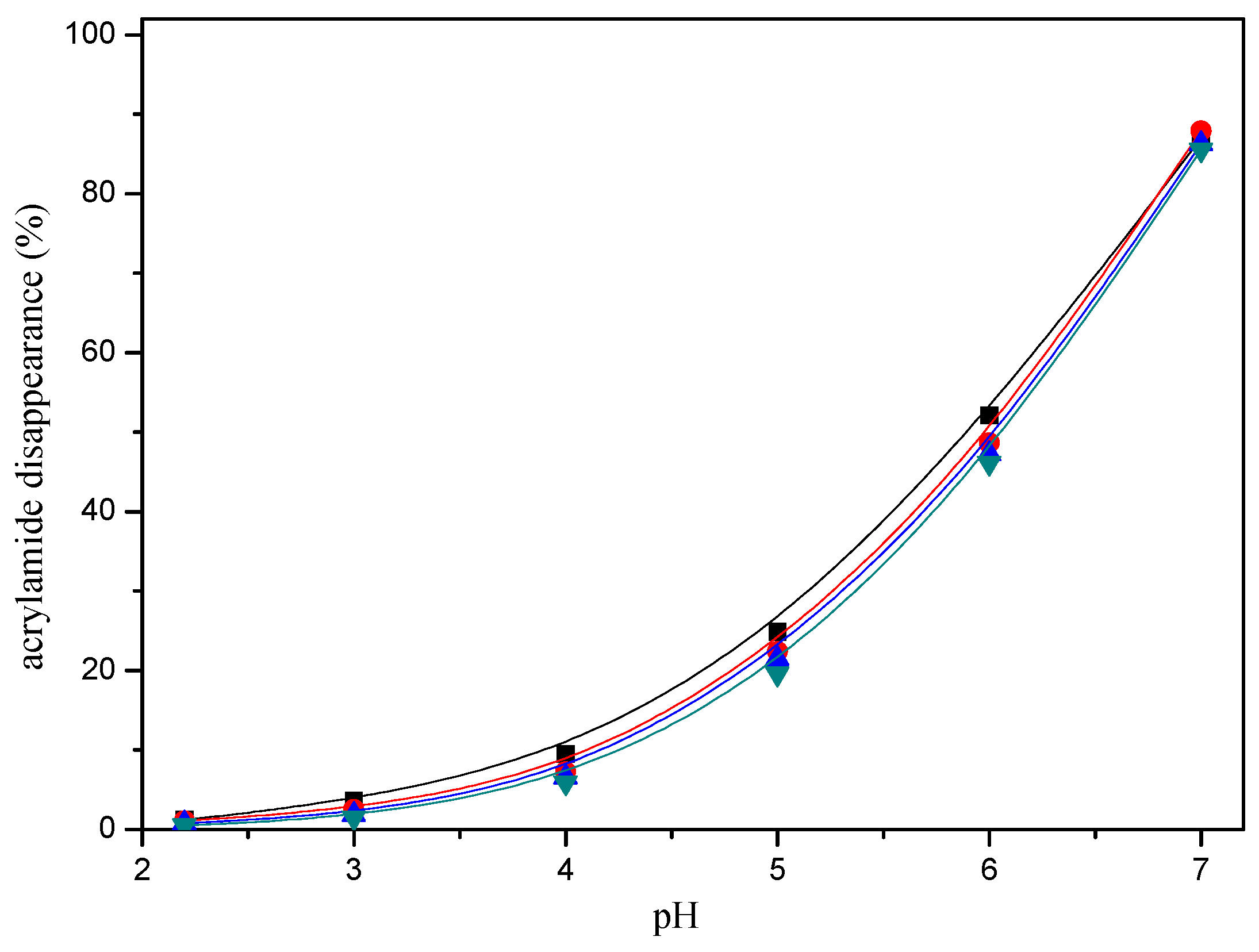
2.1. Acrylamide Disappearance in Acrylamide/mercaptan Reaction Mixtures
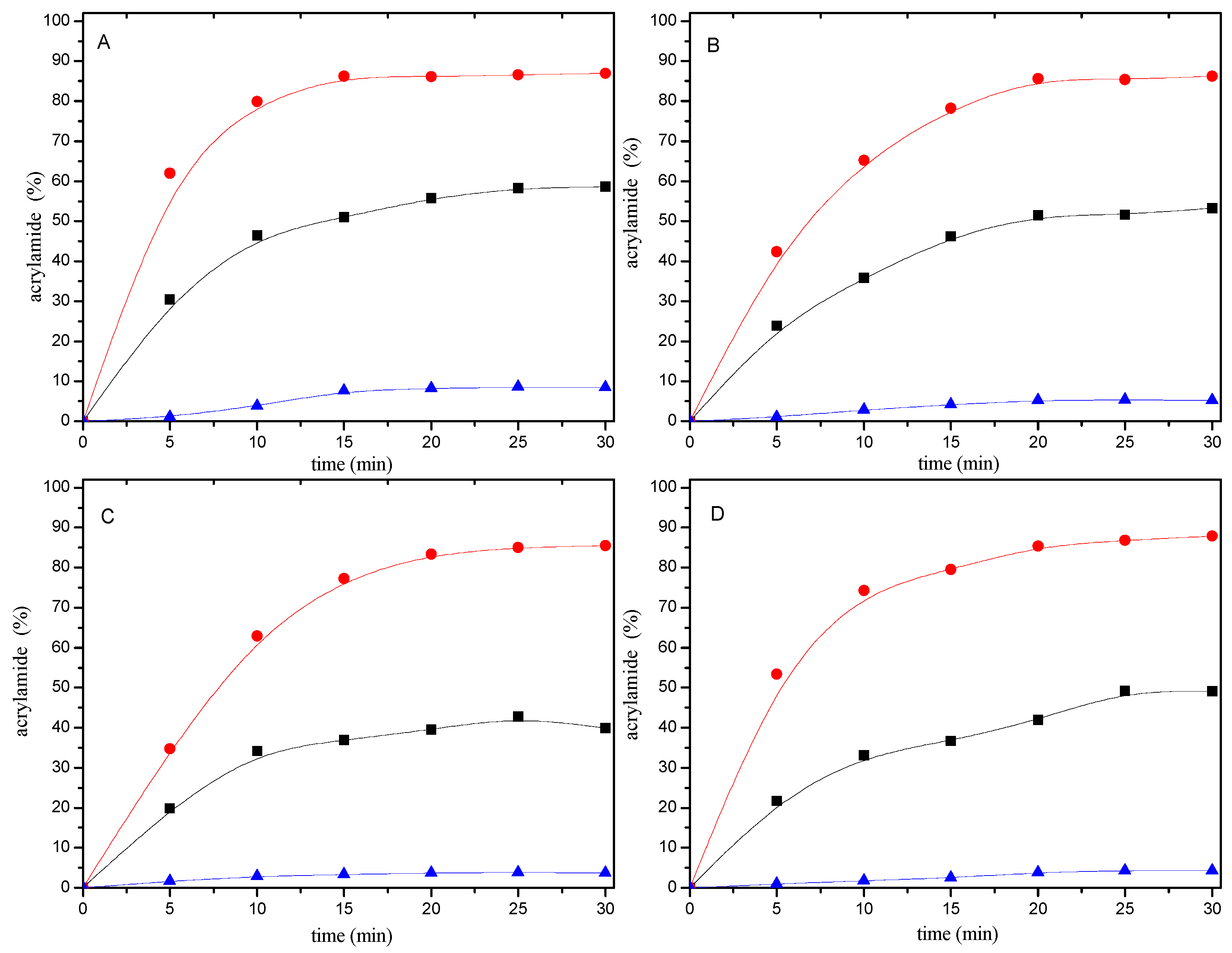
2.2. Acrylamide Formation by Thermal Decomposition of Mercaptan-AA Adducts
2.3. Acrylamide Consumption Produced to Mercaptan-Acrylamide Adducts
2.4. Comparative Reactivity of Mercaptans for Acrylamide Removal
3. Discussion
4. Materials and Methods
4.1. Materials and Reagents
4.2. Acrylamide/Mercaptan Reactions in Aqueous Model System
4.3. The Stability of Mercaptan-Acrylamide Adducts
4.4. Preparation of Brominated Samples for the Analysis of Acrylamide
4.5. Analysis of Acrylamide by GC-MS
4.6. Analysis of Mercaptan-Acrylamide Adducts by HPLC/MS
4.7. Statistical Analysis
Acknowledgment
Author Contributions
Conflicts of Interest
References
- Friedman, M. Chemistry, biochemistry, and safety of acrylamide: A review. J. Agric. Food Chem. 2003, 51, 4504–4526. [Google Scholar] [CrossRef] [PubMed]
- International Agency for Research on Cancer. IARC monographs on the evaluation of carcinogenic risk of chemicals to man. IARC Monogr. Eval. Carcinog. Risk Chem. Man 1972, 1, 184. [Google Scholar]
- McDonald, A. Some industrial chemicals: IARC monographs on the evaluation of carcinogenic risks to humans. Vol 60. Occup. Environ. Med. 1995, 52, 360. [Google Scholar] [CrossRef]
- Panel, E.C. Scientific opinion on the risks for human and animal health related to the presence of modified forms of certain mycotoxins in food and feed. EFSA J. 2014, 12, 3916. [Google Scholar]
- Granvogl, M.; Schieberle, P. Thermally generated 3-aminopropionamide as a transient intermediate in the formation of acrylamide. J. Agric. Food Chem. 2006, 54, 5933–5938. [Google Scholar] [CrossRef] [PubMed]
- Arribas-Lorenzo, G.; Morales, F.J. Effect of pyridoxamine on acrylamide formation in a glucose/asparagine model system. J. Agric. Food Chem. 2009, 57, 901–909. [Google Scholar] [CrossRef] [PubMed]
- Keramat, J.; LeBail, A.; Prost, C.; Soltanizadeh, N. Acrylamide in foods: Chemistry and analysis: A review. Food Bioprocess Technol. 2011, 4, 340–363. [Google Scholar] [CrossRef]
- Tareke, E.; Rydberg, P.; Karlsson, P.; Eriksson, S.; Törnqvist, M. Analysis of acrylamide, a carcinogen formed in heated foodstuffs. J. Agric. Food Chem. 2002, 50, 4998–5006. [Google Scholar] [CrossRef] [PubMed]
- Pedreschi, F.; Granby, K.; Risum, J.; Zude, M.; Schlüter, O.; Rosenthal, A. Acrylamide mitigation in potato chips by using NaCl. Food Bioprocess Technol. 2010, 3, 917–921. [Google Scholar] [CrossRef]
- Ou, S.; Shi, J.; Huang, C.; Zhang, G.; Teng, J.; Jiang, Y.; Yang, B. Effect of antioxidants on elimination and formation of acrylamide in model reaction systems. J. Hazard. Mater. 2010, 182, 863–868. [Google Scholar] [CrossRef] [PubMed]
- Zeng, X.; Cheng, K.W.; Du, Y.; Kong, R.; Clive, L.; Chu, I.; Chen, F.; Wang, M. Activities of hydrocolloids as inhibitors of acrylamide formation in model systems and fried potato strips. Food Chem. 2010, 121, 424–428. [Google Scholar] [CrossRef]
- Kim, C.T.; Hwang, E.S.; Lee, H.J. Reducing acrylamide in fried snack products by adding amino acids. J. Food Sci. 2005, 70, C354–C358. [Google Scholar] [CrossRef]
- Zeng, X.; Cheng, K.W.; Yue, J.; Lin, Z.X.; Shi, J.J.; Ou, S.Y.; Feng, C.; Wang, M. Inhibition of acrylamide formation by vitamins in model reactions and fried potato strips. Food Chem. 2009, 116, 34–39. [Google Scholar] [CrossRef]
- Hidalgo, F.J.; Delgado, R.M.; Zamora, R. Role of mercaptans on acrylamide elimination. Food Chem. 2010, 122, 596–601. [Google Scholar] [CrossRef]
- Friedman, M.; Cavins, J.; Wall, J. Relative nucleophilic reactivities of amino groups and mercaptide ions in addition reactions with α,β-unsaturated compounds 1, 2. J. Am. Chem. Soc. 1965, 87, 3672–3682. [Google Scholar] [CrossRef]
- Werkhoff, P.; Brüning, J.; Emberger, R.; Güntert, M.; Hopp, R. Flavor chemistry of meat volatiles: New results on flavor components from beef, pork and chicken. In Recent Developments in Flavor and Flavor and Fragrance Chemistry; Hopp, R., Mori, K., Eds.; VCH Publisher: New York, NY, USA, 1992. [Google Scholar]
- Maarse, H. Volatile Compounds in Foods and Beverages; TNO-CIVO Food Analysis Institute: Zeist, The Netherlands, 1991. [Google Scholar]
- Simpson, S. The synthesis of di-and trithiols. Can. J. Res. 1947, 25, 20–27. [Google Scholar] [CrossRef] [PubMed]
- LoPachin, R.M.; Gavin, T. Acrylamide-induced nerve terminal damage: Relevance to neurotoxic and neurodegenerative mechanisms. J. Agric. Food Chem. 2008, 56, 5994–6003. [Google Scholar] [CrossRef] [PubMed]
- Koutsidis, G.; Simons, S.P.; Thong, Y.H.; Haldoupis, Y.; Mojica-Lazaro, J.; Wedzicha, B.L.; Mottram, D.S. Investigations on the effect of amino acids on acrylamide, pyrazines, and Michael addition products in model systems. J. Agric. Food Chem. 2009, 57, 9011–9015. [Google Scholar] [CrossRef] [PubMed]
- Hidalgo, F.J.; Delgado, R.M.; Zamora, R. Positive interaction between amino and sulfhydryl groups for acrylamide removal. Food Res. Int. 2011, 44, 1083–1087. [Google Scholar] [CrossRef]
- López-López, A.; Beato, V.M.; Sánchez, A.H.; García-García, P.; Montaño, A. Effects of selected amino acids and water-soluble vitamins on acrylamide formation in a ripe olive model system. J. Food Eng. 2014, 120, 9–16. [Google Scholar] [CrossRef]
- Narita, Y.; Inouye, K. Decrease in the acrylamide content in canned coffee by heat treatment with the addition of cysteine. J. Agric. Food Chem. 2014, 62, 12218–12222. [Google Scholar] [CrossRef] [PubMed]
- Budryn, G.; Nebesny, E.; Oracz, J. Correlation between the stability of chlorogenic acids, antioxidant activity and acrylamide content in coffee beans roasted in different conditions. Int. J. Food Prop. 2015, 18, 290–302. [Google Scholar] [CrossRef]
- Welton, T. Room-temperature ionic liquids. Solvents for synthesis and catalysis. Chem. Rev. 1999, 99, 2071–2084. [Google Scholar] [CrossRef] [PubMed]
- Panpranot, J.; Phandinthong, K.; Praserthdam, P.; Hasegawa, M.; Fujita, S.; Arai, M. A comparative study of liquid-phase hydrogenation on Pd/SiO2 in organic solvents and under pressurized carbon dioxide: Activity change and metal leaching/sintering. J. Mol. Catal. A Chem. 2006, 253, 20–24. [Google Scholar] [CrossRef]
- Ranu, B.C.; Mandal, T. Water-promoted highly selective anti-Markovnikov addition of thiols to unactivated alkenes. Synlett 2007, 2007, 0925–0928. [Google Scholar] [CrossRef]
- Zamora, R.; Delgado, R.M.; Hidalgo, F.J. Model reactions of acrylamide with selected amino compounds. J. Agric. Food Chem. 2010, 58, 1708–1713. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.; Zhang, Z.; Jiang, S.; Yu, M.; Huang, C.; Qiu, R.; Zou, Y.; Zhang, Q.; Ou, S.; Zhou, H.; et al. Chlorogenic acid increased acrylamide formation through promotion of HMF formation and 3-aminopropionamide deamination. J. Hazard. Mater. 2014, 268, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Dong, Y.; Ren, Y.; Zhang, Y. Rapid determination of acrylamide contaminant in conventional fried foods by gas chromatography with electron capture detector. J. Chromatogr. A 2006, 1116, 209–216. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the four mercaptan-acrylamide adducts are available from the authors. They are all white crystals, and the spectroscopic datas are in the paper. |

| Time (Days) | 1,2-Ethanedithiol | 2-Methyl-3-furanthiol | 1-Butanethiol | 2-Methyl-1-butanethiol |
|---|---|---|---|---|
| 0 | −0.04 ± 3.10 b | −0.60 ± 2.68 b | 0.16 ± 3.58 b | 0.19 ± 3.66 b |
| 3 | 78.01 ± 3.04 c | 55.94 ± 2.12 c | 30.74 ± 1.26 c | 29.44 ± 2.96 c |
| 6 | 86.89 ± 2.12 d | 73.85 ± 3.73 d | 57.02 ± 2.30 d | 56.46 ± 1.24 d |
| 9 | 90.45 ± 1.06d e | 87.52 ± 0.86 e | 70.60 ± 0.72 e | 68.19 ± 0.30 e |
| 12 | 92.08 ± 0.21 ef | 90.61 ± 1.47 ef | 81.05 ± 1.92 f | 80.13 ± 2.05 f |
| 15 | 93.21 ± 2.52 ef | 91.29 ± 1.59 f | 82.43 ± 1.75 f | 81.19 ± 3.67 f |
| 18 | 94.61 ± 0.19 f | 92.22 ± 0.19 f | 84.29 ± 3.11 f | 80.83 ± 0.41 f |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiong, Z.; Li, B.; Li, L.; Wan, L.; Peng, X.; Yin, Y. Effect of Selected Mercapto Flavor Compounds on Acrylamide Elimination in a Model System. Molecules 2017, 22, 888. https://doi.org/10.3390/molecules22060888
Xiong Z, Li B, Li L, Wan L, Peng X, Yin Y. Effect of Selected Mercapto Flavor Compounds on Acrylamide Elimination in a Model System. Molecules. 2017; 22(6):888. https://doi.org/10.3390/molecules22060888
Chicago/Turabian StyleXiong, Zhiyong, Bing Li, Lin Li, Liting Wan, Xiaolong Peng, and Yongpo Yin. 2017. "Effect of Selected Mercapto Flavor Compounds on Acrylamide Elimination in a Model System" Molecules 22, no. 6: 888. https://doi.org/10.3390/molecules22060888





