Design, Synthesis, and Biological Activities of Novel Pyrazole Oxime Compounds Containing a Substituted Pyridyl Moiety
Abstract
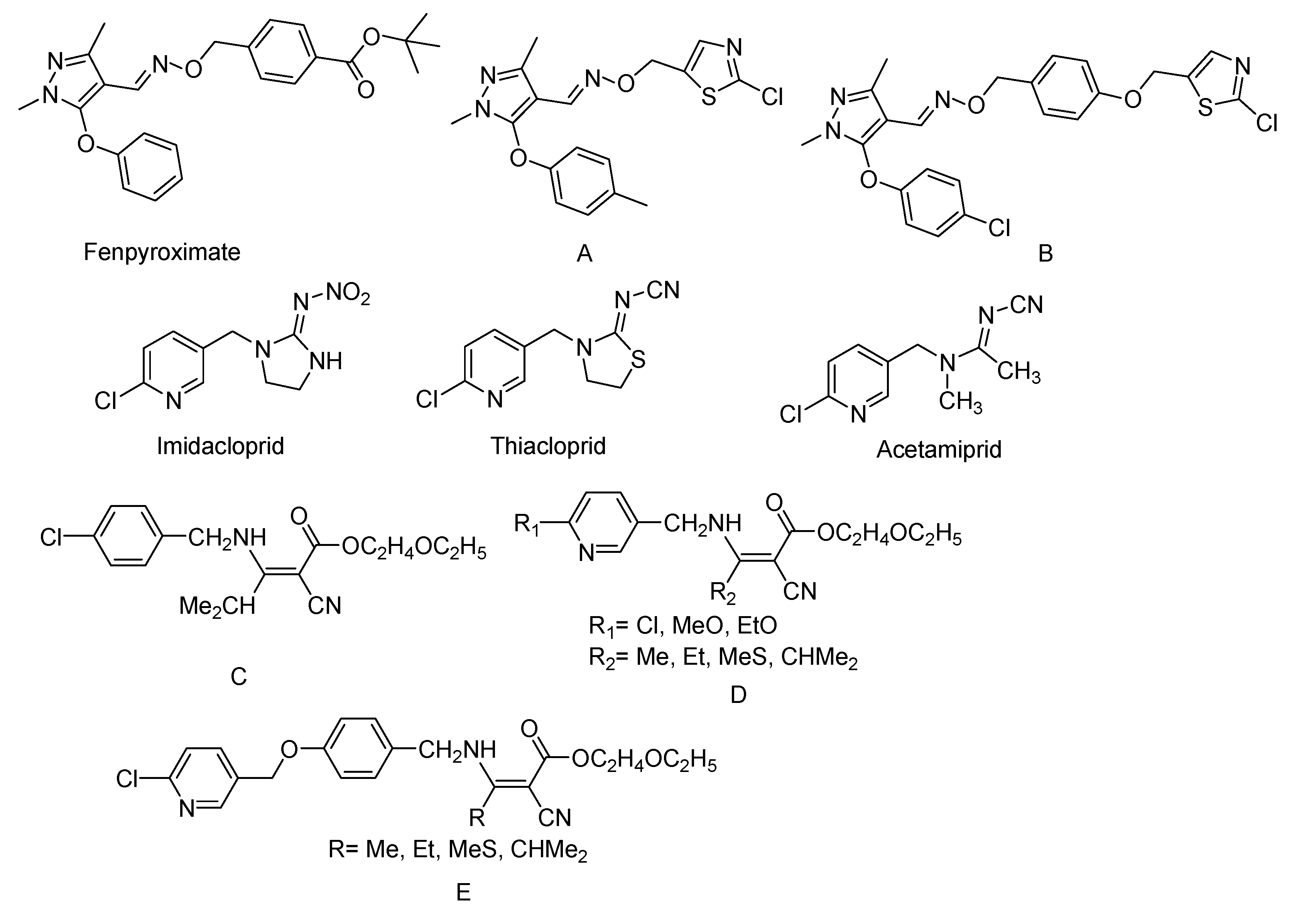
:1. Introduction
2. Results and Discussion
2.1. Chemistry
2.2. Biological Activities
2.2.1. Acaricidal Activities and Insecticidal Activity
2.2.2. Anticancer Activities
3. Experimental Section
3.1. Chemistry
3.1.1. General Procedures
3.1.2. Synthesis of Compound 2
3.1.3. Synthesis of Compound 3
3.1.4. Synthesis of Compound 4
3.1.5. Synthesis of Compound 5
3.1.6. General Procedure for the Preparation of 7a–7w
3.1.7. General Procedure for the Preparation of 8a–8w
3.1.8. General Procedure for the Preparation of 9a–9w
3.2. Biological Tests
3.2.1. Acaricidal Activity and Insecticidal Activity Assay
3.2.2. Anticancer Activity Assay
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Chen, L.; Ou, X.M.; Mao, C.H.; Shang, J.; Huang, R.Q.; Bi, F.C.; Wang, Q.M. Synthesis and bioassay evaluation of 1-(4-substituteddidene-aminooxymethyl)-phenyl-3-(2,6-difluorobenzoyl)ureas. Bioorg. Med. Chem. 2007, 15, 3678–3683. [Google Scholar] [CrossRef] [PubMed]
- Song, H.J.; Liu, Y.X.; Xiong, L.X.; Li, Y.Q.; Yang, N.; Wang, Q.M. Design, synthesis, and insecticidal evaluation of new pyrazole derivatives containing imine, oxime ether, and dihydroisoxazoline groups based on the inhibitor binding pocket of respiratory complex I. J. Agric. Food Chem. 2013, 61, 8730–8736. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhang, H.Q.; Liu, J.; Yang, X.P.; Liu, Z.J. Stereoselective synthesis and antifungal activities of (E)-α-(methoxyimino)benzeneacetate derivatives containing 1,3,5-substituted pyrazole ring. J. Agric. Food Chem. 2006, 54, 3636–3640. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.W.; Li, Y.; Ge, D.; Zhao, B.X.; Liu, Y.R.; Lv, H.S.; Ding, J.; Miao, J.Y. Synthesis of novel oxime-containing pyrazole derivatives and discovery of regulators for apoptosis and autophagy in A549 lung cancer cells. Bioorg. Med. Chem. Lett. 2010, 20, 4766–4770. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, G.P.; Cai, X.J.; Chen, Z.; Song, B.A.; Bhadury, P.S.; Yang, S.; Jin, L.H.; Xue, W.; Hu, D.Y.; Zeng, S. Synthesis and antiviral activities of pyrazole derivatives containing an oxime moiety. J. Agric. Food Chem. 2008, 56, 10160–10167. [Google Scholar] [CrossRef] [PubMed]
- Swanson, M.B.; Ivancic, W.A.; Saxena, A.M.; Allton, J.D.; O’Brien, G.K.; Suzuki, T.; Nishizawa, H.; Nokata, M. Direct photolysis of Fenpyroximate in a buffered aqueous solution under a xenon lamp. J. Agric. Food Chem. 1995, 43, 513–518. [Google Scholar] [CrossRef]
- Yang, Y.Z.; Lin, D.Y.; Fu, C.; Zou, X.M. Synthesis and biological evaluation of novel pyrazole oxime ether derivatives containing chlorothiazole group and pyrimidine rings. Chin. J. Org. Chem. 2015, 35, 100–108. [Google Scholar] [CrossRef]
- Dai, H.; Xiao, Y.S.; Li, Z.; Xu, X.Y.; Qian, X.H. The thiazoylmethoxy modification on pyrazole oximes: Synthesis and insecticidal biological evaluation beyond acaricidal activity. Chin. Chem. Lett. 2014, 25, 1014–1016. [Google Scholar] [CrossRef]
- Park, H.J.; Lee, K.; Park, S.J.; Ahn, B.; Lee, J.C.; Cho, H.; Lee, K.I. Identification of antitumor activity of pyrazole oxime ethers. Bioorg. Med. Chem. Lett. 2005, 15, 3307–3312. [Google Scholar] [CrossRef] [PubMed]
- Dai, H.; Ge, S.S.; Li, G.; Chen, J.; Shi, Y.J.; Ye, L.Y.; Ling, Y. Synthesis, and bioactivities of novel pyrazole oxime derivatives containing a 1,2,3-thiadiazole moiety. Bioorg. Med. Chem. Lett. 2016, 26, 4504–4507. [Google Scholar] [CrossRef] [PubMed]
- Li, M.Y.; Hu, P.Z.; Zhu, W.R.; Xu, K.X. Synthesis of 2,6-(substituded)pyridine derivatives using amide and imine groups. Chin. Chem. Lett. 2003, 14, 572–574. [Google Scholar]
- Zhang, J.A.; Pan, M.; Jiang, J.J.; She, Z.G.; Fan, Z.J.; Su, C.Y. Syntheses, crystal structures and antimicrobial activities of thioether ligands containing quinoline and pyridine terminal groups and their transition metal complexes. Inorg. Chim. Acta 2011, 374, 269–277. [Google Scholar] [CrossRef]
- Sun, G.X.; Yang, M.Y.; Shi, Y.X.; Sun, Z.H.; Liu, X.H.; Wu, H.K.; Li, B.J.; Zhang, Y.G. Microwave assistant synthesis, antifungal activity and DFT theoretical study of some novel 1,2,4-triazole derivatives containing pyridine moiety. Int. J. Mol. Sci. 2014, 15, 8075–8090. [Google Scholar] [CrossRef] [PubMed]
- Kloepfer, J.A.; Bradforth, S.E.; Nadeau, J.L. Photophysical properties of biologically compatible CdSe quantum dot structures. J. Phys. Chem. B 2005, 109, 9996–10003. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.J.; Yang, M.Y.; Sun, Z.H.; Tan, C.X.; Weng, J.Q.; Wu, H.K.; Liu, X.H. Synthesis and antifungal activity of 1,3,4-thiadiazole derivatives containing pyridine group. Lett. Drug Des. Discov. 2014, 11, 1107–1111. [Google Scholar] [CrossRef]
- Saito, S.; Isayama, S.; Sakamoto, N.; Umeda, K. Insecticidal activity of pyridalyl: Acute and sub-acute symptoms in Spodoptera litura larvae. J. Pestic. Sci. 2004, 29, 372–375. [Google Scholar] [CrossRef]
- Shao, X.S.; Zhang, W.W.; Peng, Y.Q.; Li, Z.; Tian, Z.Z.; Qian, X.H. cis-Nitromethylene neonicotinoids as new nicotinic family: Synthesis, structural diversity, and insecticidal evaluation of hexahydroimidazo 1,2-alpha pyridine. Bioorg. Med. Chem. Lett. 2008, 18, 6513–6516. [Google Scholar] [CrossRef] [PubMed]
- Dai, H.; Chen, J.; Li, H.; Dai, B.J.; He, H.B.; Fang, Y.; Shi, Y.J. Synthesis and bioactivities of novel pyrazole oxime derivatives containing a 5-trifluoromethylpyridyl moiety. Molecules 2016, 21, 276. [Google Scholar] [CrossRef] [PubMed]
- Scapin, G.; Reddy, S.G.; Zheng, R.; Blanchard, J.S. Three-dimensional structure of escherichia coli dihydrodipicolinate reductase in complex with NADH and the inhibitor 2,6-pyridinedicarboxylate. Biochemistry 1997, 36, 15081–15088. [Google Scholar] [CrossRef] [PubMed]
- El-Subbagh, H.I.; Abu-Zaid, S.M.; Mahran, M.A.; Badria, F.A.; Al-Obaid, A.M. Synthesis and biological evaluation of certain alpha,beta-unsaturated ketones and their corresponding fused pyridines as antiviral and cytotoxic agents. J. Med. Chem. 2000, 43, 2915–2921. [Google Scholar] [CrossRef] [PubMed]
- Vanbeusichem, M.; Farrell, N. Activation of the trans geometry in platinum antitumor complexes- synthesis, characterization, and biological-activity of complexes with the planar ligands pyridine, N-methylimidazole, thiazole, and quinoline-crystal and molecular-structure of trans-dichlorobis(thiazole)platinum(Ii). Inorg. Chem. 1992, 31, 634–639. [Google Scholar]
- Kagabu, S.; Ishihara, R.; Hieda, Y.; Nishimura, K.; Naruse, Y. Insecticidal and neuroblocking potencies of variants of the imidazolidine moiety of imidacloprid-related neonicotinoids and the relationship to partition coefficient and charge density on the pharmacophore. J. Agric. Food Chem. 2007, 55, 812–818. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.M.; Sun, H.K.; Cao, H.Y.; Cheng, M.R.; Huang, R.Q. Synthesis and herbicidal activity of 2-cyano-3-substituted-pyridinemethylaminoacrylates. J. Agric. Food Chem. 2003, 51, 5030–5035. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.S.; Miao, W.K.; Wang, T.T.; Fang, J.X. Synthesis and herbicidal activities of 2-cyanocarylates with 4-(6-chloropyridin-3-yl)methoxy-benzylamine moieties. Chin. J. Org. Chem. 2015, 35, 1484–1492. [Google Scholar] [CrossRef]
- Park, M.S.; Park, H.J.; Park, K.H.; Lee, K.I. Introduction of N-containing heterocycles into pyrazole by nucleophilic aromatic substitution. Synth. Commun. 2004, 34, 1541–1550. [Google Scholar] [CrossRef]
- Dai, H.; Chen, J.; Li, G.; Ge, S.S.; Shi, Y.J.; Fang, Y.; Ling, Y. Design, synthesis, and bioactivities of novel oxadiazole-substituted pyrazole oximes. Bioorg. Med. Chem. Lett. 2017, 27, 950–953. [Google Scholar] [CrossRef] [PubMed]
- Ling, Y.; Wang, Z.Q.; Xiao, Y.A.; Zhu, C.Y.; Shen, L.C.; Wang, X.M.; Hui, Y.; Wang, X.Y. Benzylidene 2-aminoimidazolones derivatives: Synthesis and in vitro evaluation of anti-tumor carcinoma activity. Chem. Pharm. Bull. 2013, 61, 1081–1084. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds 9a–9w are available from the authors. |


| Compd. | Tetranychus cinnabarinus | ||
|---|---|---|---|
| 500 μg/mL | 100 μg/mL | 20 μg/mL | |
| 9a | 0 | — b | — |
| 9b | 0 | — | — |
| 9c | 0 | — | — |
| 9d | 0 | — | — |
| 9e | 0 | — | — |
| 9f | 0 | — | — |
| 9g | 0 | — | — |
| 9h | 0 | — | — |
| 9i | 0 | — | — |
| 9j | 0 | — | — |
| 9k | 0 | — | — |
| 9l | 50.39 ± 0.83 a | — | — |
| 9m | 80.65 ± 1.32 | 0 | — |
| 9n | 0 | — | — |
| 9o | 0 | — | — |
| 9p | 80.56 ± 1.05 | 0 | — |
| 9q | 80.78 ± 0.76 | 70.89 ± 1.13 | 30.55 ± 0.35 |
| 9r | 40.92 ± 1.68 | — | — |
| 9s | 0 | — | — |
| 9t | 0 | — | — |
| 9u | 0 | — | — |
| 9v | 0 | — | — |
| 9w | 0 | — | — |
| Fenpyroximate | 100.00 ± 0.00 | 100.00 ± 0.00 | 100.00 ± 0.00 |
| Compd. | Oriental armyworm | Aphis medicaginis | Nilaparvata lugens | |||
|---|---|---|---|---|---|---|
| 500 μg/mL | 100 μg/mL | 500 μg/mL | 100 μg/mL | 500 μg/mL | 100 μg/mL | |
| 9a | 100.00 ± 0.00 a | 0 | 0 | — | 0 | — |
| 9b | 80.23 ± 1.22 | 0 | 0 | — | 0 | — |
| 9c | 100.00 ± 0.00 | 0 | 0 | — | 0 | — |
| 9d | 100.00 ± 0.00 | 0 | 0 | — | 70.57 ± 1.26 | 0 |
| 9e | 100.00 ± 0.00 | 20.41 ± 1.02 | 0 | — | 0 | — |
| 9f | 100.00 ± 0.00 | 0 | 0 | — | 50.68 ± 1.72 | — |
| 9g | 100.00 ± 0.00 | 0 | 0 | — | 60.36 ± 0.96 | — |
| 9h | 90.36 ± 1.35 | 0 | 0 | — | 0 | — |
| 9i | 100.00 ± 0.00 | 0 | 0 | — | 0 | — |
| 9j | 100.00 ± 0.00 | 0 | 0 | — | 0 | — |
| 9k | 100.00 ± 0.00 | 0 | 0 | — | 0 | — |
| 9l | 100.00 ± 0.00 | 0 | 100.00 ± 0.00 | 20.88 ± 1.52 | 100.00 ± 0.00 | 0 |
| 9m | 100.00 ± 0.00 | 0 | 100.00 ± 0.00 | 0 | 100.00 ± 0.00 | 0 |
| 9n | 100.00 ± 0.00 | 0 | 0 | — | 0 | — |
| 9o | 100.00 ± 0.00 | 0 | 0 | — | 95.54 ± 1.62 | 0 |
| 9p | 60.45 ± 0.82 | — b | 100.00 ± 0.00 | 0 | 100.00 ± 0.00 | 0 |
| 9q | 90.56 ± 1.91 | 0 | 100.00 ± 0.00 | 0 | 100.00 ± 0.00 | 0 |
| 9r | 100.00 ± 0.00 | 30.62 ± 0.87 | 100.00 ± 0.00 | 0 | 90.66 ± 1.08 | 0 |
| 9s | 100.00 ± 0.00 | 0 | 100.00 ± 0.00 | 0 | 100.00 ± 0.00 | 0 |
| 9t | 100.00 ± 0.00 | 40.37 ± 1.18 | 100.00 ± 0.00 | 0 | 60.53 ± 1.37 | — |
| 9u | 100.00 ± 0.00 | 0 | 100.00 ± 0.00 | 0 | 80.68 ± 0.29 | 0 |
| 9v | 80.33 ± 0.79 | 0 | 50.77 ± 0.42 | — | 0 | — |
| 9w | 80.25 ± 1.22 | 0 | 0 | — | 0 | — |
| Abamectin | 100.00 ± 0.00 | 100.00 ± 0.00 | 100.00 ± 0.00 | 100.00 ± 0.00 | 100.00 ± 0.00 | 100.00 ± 0.00 |
| Compd. | IC50, μM | ||
|---|---|---|---|
| Panc-1 | HepG2 | SGC-7901 | |
| 9a | >40 | >40 | >40 |
| 9b | >40 | 12.65 ± 0.75 | >40 |
| 9c | >40 | >40 | >40 |
| 9d | >40 | 39.35 ± 0.60 | >40 |
| 9e | >40 | >40 | >40 |
| 9f | >40 | 39.52 ± 0.51 | >40 |
| 9g | >40 | 17.27 ± 2.02 | >40 |
| 9h | >40 | 39.02 ± 0.93 | >40 |
| 9i | >40 | >40 | >40 |
| 9j | >40 | >40 | >40 |
| 9k | >40 | >40 | >40 |
| 9l | >40 | 8.72 ± 1.23 | >40 |
| 9m | >40 | >40 | >40 |
| 9n | >40 | 33.46 ± 2.55 | >40 |
| 9o | >40 | >40 | >40 |
| 9p | >40 | 7.24 ± 0.54 | >40 |
| 9q | >40 | 8.27 ± 1.47 | >40 |
| 9r | >40 | 1.53 ± 0.79 | >40 |
| 9s | >40 | 9.76 ± 2.32 | >40 |
| 9t | >40 | 9.13 ± 1.73 | >40 |
| 9u | >40 | 15.24 ± 3.05 | >40 |
| 9v | >40 | 11.93 ± 2.24 | >40 |
| 9w | >40 | >40 | >40 |
| Sorafenib | 11.50 ± 2.32 | — | 12.10 ± 2.68 |
| 5-Fluorouracil | — | 35.67 ± 3.15 | — |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, C.; Chen, J.; Gu, H.; Bao, N.; Dai, H. Design, Synthesis, and Biological Activities of Novel Pyrazole Oxime Compounds Containing a Substituted Pyridyl Moiety. Molecules 2017, 22, 878. https://doi.org/10.3390/molecules22060878
Chen C, Chen J, Gu H, Bao N, Dai H. Design, Synthesis, and Biological Activities of Novel Pyrazole Oxime Compounds Containing a Substituted Pyridyl Moiety. Molecules. 2017; 22(6):878. https://doi.org/10.3390/molecules22060878
Chicago/Turabian StyleChen, Cuili, Jia Chen, Haiying Gu, Ning Bao, and Hong Dai. 2017. "Design, Synthesis, and Biological Activities of Novel Pyrazole Oxime Compounds Containing a Substituted Pyridyl Moiety" Molecules 22, no. 6: 878. https://doi.org/10.3390/molecules22060878






