Cholinesterase Inhibitory Activities of Adamantyl-Based Derivatives and Their Molecular Docking Studies
Abstract
:1. Introduction
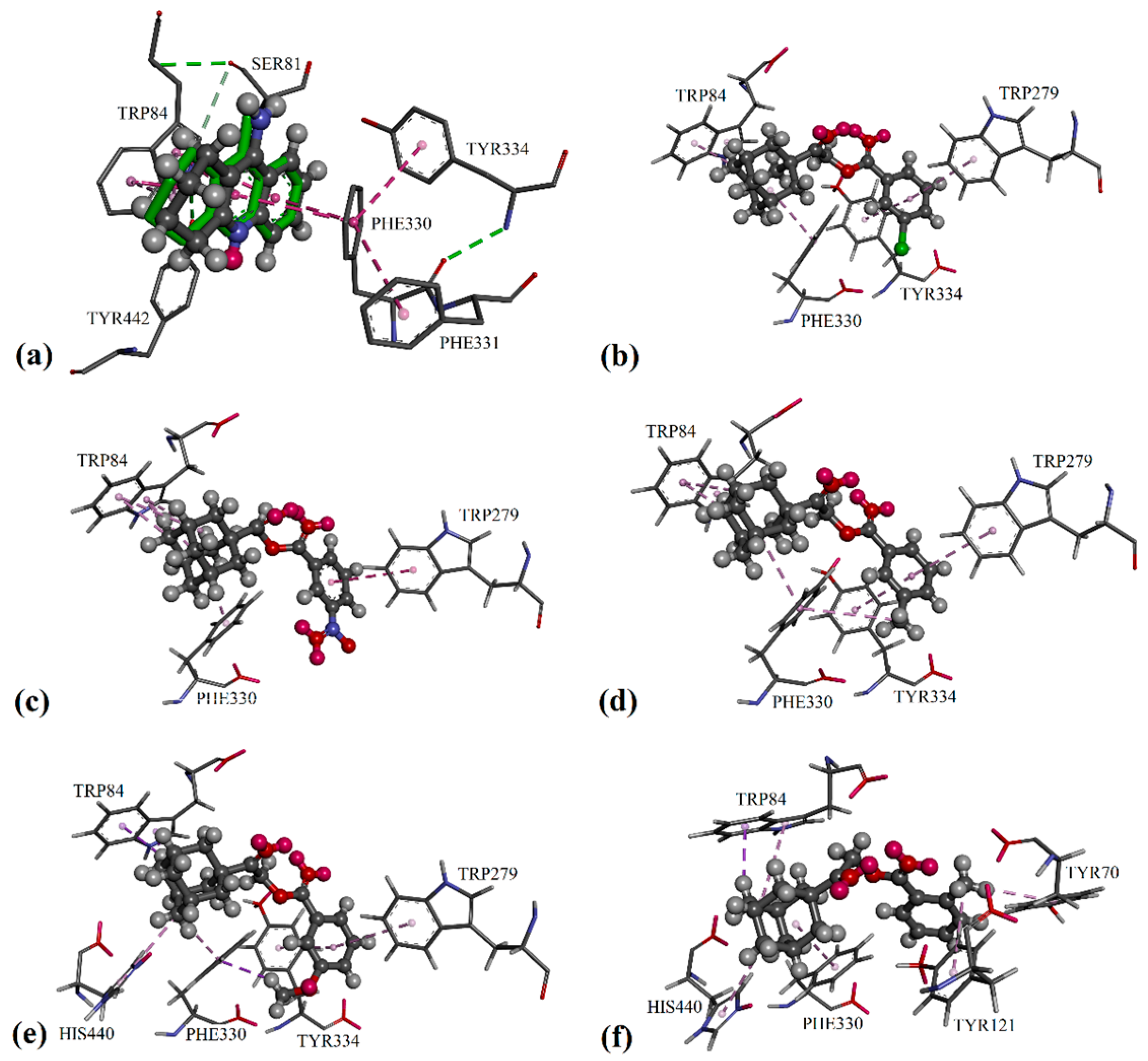
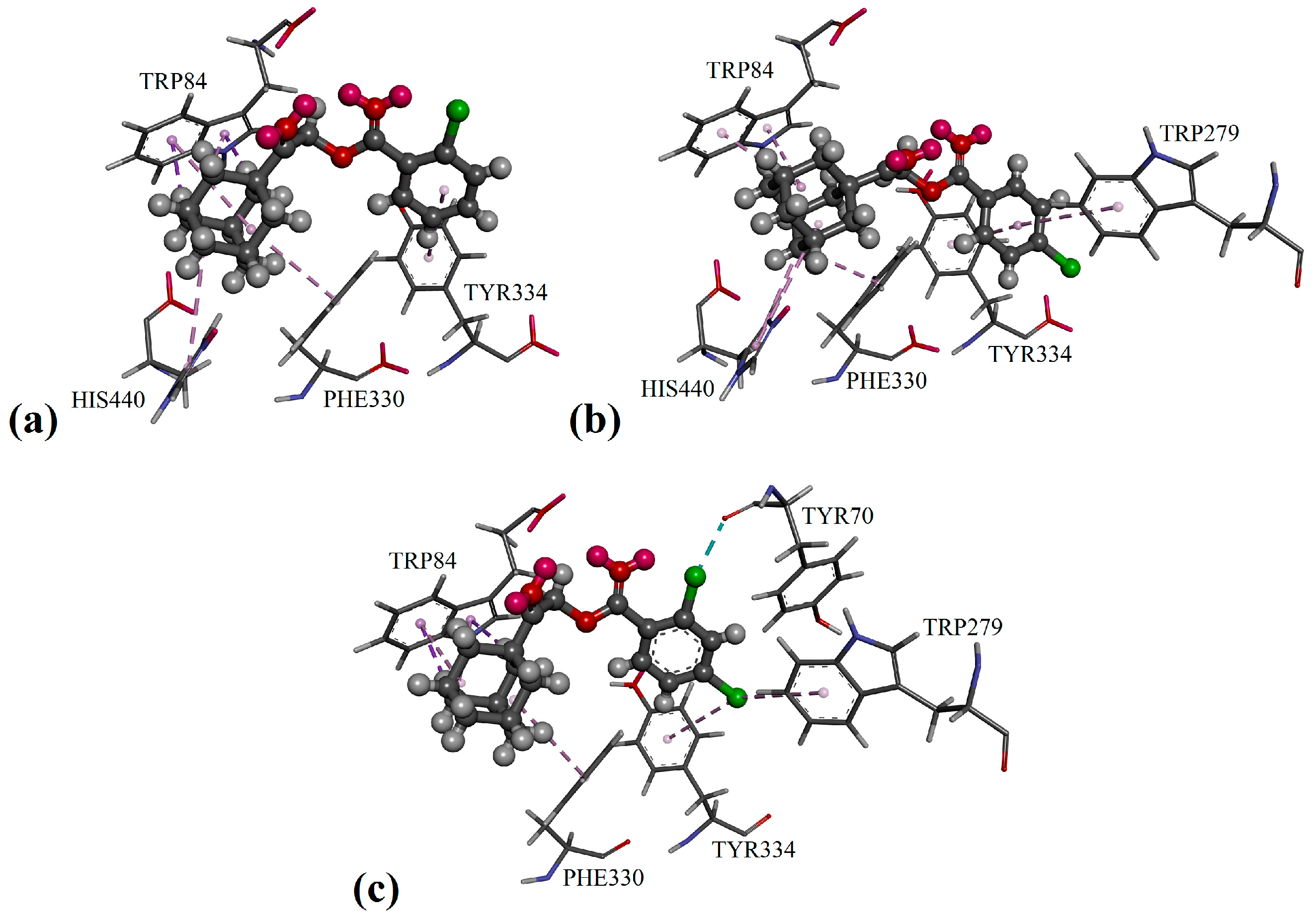
2. Results and Discussion
3. Materials and Methods
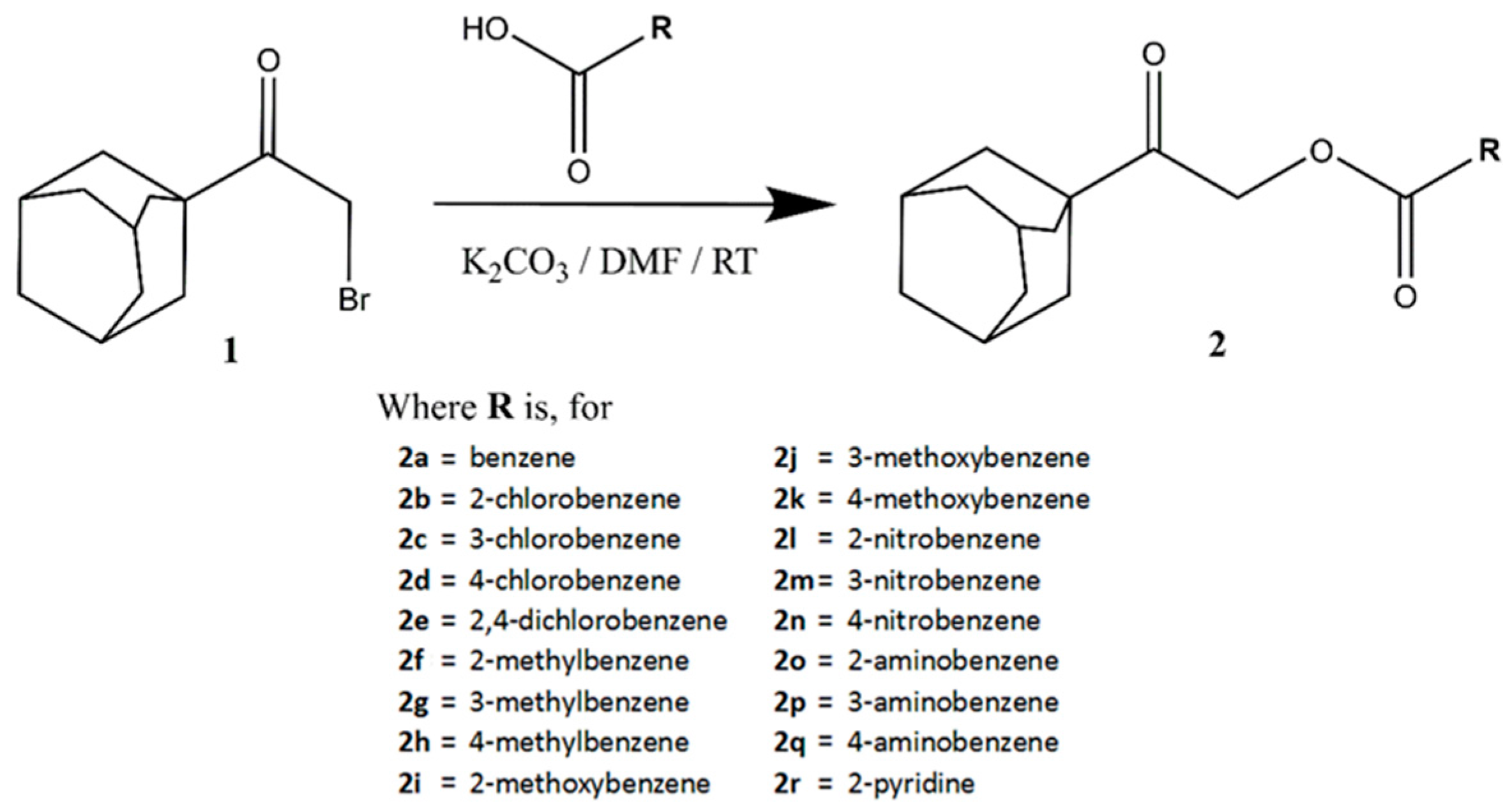
3.1. Synthesis and Structural Elucidation of Adamantane

3.2. Cholinesterases Inhibitory Assay
3.3. Statistical Analysis
3.4. Docking Protocol
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Alzheimer’s Association. 2015 Alzheimer’s disease facts and figures. Alzheimers Dement. 2015, 11, 332. [Google Scholar]
- Francis, P.T.; Palmer, A.M.; Snape, M.; Wilcock, G.K. The cholinergic hypothesis of alzheimer’s disease: A review of progress. J. Neurol. Neurosurg. Psychiatry 1999, 66, 137–147. [Google Scholar] [CrossRef] [PubMed]
- Terry, A.V.; Buccafusco, J.J. The cholinergic hypothesis of age and alzheimer’s disease-related cognitive deficits: Recent challenges and their implications for novel drug development. J. Pharmacol. Exp. Ther. 2003, 306, 821–827. [Google Scholar] [CrossRef] [PubMed]
- Scott, L.J.; Goa, K.L. Galantamine. Drugs 2000, 60, 1095–1122. [Google Scholar] [CrossRef] [PubMed]
- Rosenberry, T.L.; Mallender, W.D.; Thomas, P.J.; Szegletes, T. A steric blockade model for inhibition of acetylcholinesterase by peripheral site ligands and substrate. Chem.-Biol. Interact. 1999, 119, 85–97. [Google Scholar] [CrossRef]
- Lagadic-Gossmann, D.; Rissel, M.; Le Bot, M.A.; Guillouzo, A. Toxic effects of tacrine on primary hepatocytes and liver epithelial cells in culture. Cell Biol. Toxicol. 1998, 14, 361–373. [Google Scholar] [CrossRef] [PubMed]
- Touchon, J.; Bergman, H.; Bullock, R.; Rapatz, G.; Nagel, J.; Lane, R. Response to rivastigmine or donepezil in alzheimer’s patients with symptoms suggestive of concomitant lewy body pathology. Curr. Med. Res. Opin. 2006, 22, 49–59. [Google Scholar] [CrossRef] [PubMed]
- Ali, T.B.; Schleret, T.R.; Reilly, B.M.; Chen, W.Y.; Abagyan, R. Adverse effects of cholinesterase inhibitors in dementia, according to the pharmacovigilance databases of the United-States and Canada. PLoS ONE 2015, 10, e0144337. [Google Scholar] [CrossRef] [PubMed]
- Landa, S.; Macháček, V. Sur l’adamantane. nouvel hydrocarbure extrait du naphte. Collect. Czechoslov. Chem. Commun. 1933, 5, 1–5. [Google Scholar] [CrossRef]
- Prelog, V.; Seiwerth, R. Über die synthese des adamantans. Eur. J. Inorg. Chem. 1941, 74, 1644–1648. [Google Scholar] [CrossRef]
- Henry, G.E.; Jacobs, H.; Carrington, C.M.S.; McLean, S.; Reynolds, W.F. Plukenetione A. An unusual adamantyl ketone from Clusia plukenetii (guttiferae). Tetrahedron Lett. 1996, 37, 8663–8666. [Google Scholar] [CrossRef]
- Díaz-Carballo, D.; Malak, S.; Bardenheuer, W.; Freistuehler, M.; Peter Reusch, H. The contribution of plukenetione a to the anti-tumoral activity of cuban propolis. Bioorg. Med. Chem. 2008, 16, 9635–9643. [Google Scholar] [CrossRef] [PubMed]
- Maugh, T. Panel urges wide use of antiviral drug. Science 1979, 206, 1058–1060. [Google Scholar] [CrossRef] [PubMed]
- Hay, A.J.; Wolstenholme, A.J.; Skehel, J.J.; Smith, M.H. The molecular basis of the specific anti-influenza action of amantadine. EMBO J. 1985, 4, 3021–3024. [Google Scholar] [PubMed]
- Piérard, G.E.; Piérard-Franchimont, C.; Paquet, P.; Quatresooz, P. Spotlight on adapalene. Exp. Opin. Drug Metab. Toxicol. 2009, 5, 1565–1575. [Google Scholar] [CrossRef] [PubMed]
- Rosenthal, K.S.; Sokol, M.S.; Ingram, R.L.; Subramanian, R.; Fort, R.C. Tromantadine: Inhibitor of early and late events in herpes simplex virus replication. Antimicrob. Agents Chemother. 1982, 22, 1031–1036. [Google Scholar] [CrossRef] [PubMed]
- Kelly, J.M.; Miles, M.A.; Skinner, A.C. The anti-influenza virus drug rimantadine has trypanocidal activity. Antimicrob. Agents Chemother. 1999, 43, 985–987. [Google Scholar] [PubMed]
- De Clercq, E. Antiviral agents active against influenza a viruses. Nat. Rev. Drug Discov. 2006, 5, 1015–1025. [Google Scholar] [CrossRef] [PubMed]
- Cady, S.D.; Luo, W.; Hu, F.; Hong, M. Structure and function of the influenza A M2 proton channel. Biochemistry 2009, 48, 7356–7364. [Google Scholar] [CrossRef] [PubMed]
- Zoidis, G.; Kolocouris, N.; Kelly, J.M.; Prathalingam, S.R.; Naesens, L.; De Clercq, E. Design and synthesis of bioactive adamantanaminoalcohols and adamantanamines. Eur. J. Med. Chem. 2010, 45, 5022–5030. [Google Scholar] [CrossRef] [PubMed]
- Von Geldern, T.W.; Trevillyan, J.M. “The next big thing” in diabetes: Clinical progress on DPP-IV inhibitors. Drug Dev. Res. 2006, 67, 627–642. [Google Scholar] [CrossRef]
- Havale, S.H.; Pal, M. Medicinal chemistry approaches to the inhibition of dipeptidyl peptidase-4 for the treatment of type 2 diabetes. Bioorg. Med. Chem. 2009, 17, 1783–1802. [Google Scholar] [CrossRef] [PubMed]
- Zettl, H.; Schubert-Zsilavecz, M.; Steinhilber, D. Medicinal chemistry of incretin mimetics and dpp-4 inhibitors. ChemMedChem 2010, 5, 179–185. [Google Scholar] [CrossRef] [PubMed]
- Reisberg, B.; Doody, R.; Stöffler, A.; Schmitt, F.; Ferris, S.; Möbius, H.J. Memantine in moderate-to-severe alzheimer’s disease. N. Engl. J. Med. 2003, 348, 1333–1341. [Google Scholar] [CrossRef] [PubMed]
- Kumar, C.; Kwong, H.; Mah, S.; Chia, T.; Loh, W.S.; Quah, C.; Lim, G.; Chandraju, S.; Fun, H.K. Synthesis and crystallographic insight into the structural aspects of some novel adamantane-based ester derivatives. Molecules 2015, 20, 18827–18846. [Google Scholar] [CrossRef] [PubMed]
- Ellman, G.L.; Courtney, K.D.; Andres jr, V.; Featherstone, R.M. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol. 1961, 7, 88–95. [Google Scholar] [CrossRef]
- Mohammadi-Farani, A.; Ahmadi, A.; Nadri, H.; Aliabadi, A. Synthesis, docking and acetylcholinesterase inhibitory assessment of 2-(2-(4-benzylpiperazin-1-yl)ethyl)isoindoline-1,3-dione derivatives with potential anti-alzheimer effects. DARU J. Pharm. Sci. 2013, 21, 47. [Google Scholar] [CrossRef] [PubMed]
- Nicolet, Y.; Lockridge, O.; Masson, P.; Fontecilla-Camps, J.C.; Nachon, F. Crystal structure of human butyrylcholinesterase and of its complexes with substrate and products. J. Biol. Chem. 2003, 278, 41141–41147. [Google Scholar] [CrossRef] [PubMed]
- Johnson, G.; Moore, S.W. The peripheral anionic site of acetylcholinesterase: Structure, functions and potential role in rational drug design. Curr. Pharm. Des. 2006, 12, 217–225. [Google Scholar] [CrossRef] [PubMed]
- Jones, G.; Willett, P.; Glen, R.C. Molecular recognition of receptor sites using a genetic algorithm with a description of desolvation. J. Mol. Biol. 1995, 245, 43–53. [Google Scholar] [CrossRef]
- Jones, G.; Willett, P.; Glen, R.C.; Leach, A.R.; Taylor, R. Development and validation of a genetic algorithm for flexible docking. J. Mol. Biol. 1997, 267, 727–748. [Google Scholar] [CrossRef] [PubMed]
- Verdonk, M.L.; Cole, J.C.; Hartshorn, M.J.; Murray, C.W.; Taylor, R.D. Improved protein–ligand docking using gold. Proteins Struct. Funct. Bioinform. 2003, 52, 609–623. [Google Scholar] [CrossRef] [PubMed]
- Harel, M.; Schalk, I.; Ehret-Sabatier, L.; Bouet, F.; Goeldner, M.; Hirth, C.; Axelsen, P.H.; Silman, I.; Sussman, J.L. Quaternary ligand binding to aromatic residues in the active-site gorge of acetylcholinesterase. Proc. Natl. Acad. Sci. USA 1993, 90, 9031–9035. [Google Scholar] [CrossRef] [PubMed]
- Nachon, F.; Carletti, E.; Ronco, C.; Trovaslet, M.; Nicolet, Y.; Jean, L.; Renard, P.Y. Crystal structures of human cholinesterases in complex with huprine w and tacrine: Elements of specificity for anti-alzheimer’s drugs targeting acetyl- and butyryl-cholinesterase. Biochem. J. 2013, 453, 393–399. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are available from the authors. |
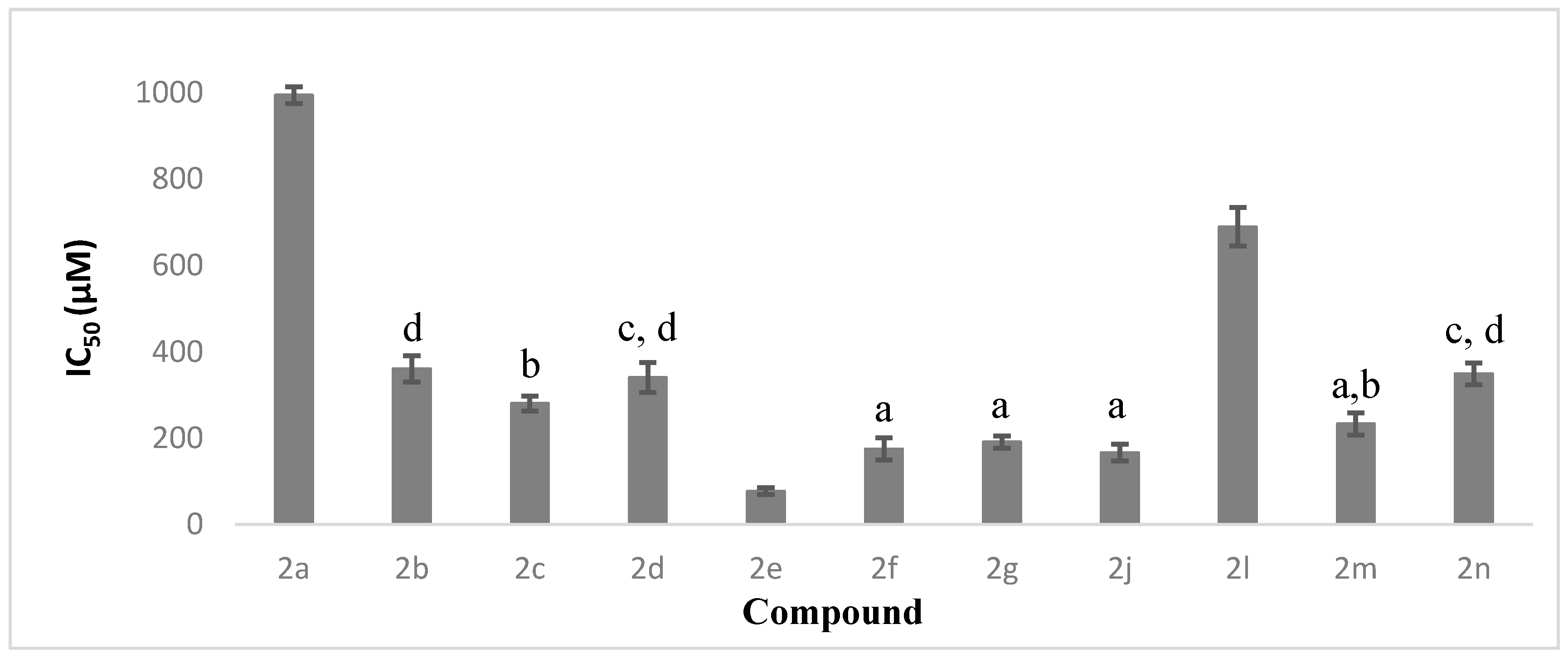

| Compounds | Substituent | AChE IC50 (µM) | BChE IC50 (µM) |
|---|---|---|---|
| 2a | benzene | 994.26 ± 19.35 | >1500 |
| 2b | 2-chlorobenzene | 360.56 ± 30.05 | >1500 |
| 2c | 3-chlorobenzene | 280.43 ± 17.35 | 971.50 ± 62.55 |
| 2d | 4-chlorobenzene | 340.52 ± 34.69 | 1291.99 ± 30.05 |
| 2e | 2,4-dichlorobenzene | 77.15 ± 7.86 | 306.77 ± 25.15 |
| 2f | 2-methybenzene | 174.98 ± 25.87 | >1500 |
| 2g | 3-methybenzene | 190.99 ± 14.43 | >1500 |
| 2h | 4-methybenzene | >1500 | >1500 |
| 2i | 2-methoxybenzene | >1500 | >1500 |
| 2j | 3-methoxybenzene | 166.46 ± 19.58 | 223.30 ± 17.58 |
| 2k | 4-methoxybenzene | >1500 | >1500 |
| 2l | 2-nitrobenzene | 689.23 ± 44.48 | >1500 |
| 2m | 3-nitrobenzene | 232.98 ± 25.88 | >1500 |
| 2n | 4-nitrobenzene | 349.47 ± 25.22 | 776.59 ± 33.63 |
| 2o | 2-aminobenzene | >1500 | >1500 |
| 2p | 3-aminobenzene | >1500 | >1500 |
| 2q | 4-aminobenzene | >1500 | >1500 |
| 2r | 2-pyridine | >1500 | >1500 |
| Tacrine | 0.21 ± 0.04 | 0.06 ± 0.01 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kwong, H.C.; Mah, S.H.; Chia, T.S.; Quah, C.K.; Lim, G.K.; Kumar, C.S.C. Cholinesterase Inhibitory Activities of Adamantyl-Based Derivatives and Their Molecular Docking Studies. Molecules 2017, 22, 1005. https://doi.org/10.3390/molecules22061005
Kwong HC, Mah SH, Chia TS, Quah CK, Lim GK, Kumar CSC. Cholinesterase Inhibitory Activities of Adamantyl-Based Derivatives and Their Molecular Docking Studies. Molecules. 2017; 22(6):1005. https://doi.org/10.3390/molecules22061005
Chicago/Turabian StyleKwong, Huey Chong, Siau Hui Mah, Tze Shyang Chia, Ching Kheng Quah, Gin Keat Lim, and C. S. Chidan Kumar. 2017. "Cholinesterase Inhibitory Activities of Adamantyl-Based Derivatives and Their Molecular Docking Studies" Molecules 22, no. 6: 1005. https://doi.org/10.3390/molecules22061005






