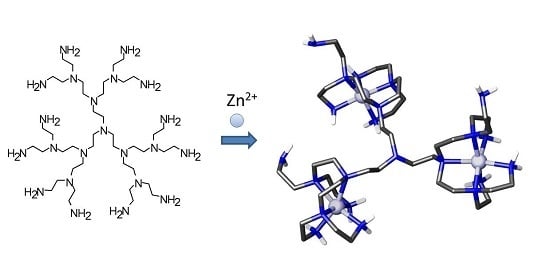
Cation, Anion and Ion-Pair Complexes with a G-3 Poly(ethylene imine) Dendrimer in Aqueous Solution
Abstract
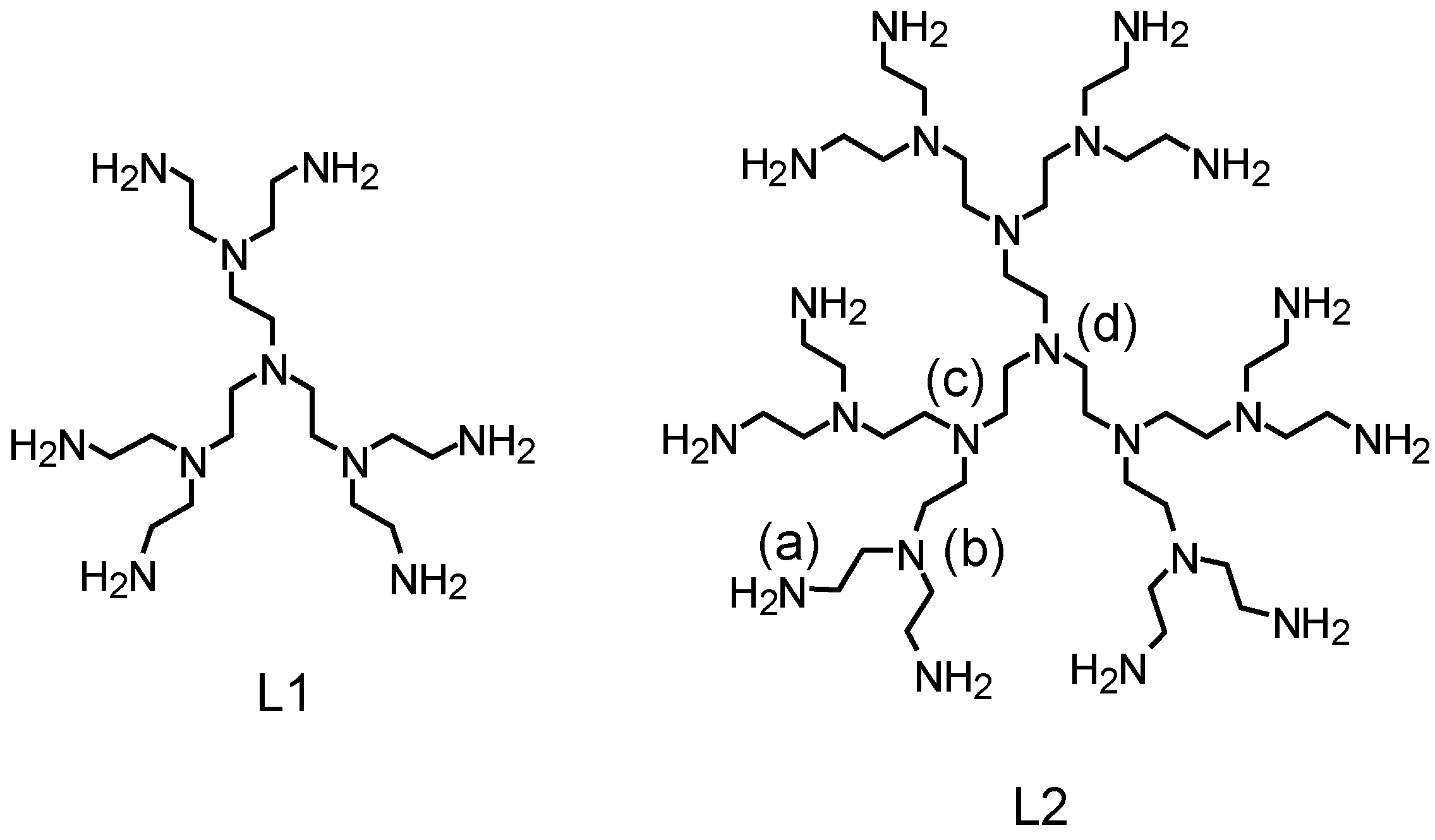
:1. Introduction
2. Results and Discussion
2.1. Formation of Metal Complexes
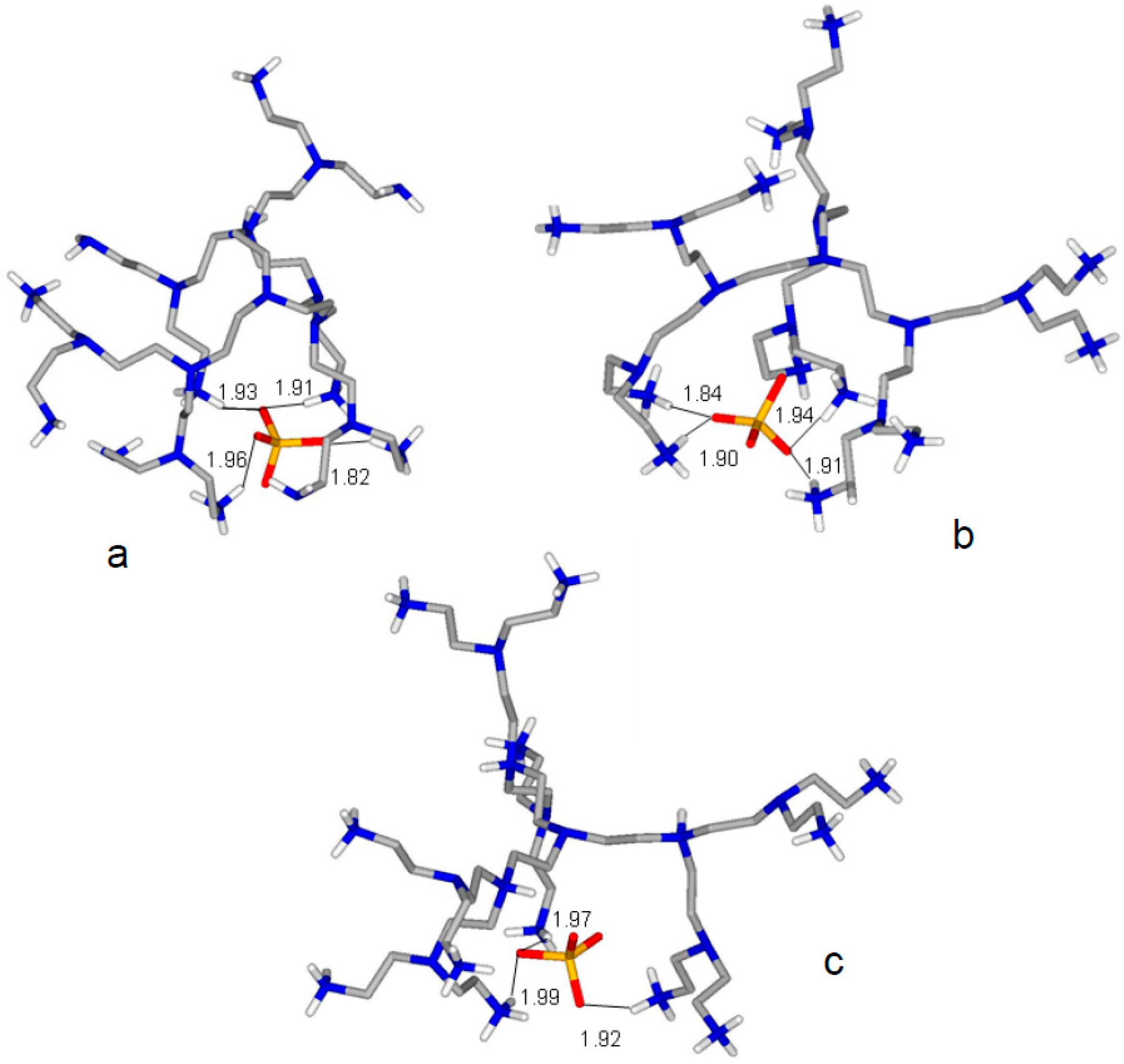
2.2. Formation of Anion and Ion-Pair Complexes
3. Materials and Methods
3.1. General Information
3.2. Potentiometric Measurements
3.3. Molecular Modelling
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Bazzicalupi, C.; Bianchi, A.; Giorgi, C.; Gratteri, P.; Mariani, P.; Valtancoli, B. Metal ion binding by a G-2 Poly(ethylene imine) dendrimer. Ion-directed self-assembling of hierearchical mono- and two-dimensional nanostructured materials. Inorg. Chem. 2013, 52, 2125–2137. [Google Scholar] [CrossRef] [PubMed]
- Bazzicalupi, C.; Bianchi, A.; Giorgi, C.; Gratteri, P.; Mariani, P.; Valtancoli, B. Anion and ion-pair binding by a G-2 Poly(ethylene imine) dendrimer. Dalton Trans. 2013, 42, 12130–12138. [Google Scholar] [CrossRef] [PubMed]
- Bazzicalupi, C.; Bianchi, A.; Giorgi, C.; Valtancoli, B. Zn(II) enhances nucleotide binding and dephosphorilation in the presence of a Poly(ethylene imine) dendrimer. Inorg. Chim. Acta 2014, 417, 163–170. [Google Scholar] [CrossRef]
- Salvador Serrano, E.; Savastano, M.; Bianchi, A. Inorganic Mercury Sequestration by a Poly(ethylene imine) Dendrimer in Aqueous Solution. Molecules 2015, 20, 3783–3790. [Google Scholar] [CrossRef] [PubMed]
- Bazzicalupi, C.; Bencini, A.; Bianchi, A.; Danesi, A.; Giorgi, C.; Valtancoli, B. Anion Binding by Protonated Forms of the Tripodal Ligand Tren. Inorg. Chem. 2009, 48, 2391–2398. [Google Scholar] [CrossRef] [PubMed]
- Bazzicalupi, C.; Bianchi, A.; Giorgi, C.; Savastano, M.; Morales-Lara, F. ATP dephosphorylation can be either enhanced or inhibited by pH-controlled interaction with a dendrimer molecule. Chem. Commun. 2015, 51, 3907–3910. [Google Scholar] [CrossRef] [PubMed]
- Astruc, D.; Wang, D.; Deraedt, C.; Liang, L.; Ciganda, R.; Ruiz, J. Catalysis Inside Dendrimers. Synthesis 2015, 47, 2017–2031. [Google Scholar] [CrossRef]
- Wang, D; Astruc, D. Dendritic catalysis—Basic concepts and recent trends. Coord. Chem. Rev. 2013, 257, 2317–2334. [Google Scholar] [CrossRef]
- Myers, V.S.; Weir, M.G.; Carino, E.V.; Yancey, D.F.; Pande, S.; Crooks, R.M. Dendrimer-encapsulated nanoparticles: New synthetic and characterization methods and catalytic applications. Chem. Sci. 2011, 2, 1632–1646. [Google Scholar] [CrossRef]
- Hwang, S.-H.; Shreiner, C.D.; Moorefield, C.N.; Newkome, G.W. Recent progress and applications for metallodendrimers. New J. Chem. 2007, 31, 1192–1217. [Google Scholar] [CrossRef]
- Jarvis, N.V.; Wagener, J.M. Mechanistic studies of metal ion binding to water-soluble polymers using potentiometry. Talanta 1995, 42, 219–226. [Google Scholar] [CrossRef]
- Gans, P.; Sabatini, A.; Vacca, A. Investigation of equilibria in solution. Determination of equilibrium constants with the HYPERQUAD suite of programs. Talanta 1996, 43, 1739–1753. [Google Scholar] [CrossRef]
- Aragó, J.; Bencini, A.; Bianchi, A.; Garcia-España, E.; Micheloni, M.; Paoletti, P.; Ramirez, J.A.; Paoli, P. Interaction of “long” open-chain polyazaalkanes with hydrogen and copper(II) ions. Inorg. Chem. 1991, 30, 1843–1849. [Google Scholar] [CrossRef]
- Bencini, A.; Bianchi, A.; Micheloni, M.; Paoletti, P.; Garcia-España, E.; Niño, M.A. Co-ordination tendency of [3k]aneNk polyazacycloalkanes. Thermodynamic study of solution equilibria. J. Chem. Soc. Dalton Trans. 1991, 1171–1174. [Google Scholar] [CrossRef]
- Aragó, J.; Bencini, A.; Bianchi, A.; Garcia-España, E.; Micheloni, M.; Paoletti, P.; Ramirez, J.A.; Rodriguez, A. Interaction of long polyazaalkanes with zinc(II) and cadmium(II) ions. A thermodynamic and 13C nuclear magnetic resonance study. J. Chem. Soc. Dalton Trans. 1991, 3077–3083. [Google Scholar] [CrossRef]
- Bencini, A.; Bianchi, A.; Dapporto, P.; Garcia-España, E.; Micheloni, M.; Paoletti, P. Polynuclear zinc(II) complexes with large polyazacycloalkanes. 2. Equilibrium study and crystal structure of the binuclear complex [Zn2LCl2](Cl)ClO4∙H2O (L = 1,4,7,10,13,16,19,22-octaazacyclotetracosane). Inorg. Chem. 1989, 28, 1188–1191. [Google Scholar] [CrossRef]
- Anion Coordination Chemistry; Bowman-James, K.; Bianchi, A.; García-España, E. (Eds.) Wiley-VCH: New York, NY, USA, 2012. [Google Scholar]
- Mateus, P.; Bernier, N.; Delgado, R. Recognition of anions by polyammonium macrocyclic and cryptand receptors: Influence of the dimensionality on the binding behavior. Coord. Chem. Rev. 2010, 254, 1726–1747. [Google Scholar] [CrossRef]
- Bowman-James, K. Alfred Werner Revisited: The Coordination Chemistry of Anions. Acc. Chem. Res. 2005, 38, 671–678. [Google Scholar] [CrossRef] [PubMed]
- Garcia-España, E.; Díaz, P.; Llinares, J.M.; Bianchi, A. Anion coordination chemistry in aqueous solution of polyammonium receptors. Coord. Chem. Rev. 2006, 250, 2952–2986. [Google Scholar] [CrossRef]
- Bianchi, A.; Micheloni, M.; Paoletti, P. Supramolecular interaction between adenosine 5′-triphosphate (ATP) and polycharged tetraazamacrocycles. Thermodynamic and 31P NMR studies. Inorg. Chim. Acta 1988, 151, 269–272. [Google Scholar] [CrossRef]
- Arranz, P.; Bencini, A.; Bianchi, A.; Díaz, P.; Garcia-España, E.; Giorgi, C.; Luis, S.V.; Querol, M.; Valtancoli, B. Thermodynamics of sulfate anion binding by macrocyclic polyammonium receptors. J. Chem. Soc. Perkin Trans. 2001, 1765–1770. [Google Scholar] [CrossRef]
- Bazzicalupi, C.; Bianchi, A.; Giorgi, C.; Clares, M.P.; Garcia-España, E. Addressing selectivity criteria in binding equilibria. Coord. Chem. Rev. 2011, 256, 13–27. [Google Scholar] [CrossRef]
- Lee, S.H.; Kim, D.-J.; Chang, C.-C.; Hah, S.S.; Suh, J. An efficient synthesis of ethylenimine dendrimer. Bull. Korean Chem. Soc. 1998, 19, 1270–1273. [Google Scholar]
- Bazzicalupi, C.; Bianchi, A.; Biver, T; Giorgi, C.; Santarelli, S.; Savastano, M. Formation of double-strand dimetallic helicates with a terpyridine-based macrocycle. Inorg. Chem. 2014, 53, 12215–12224. [Google Scholar] [CrossRef] [PubMed]
- Gran, G. Determination of the equivalence point in potentiometric titration, Part II. Analyst 1952, 77, 661–671. [Google Scholar] [CrossRef]
- Hyperchem, Release 7.51 for Windows MM System, Hypercube, Inc.: Gainesville, FL, USA, 2002.
- Stewart, J.J.P. Optimization of parameters for semiempirical methods I. Method. J. Comput. Chem. 1989, 10, 209–220. [Google Scholar] [CrossRef]
- Stewart, J.J.P. Optimization of parameters for semiempirical methods II. Applications. J. Comput. Chem. 1989, 10, 221–264. [Google Scholar] [CrossRef]
- Banks, J.L.; Beard, H.S.; Cao, Y.; Cho, A.E.; Damm, W.; Farid, R.; Felts, A.K.; Halgren, T.A.; Mainz, D.T.; Maple, J.R.; et al. Integrated Modeling Program, Applied Chemical Theory (IMPACT). J. Comput. Chem. 2005, 26, 1752–1780. [Google Scholar] [CrossRef] [PubMed]
- Kohn, W.; Sham, L.J. Self-Consistent Equations Including Exchange and Correlation Effects. Phys. Rev. 1965, 140, A1133–A1138. [Google Scholar] [CrossRef]
- Zhao, Y.; Truhlar, D.G. The M06 suite of density functionals for main group thermochemistry, thermochemical kinetics, noncovalent interactions, excited states, and transition elements: Two new functionals and systematic testing of four M06-class functionals and 12 other functionals. Theor. Chem. Acc. 2008, 120, 215–241. [Google Scholar]
- Ditchfield, R.; Hehre, W.J.; Pople, J.A. Self-Consistent Molecular-Orbital Methods. IX. An Extended Gaussian-Type Basis for Molecular-Orbital Studies of Organic Molecules. J. Chem. Phys. 1971, 54, 724–728. [Google Scholar] [CrossRef]
- Hehre, W.J.; Pople, J.A. Self-Consistent Molecular Orbital Methods. XIII. An Extended Gaussian Type Basis for Boron. J. Chem. Phys. 1972, 56, 4233–4234. [Google Scholar] [CrossRef]
- Binkley, J.S.; Pople, J.A. Self-Consistent Molecular Orbital Methods. XIX. Split Valence Gaussian-type Basis Sets for Beryllium. J. Chem. Phys. 1977, 66, 879–880. [Google Scholar] [CrossRef]
- Hariharan, P.C.; Pople, J.A. The Influence of Polarization Functions on Molecular Orbital Hydrogenation Energies. Theor. Chim. Acta 1973, 28, 213–222. [Google Scholar] [CrossRef]
- Mitin, A.V.; Baker, J.; Pulay, P. An improved 6–31G* basis set for first-row transition metals. J. Chem. Phys. 2003, 119, 7775–7782. [Google Scholar] [CrossRef]
- Cortis, M.C.; Friesner, R.A. Poisson-Boltzmann Calculations of Nonspecific Salt Effects on Protein-Protein Binding Free Energies. J. Comput. Chem. 1997, 18, 1591–1608. [Google Scholar] [CrossRef]
- Peñas-Sanjuán, A.; López-Garzón, R.; López-Garzón, J.; Pérez-Mendoza, M.; Melguizo, M. Preparation of a poly-alkylamine surface-functionalized carbon with excellent performance as a Pd(II) scavenger. Carbon 2012, 50, 2350–2352. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compound L2 is available from the authors. |


| Equilibria | logK | Equilibria | logK |
|---|---|---|---|
| Cu2+ + L2 = CuL22+ | 23.66 (5) | Cu2H7L211+ + H+ = Cu2H8L212+ | 7.46 (8) |
| CuL22+ + 2H+ = CuH2L24+ | 22.88 (7) | Cu2H8L212+ + H+ = Cu2H9L213+ | 6.25 (8) |
| CuH2L24+ + H+ = CuH3L25+ | 9.93 (5) | Cu2H9L213+ + H+ = Cu2H10L214+ | 4.98 (7) |
| CuH3L25+ + H+ = CuH4L26+ | 10.07 (5) | Cu2H10L214+ + H+ = Cu2H11L215+ | 4.07 (7) |
| CuH4L26+ + H+ = CuH5L27+ | 9.42 (3) | ||
| CuH5L27+ + H+ = CuH6L28+ | 9.21 (7) | 3Cu2+ + L2 = Cu3L26+ | 56.55 (7) |
| CuH6L28+ + H+ = CuH7L29+ | 9.09 (7) | Cu2L24+ + Cu2+ = Cu3L26+ | 10.0 (1) |
| CuH7L29+ + H+ = CuH8L210+ | 8.63 (5) | Cu3L26+ + 2H+ = Cu3H2L28+ | 22.72 (6) |
| CuH8L210+ + H+ = CuH9L211+ | 8.50 (4) | Cu3H2L28+ + H+ = Cu3H3L29+ | 10.31 (7) |
| CuH9L211+ + H+ = CuH10L212+ | 8.13 (4) | Cu3H3L29+ + H+ = Cu3H4L210+ | 8.87 (8) |
| CuH10L212+ + H+ = CuH11L213+ | 7.49 (4) | Cu3H4L210+ + H+ = Cu3H5L211+ | 8.50 (8) |
| CuH11L213+ + H+ = CuH12L214+ | 5.87 (4) | Cu3H5L211+ + H+ = Cu3H6L212+ | 7.39 (7) |
| CuH12L214+ + H+ = CuH13L215+ | 5.18 (4) | Cu3H6L212+ + H+ = Cu3H7L213+ | 6.82 (8) |
| CuH13L215+ + H+ = CuH14L216+ | 3.94 (5) | Cu3H7L213+ + H+ = Cu3H8L214+ | 5.81 (8) |
| CuH14L216+ + H+ = CuH15L217+ | 2.59 (5) | ||
| CuH15L217+ + H+ = CuH16L218+ | 2.89 (6) | 4Cu2+ + L2 = Cu4L28+ | 72.6 (1) |
| Cu3L26+ + Cu2+ = Cu4L28+ | 16.0 (1) | ||
| 2Cu2+ + L2 = Cu2L24+ | 46.53 (7) | Cu4L28+ + 2H+ = Cu4H2L28+ | 22.5 (1) |
| CuL22+ + Cu2+ = Cu2L24+ | 22.9 (1) | Cu4H2L210+ + H+ = Cu4H3L211+ | 8.54 (1) |
| Cu2L24+ + H+ = Cu2HL25+ | 11.51 (6) | Cu4H3L211+ + H+ = Cu4H4L212+ | 7.3 (1) |
| Cu2HL25+ + H+ = Cu2H2L26+ | 10.20 (7) | Cu4H4L212+ + H+ = Cu4H5L213+ | 6.9 (1) |
| Cu2H2L26+ + H+ = Cu2H3L27+ | 9.24 (7) | Cu4H5L213+ + H+ = Cu4H6L214+ | 3.9 (1) |
| Cu2H3L27+ + H+ = Cu2H4L28+ | 9.61 (6) | ||
| Cu2H4L28+ + H+ = Cu2H5L29+ | 8.31 (7) | 5Cu2+ + L2 = Cu5L210+ | 82.0 (2) |
| Cu2H5L29+ + H+ = Cu2H6L210+ | 8.22 (7) | Cu4L28+ + Cu2+ = Cu5L210+ | 9.4 (3) |
| Cu2H6L210+ + H+ = Cu2H7L211+ | 8.18 (7) | Cu5L210+ + 2OH− =[Cu5L2(OH)2]8+ | 8.5 (2) |
| Equilibria | logK | Equilibria | logK |
|---|---|---|---|
| Zn2+ + L2 = ZnL22+ | 17.18 (5) | Zn2H6L210+ + H+ = Zn2H7L211+ | 8.13 (8) |
| ZnL22+ + 2H+ = ZnH2L24+ | 22.50 (8) | Zn2H7L211+ + H+ = Zn2H8L212+ | 7.36 (7) |
| ZnH2L24+ + H+ = ZnH3L25+ | 10.04 (5) | Zn2H8L212+ + H+ = Zn2H9L213+ | 6.47 (5) |
| ZnH3L25+ + H+ = ZnH4L26+ | 9.59 (6) | ||
| ZnH4L26+ + H+ = ZnH5L27+ | 10.01 (7) | 3Zn2+ + L2 = Zn3L26+ | 41.36 (5) |
| ZnH5L27+ + 2H+ = ZnH7L29+ | 18.14 (7) | Zn2L24+ + Zn2+ = Zn3L26+ | 11.0 (1) |
| ZnH7L29+ + H+ = ZnH8L210+ | 8.25 (6) | Zn3L26+ + 2H+ = Zn3H2L28+ | 22.52 (6) |
| ZnH8L210+ + H+ = ZnH9L211+ | 8.64 (7) | Zn3H2L28+ + H+ = Zn3H3L29+ | 9.34 (8) |
| ZnH9L211+ + H+ = ZnH10L212+ | 7.97 (6) | Zn3H3L29+ + H+ = Zn3H4L210+ | 8.12 (8) |
| ZnH10L212+ + H+ = ZnH11L213+ | 6.92 (5) | Zn3H4L210+ + H+ = Zn3H5L211+ | 8.00 (8) |
| ZnH11L213+ + H+ = ZnH12L214+ | 5.75 (4) | Zn3H5L211+ + H+ = Zn3H6L212+ | 6.94 (6) |
| ZnH12L214+ + H+ = ZnH13L215+ | 5.38 (5) | Zn3H6L212+ + H+ = Zn3H7L213+ | 6.26 (6) |
| 2Zn2+ + L2 = Zn2L24+ | 30.35 (7) | 4Zn2+ + L2 = Zn4L28+ | 52.08 (8) |
| ZnL22+ + Zn2+ = Zn2L24+ | 13.2 (1) | Zn3L26+ + Zn2+ = Zn4L28+ | 10.7 (1) |
| Zn2L24+ + H+ = Zn2HL25+ | 11.27 (8) | Zn4L28+ + H+ = Zn4HL29+ | 9.48 (8) |
| Zn2HL25+ + H+ = Zn2H2L26+ | 11.44 (8) | Zn4HL29+ + H+ = Zn4H2L210+ | 8.90 (8) |
| Zn2H2L26+ + H+ = Zn2H3L27+ | 9.53 (8) | Zn4H2L210+ + H+ = Zn4H3L211+ | 8.24 (8) |
| Zn2H3L27+ + H+ = Zn2H4L28+ | 9.54 (8) | Zn4H3L211+ + H+ = Zn4H4L212+ | 7.35 (9) |
| Zn2H4L28+ + H+ = Zn2H5L29+ | 8.80 (9) | Zn4L28+ + OH− = [Zn4L2(OH)]7+ | 2.2 (1) |
| Zn2H5L29+ + H+ = Zn2H6L210+ | 8.60 (8) |
| Equilibria | logK | Equilibria | logK |
|---|---|---|---|
| L2 + 3H+ + SO42− = [H3L2(SO4)]+ | 38.09 (5) | H11L211+ + SO42− = [H11L2(SO4)]9+ | 3.10 (7) |
| L2 + 5H+ + SO42− = [H5L2(SO4)]3+ | 57.88 (5) | H12L212+ + SO42− = [H12L2(SO4)]10+ | 2.81 (7) |
| L2 + 7H+ + SO42− = [H7L2(SO4)]5+ | 76.63 (5) | H13L213+ + SO42− = [H13L2(SO4)]11+ | 2.46 (7) |
| L2 + 9H+ + SO42− = [H9L2(SO4)]7+ | 94.62 (5) | H15L215+ + SO42− = [H15L2(SO4)]13+ | 2.59 (7) |
| L2 + 11H+ + SO42− = [H11L2(SO4)]9+ | 111.54 (5) | H16L216+ + SO42− = [H16L2(SO4)]14+ | 2.76 (7) |
| L2 + 12H+ + SO42− = [H12L2(SO4)]10+ | 119.58 (5) | H17L217+ + SO42− = [H17L2(SO4)]15+ | 2.91 (7) |
| L2 + 13H+ + SO42− = [H13L2(SO4)]11+ | 127.24 (5) | H18L218+ + SO42− = [H18L2(SO4)]16+ | 3.32 (7) |
| L2 + 15H+ + SO42− = [H15L2(SO4)]13+ | 139.90 (5) | ||
| L2 + 16H+ + SO42− = [H16L2(SO4)]14+ | 145.53 (5) | ||
| L2 + 17H+ + SO42− = [H17L2(SO4)]15+ | 149.44 (5) | ||
| L2 + 18H+ + SO42− = [H18L2(SO4)]16+ | 152.12 (5) |
| Equilibria | logK |
|---|---|
| CuH3L25+ + SO42− = [CuH3L2(SO4)]3+ | 3.10 (8) |
| CuH5L27+ + SO42− = [CuH5L2(SO4)]5+ | 3.33 (5) |
| CuH7L29+ + SO42− = [CuH7L2(SO4)]7+ | 3.51 (5) |
| CuH9L211+ + SO42− = [CuH9L2(SO4)]9+ | 3.62 (5) |
| CuH10L212+ + SO42− = [CuH10L2(SO4)]10+ | 3.44 (5) |
| CuH11L213+ + SO42− = [CuH11L2(SO4)]11+ | 3.69 (5) |
| CuH12L214+ + SO42− = [CuH12L2(SO4)]12+ | 3.96 (5) |
| CuH13L215+ + SO42− = [CuH13L2(SO4)]13+ | 4.31 (5) |
| CuH14L216+ + SO42− = [CuH14L2(SO4)]14+ | 4.64 (5) |
| CuH16L218+ + SO42− = [CuH16L2(SO4)]16+ | 5.20 (5) |
| [CuH16L2(SO4)]16+ + H+ = [CuH17L2(SO4)]17+ | 2.78 (5) |
| Cu2L24+ + SO42− = [Cu2L2(SO4)]2+ | 4.01 (5) |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Savastano, M.; Bazzicalupi, C.; Giorgi, C.; Gratteri, P.; Bianchi, A. Cation, Anion and Ion-Pair Complexes with a G-3 Poly(ethylene imine) Dendrimer in Aqueous Solution. Molecules 2017, 22, 816. https://doi.org/10.3390/molecules22050816
Savastano M, Bazzicalupi C, Giorgi C, Gratteri P, Bianchi A. Cation, Anion and Ion-Pair Complexes with a G-3 Poly(ethylene imine) Dendrimer in Aqueous Solution. Molecules. 2017; 22(5):816. https://doi.org/10.3390/molecules22050816
Chicago/Turabian StyleSavastano, Matteo, Carla Bazzicalupi, Claudia Giorgi, Paola Gratteri, and Antonio Bianchi. 2017. "Cation, Anion and Ion-Pair Complexes with a G-3 Poly(ethylene imine) Dendrimer in Aqueous Solution" Molecules 22, no. 5: 816. https://doi.org/10.3390/molecules22050816