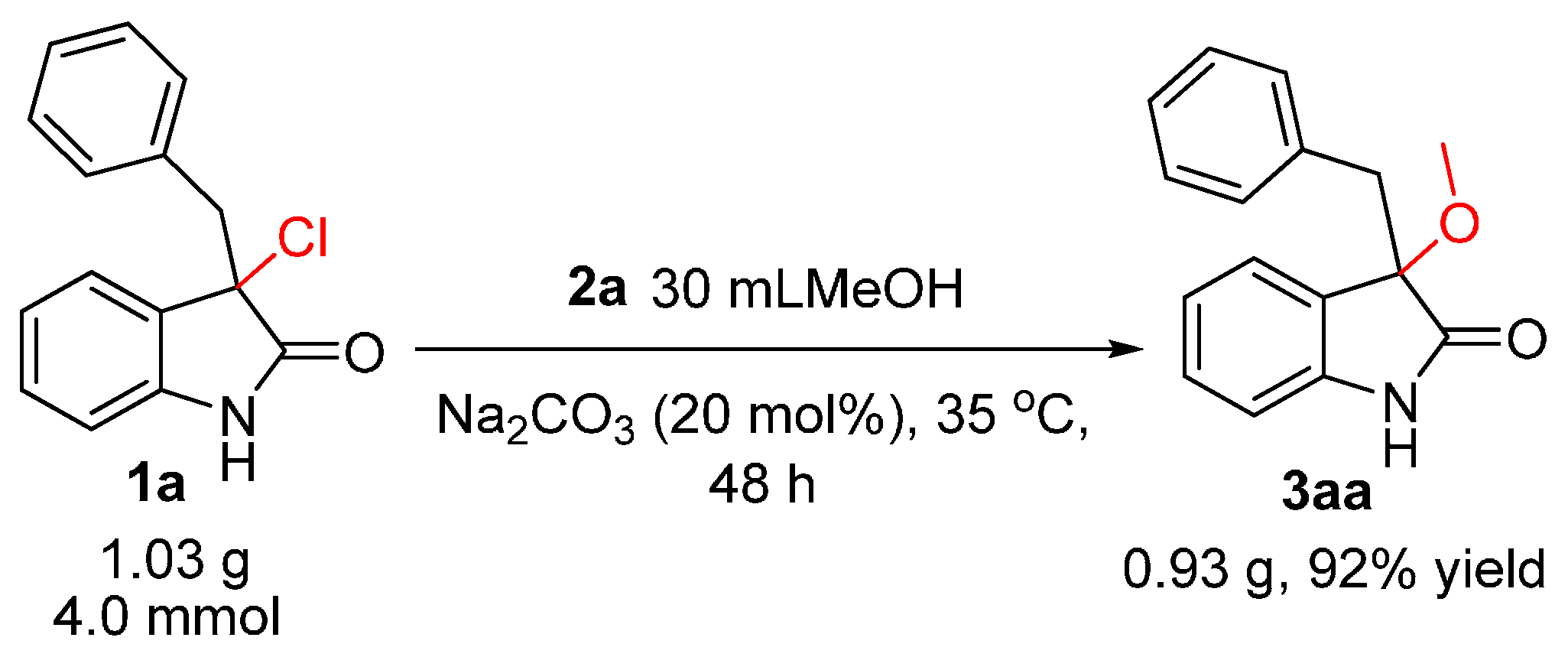3.3. Characterization Data of Compounds 3
3-Benzyl-3-methoxyindolin-2-one (
3aa). Light orange solid, m.p. 120.3–122.5 °C; yield 94%;
1H-NMR (CDCl
3) δ: 3.08 (s, 3H), 3.11 (d,
J = 12.8 Hz, 1H), 3.32 (d,
J = 12.8 Hz, 1H), 6.80–6.83 (m, 1H), 6.93–6.96 (m, 2H), 7.02–7.11 (m, 5H), 7.22–7.26 (m, 1H), 9.13 (br s, 1H);
13C-NMR (CDCl
3) δ: 43.6, 5.3.3, 84.2, 110.4, 122.6, 125.3, 126.3, 126.7, 127.6, 129.8, 130.6, 133.9, 141.2, 178.6; HRMS (ESI-TOF)
m/
z: Calcd. for C
16H
15NNaO
2 [M + Na]
+: 276.1000; Found: 276.1004. Spectra are in
Supplementary Materials.
![Molecules 22 00801 i013]()
3-Methoxy-3-(4-methoxybenzyl)indolin-2-one (3ba). Light orange solid, m.p. 142.3–144.1 °C; yield 92%; 1H-NMR (CDCl3) δ: 3.03 (d, J = 12.8 Hz, 1H), 3.05 (s, 3H), 3.23 (d, J = 12.8 Hz, 1H), 3.66 (s, 3H), 6.57–6.61 (m, 2H), 6.79–6.85 (m, 3H), 7.01–7.03 (m, 2H), 7.20–7.25 (m, 1H), 8.98 (br s, 1H); 13C- NMR (CDCl3) δ: 42.9, 53.4, 55.1, 84.4, 110.5, 113.1, 122.7, 125.4, 126.0, 126.6, 129.9, 131.7, 141.4, 158.5, 178.8; HRMS (ESI-TOF) m/z: Calcd. for C17H17NNaO3 [M + Na]+: 306.1106; Found: 306.1107.
3-Methoxy-3-(4-methylbenzyl)indolin-2-one (3ca). Light orange solid, m.p. 130.7–134.2 °C; yield 91%; 1H-NMR (CDCl3) δ: 2.13 (s, 3H), 2.97–3.01 (m, 4H), 3.19 (d, J = 12.8 Hz, 1H), 6.72–6.81 (m, 5H), 6.94–6.99 (m, 2H), 7.13–7.18 (m, 1H), 8.94 (br s, 1H); 13C-NMR (CDCl3) δ: 21.0, 43.2, 53.2, 84.2, 110.4, 122.5, 125.3, 126.5, 128.3, 129.7, 130.4, 130.7, 136.2, 141.2, 178.7; HRMS (ESI-TOF) m/z: Calcd. for C17H17NNaO2 [M + Na]+: 290.1157; Found: 290.1154.
3-(4-Bromobenzyl)-3-methoxyindolin-2-one (3da). Light orange solid, m.p. 120.3–122.4 °C; yield 91%; 1H-NMR (CDCl3) δ: 3.05 (d, J = 13.2 Hz, 1H), 3.08 (s, 3H), 3.26 (d, J = 13.2 Hz, 1H), 6.81–6.84 (m, 3H), 7.03–7.06 (m, 2H), 7.20–7.26 (m, 3H), 8.82 (br s, 1H); 13C-NMR (CDCl3) δ: 43.0, 53.3, 83.8, 110.5, 121.0, 122.7, 125.2, 126.0, 130.0, 130.8, 132.3, 132.9, 141.0, 178.2; HRMS (ESI-TOF) m/z: Calcd. for C16H14BrNNaO2 [M + Na]+: 354.0106; Found: 354.0106.
3-(2-Bromobenzyl)-3-methoxyindolin-2-one (3ea). Light orange solid, m.p. 184.5–187.1 °C; yield 91%; 1H- NMR (CDCl3) δ: 3.09 (s, 3H), 3.36 (d, J = 11.2 Hz, 1H), 3.48 (d, J = 11.2 Hz, 1H), 6.68 (d, J = 6.0 Hz, 1H), 6.89–6.95 (m, 2H), 7.05–7.09 (m, 1H), 7.20–7.27 (m, 2H), 7.40–7.42 (m, 1H), 7.48–7.50 (m, 1H), 9.32 (br s, 1H); 13C-NMR (CDCl3) δ: 42.0, 53.2, 83.2, 110.5, 122.6, 125.6, 126.8, 128.5, 129.8, 132.4, 132.8, 134.4, 140.9, 179.0; HRMS (ESI-TOF) m/z: Calcd. for C16H14BrNNaO2 [M + Na]+: 354.0106; Found: 354.0105.
![Molecules 22 00801 i017]()
3-(2-Chlorobenzyl)-3-methoxyindolin-2-one (3fa). Light orange solid, m.p. 138.2–139.8 °C; yield 93%; 1H-NMR (CDCl3) δ: 3.09 (s, 3H), 3.35 (d, J = 13.5 Hz, 1H), 3.47 (d, J = 13.5 Hz, 1H), 6.77 (d, J = 7.2 Hz, 1H), 6.89–6.97 (m, 2H), 7.13–7.26 (m, 4H), 7.42–7.44 (m, 1H), 9.40 (br s, 1H); 13C-NMR (CDCl3) δ: 39.4, 53.2, 83.3, 110.4, 122.6, 125.5, 125.9, 126.2, 128.3, 129.1, 129.8, 132.5, 132.7, 135.3, 140.5, 141.0, 179.0; HRMS (ESI-TOF) m/z: Calcd. for C16H14ClNNaO2 [M + Na]+: 310.0611; Found: 310.0614.
3-(4-Chlorobenzyl)-3-methoxyindolin-2-one (3ga). Light orange solid, m.p. 125.2–127.3 °C; yield 91%; 1H-NMR (CDCl3) δ: 3.05 (d, J = 12.8 Hz, 1H), 3.08 (s, 3H), 3.27 (d, J = 12.8 Hz, 1H), 6.83 (d, J = 7.6 Hz, 1H), 6.88 (d, J = 8.4 Hz, 2H), 7.02–7.08 (m, 4H), 7.24–7.28 (m, 1H), 8.93 (br s, 1H); 13C-NMR (CDCl3) δ: 42.9, 53.3, 83.9, 110.5, 122.8, 125.3, 126.1, 127.8, 130.0, 131.9, 132.4, 132.8, 141.1, 178.3; HRMS (ESI-TOF) m/z: Calcd. for C16H14ClNNaO2 [M + Na]+: 310.0611; Found: 310.0610.
![Molecules 22 00801 i019]()
3-(3-Fluorobenzyl)-3-methoxyindolin-2-one (3ha). Light orange solid, m.p. 135.7–138.2 °C; yield 90%; 1H-NMR (CDCl3, 500 MHz) δ: 3.07 (d, J = 13.5 Hz, 1H), 3.09 (s, 3H), 3.31 (d, J = 13.5 Hz, 1H), 6.70–6.75 (m, 2H), 6.83–6.85 (m, 2H), 6.98–7.06 (m, 3H), 7.24–7.27 (m, 1H), 9.12 (br s, 1H); 13C-NMR (CDCl3, 125 MHz) δ: 43.2, 53.3, 83.8, 110.5, 110.6, 113.7 (d, JCF = 20.8 Hz), 117.4 (d, JCF = 21.3 Hz), 122.7, 125.3, 126.0, 126.3, 126.4, 128.9, 129.0, 130.0, 136.5, 136.6, 141.1, 141.2, 162.1 (d, JCF = 245.8 Hz), 178.5; HRMS (ESI-TOF) m/z: Calcd. for C16H14FNNaO2 [M + Na]+: 294.0906; Found: 294.0908.
![Molecules 22 00801 i020]()
3-Benzyl-5-chloro-3-methoxyindolin-2-one (3ia). Light orange solid, m.p. 130.4–133.3 °C; yield 90%; 1H-NMR (CDCl3) δ: 3.00 (s, 3H), 3.03 (d, J = 12.8 Hz, 1H), 3.21 (d, J = 12.8 Hz, 1H), 6.66 (d, J = 8.4 Hz, 1H), 6.87–6.92 (m, 3H), 7.01–7.08 (m, 3H), 7.13–7.19 (m, 1H), 8.83 (br s, 1H); 13C-NMR (CDCl3) δ: 43.6, 53.5, 84.3, 111.4, 125.6, 127.0, 127.8, 128.2, 128.3, 129.8, 130.5, 133.4, 139.6, 178.2; HRMS (ESI-TOF) m/z: Calcd. for C16H14ClNNaO2 [M + Na]+: 310.0611; Found: 310.0611.
3-Benzyl-6-chloro-3-methoxyindolin-2-one (3ja). Light orange solid, m.p. 198.4–201.9 °C; yield 91%; 1H-NMR (DMSO-d6) δ: 2.91 (s, 3H), 3.00 (d, J = 12.8 Hz, 1H), 3.20 (d, J = 12.8 Hz, 1H), 6.66 (s, 1H), 6.67–6.90 (m, 2H), 7.03 (d, J = 2.0 Hz, 1H), 7.09–7.13 (m, 4H), 10.5 (br s, 1H); 13C-NMR (DMSO-d6) δ: 42.3, 52.3, 83.1, 109.9, 121.5, 125.0, 126.6, 126.8, 127.7, 130.3, 134.0, 134.1, 143.9, 176.2; HRMS (ESI-TOF) m/z: Calcd. for C16H14ClNNaO2 [M + Na]+: 310.0611; Found: 310.0611.
3-Benzyl-3-ethoxyindolin-2-one (3ab). Light orange solid, m.p. 112.0–113.8 °C; yield 89%; 1H-NMR (CDCl3) δ: 1.14–1.18 (m, 3H), 3.10–3.15 (m, 2H), 3.22–3.26 (m, 1H), 3.31 (d, J = 12.8 Hz, 1H), 6.78 (d, J = 7.6 Hz, 1H), 6.92–6.93 (m, 2H), 6.94–7.10 (m, 5H), 7.20–7.26 (m, 1H), 9.01 (br s, 1H); 13C- NMR (CDCl3) δ: 15.3, 43.8, 61.2, 83.6, 110.3, 122.5, 125.1, 126.7, 127.1, 127.6, 129.6, 130.5, 134.0, 141.0, 179.0; HRMS (ESI-TOF) m/z: Calcd. for C17H17NNaO2 [M + Na]+: 290.1157; Found: 290.1154.
3-Ethoxy-3-(4-methoxybenzyl)indolin-2-one (3bb). Light orange solid, m.p. 151.2–153.1°C; yield 92%; 1H-NMR (CDCl3) δ: 1.12–1.16 (m, 3H), 3.02–3.12 (m, 2H), 3.19–3.26 (m, 2H), 3.65 (s, 3H), 6.56–6.58 (m, 2H), 6.76–6.84 (m, 3H), 7.01–7.06 (m, 2H), 7.18–7.25 (m, 1H), 8.93 (br s, 1H); 13C-NMR (CDCl3) δ: 15.5, 43.1, 55.1, 61.3, 83.8, 110.4, 113.1, 122.6, 125.2, 126.1, 127.5, 129.7, 131.6, 141.2, 158.4, 179.1; HRMS (ESI-TOF) m/z: Calcd. for C18H19NNaO3 [M + Na]+: 320.1263; Found: 320.1263.
3-Ethoxy-3-(4-methylbenzyl)indolin-2-one (3cb). Light orange solid, m.p. 138.8–141.9 °C; yield 91%; 1H-NMR (CDCl3) δ: 1.07–1.10 (m, 3H), 2.13 (s, 3H), 2.99–3.07 (m, 2H), 3.14–3.22 (m, 2H), 6.69–6.79 (m, 5H), 6.94–7.01 (m, 2H), 7.12–7.18 (m, 1H), 8.82 (br s, 1H); 13C-NMR (CDCl3) δ: 15.3, 21.0, 43.4, 61.1, 83.6, 110.2, 122.5, 125.1, 127.3, 128.3, 129.6, 130.4, 130.8, 136.1, 141.0, 178.9; HRMS (ESI-TOF) m/z: Calcd. for C18H19NNaO2 [M + Na]+: 304.1313; Found: 304.1315.
3-(4-Bromobenzyl)-3-ethoxyindolin-2-one (3db). Light orange solid, m.p. 142.1–144.6 °C; yield 90%; 1H-NMR (CDCl3) δ: 1.15–1.18 (m, 3H), 3.04 (d, J = 13.2 Hz, 1H), 3.11–3.15 (m, 1H), 3.22–3.28 (m, 2H), 6.78–6.84 (m, 3H), 7.03–7.05 (m, 2H), 7.18–7.27 (m, 3H), 8.82 (br s, 1H); 13C-NMR (CDCl3) δ: 15.3, 43.2, 61.3, 83.2, 110.4, 120.9, 122.7, 125.1, 126.8, 129.8, 130.7, 132.3, 133.0, 140.8, 178.6; HRMS (ESI-TOF) m/z: Calcd. for C17H16BrNNaO2 [M + Na]+: 368.0262; Found: 368.0265.
![Molecules 22 00801 i026]()
3-(2-Bromobenzyl)-3-ethoxyindolin-2-one (3eb). Light orange solid, m.p. 185.1–186.2 °C; yield 88%; 1H-NMR (CDCl3) δ: 1.14–1.18 (m, 3H), 3.07–3.11 (m, 1H), 3.23–3.28 (m, 1H), 3.32 (d, J = 13.6 Hz, 1H), 3.47 (d, J = 13.6 Hz, 1H), 6.65 (d, J = 7.2 Hz, 1H), 6.86–6.92 (m, 2H), 7.05–7.07 (m, 1H), 7.18–7.25 (m, 2H), 7.38–7.40 (m, 1H), 7.49–7.52 (m, 1H); 13C-NMR (CDCl3) δ: 15.4, 42.2, 61.2, 82.7, 110.5, 122.6, 125.6, 126.5, 126.7, 126.9, 128.6, 129.7, 132.4, 132.9, 134.7, 140.9, 179.4; HRMS (ESI-TOF) m/z: Calcd. for C17H16BrNNaO2 [M + Na]+: 368.0262; Found: 368.0262.
![Molecules 22 00801 i027]()
3-(2-Chlorobenzyl)-3-ethoxyindolin-2-one (3fb). Light orange solid, m.p. 207.1–209.2 °C; yield 90%; 1H-NMR (DMSO-d6) δ: 1.01–1.05 (m, 3H), 2.91–2.95 (m, 1H), 3.05–3.08 (m, 1H), 3.14 (d, J = 12.8 Hz, 1H), 3.32 (d, J = 12.8 Hz, 1H), 6.72 (d, J = 7.8 Hz, 1H), 6.80–6.87 (m, 2H), 7.14–7.28 (m, 5H), 10.5 (br s, 1H); 13C-NMR (DMSO-d6) δ: 15.3, 40.1, 60.0, 82.0, 109.8, 121.5, 124.8, 126.5, 128.6, 129.0, 129.7, 132.2, 132.5, 142.0, 176.7; HRMS (ESI-TOF) m/z: Calcd. for C17H16ClNNaO2 [M + Na]+: 324.0767; Found: 324.0769.
![Molecules 22 00801 i028]()
3-(4-Chlorobenzyl)-3-ethoxyindolin-2-one (3gb). Light orange solid, m.p. 154.1–156.2 °C; yield 91%; 1H-NMR (CDCl3) δ: 1.14–1.18 (m, 3H), 3.06 (d, J = 12.8 Hz, 1H), 3.11–3.15 (m, 1H), 3.21–3.25 (m, 1H), 3.28 (d, J = 12.8 Hz, 1H), 6.81 (d, J = 8.0 Hz, 1H), 6.88 (d, J = 8.4 Hz, 2H), 7.02–7.05 (m, 4H), 7.22–7.27 (m, 1H), 9.04 (br s, 1H); 13C-NMR (CDCl3) δ: 15.3, 43.1, 61.3, 83.3, 110.4, 122.7, 125.1, 126.9, 127.7, 129.8, 131.9, 132.5, 132.7, 140.9, 178.7; HRMS (ESI-TOF) m/z: Calcd. for C17H16ClNNaO2 [M + Na]+: 324.0767; Found: 324.0766.
![Molecules 22 00801 i029]()
3-Ethoxy-3-(3-fluorobenzyl)indolin-2-one (3hb). Light orange solid, m.p. 118.3–119.8 °C; yield 89%; 1H-NMR (CDCl3, 500 MHz) δ: 1.16–1.19 (m, 3H), 3.08 (d, J = 13.0 Hz, 1H), 3.12–3.15 (m, 1H), 3.24–3.27 (m, 1H), 3.31 (d, J = 13.0 Hz, 1H), 6.69–6.75 (m, 2H), 6.80–6.83 (m, 2H), 7.00–7.05 (m, 3H), 7.22–7.26 (m, 1H), 9.10 (br s, 1H); 13C-NMR (CDCl3, 125 MHz) δ: 15.3, 43.4, 61.2, 83.2, 110.4, 110.5, 113.6, 113.7, 117.4 (d, JCF = 21.3 Hz), 122.7, 125.1, 126.3, 126.4, 126.8, 128.9 (d, JCF = 8.8 Hz), 129.8, 136.6 (d, JCF = 7.5 Hz), 140.9, 162.1 (d, JCF = 243.8 Hz), 178.7; HRMS (ESI-TOF) m/z: Calcd. for C17H16FNNaO2 [M + Na]+: 308.1063; Found: 308.1067.
![Molecules 22 00801 i030]()
3-Benzyl-3-propoxyindolin-2-one (3ac). Light orange solid, m.p. 146.1–148.3 °C; yield 86%; 1H-NMR (CDCl3) δ: 0.84–0.88 (m, 3H), 1.54–1.60 (m, 2H), 2.95–3.01 (m, 1H), 3.08 (d, J = 12.8 Hz, 1H), 3.15–3.20 (m, 1H), 3.33 (d, J = 12.8 Hz, 1H), 6.78 (d, J = 7.6 Hz, 1H), 6.96–7.13 (m, 7H), 7.19–7.26 (m, 1H), 8.90 (br s, 1H); 13C-NMR (CDCl3) δ: 10.5, 23.1, 43.8, 67.2, 83.4, 110.2, 122.5, 125.3, 126.7, 127.2, 127.6, 129.6, 130.7, 134.1, 140.9, 178.9; HRMS (ESI-TOF) m/z: Calcd. for C18H19NNaO2 [M + Na]+: 304.1313; Found: 304.1315.
![Molecules 22 00801 i031]()
3-(4-Methoxybenzyl)-3-propoxyindolin-2-one (3bc). Light orange solid, m.p. 145.5–146.9 °C; yield 82%; 1H-NMR (CDCl3) δ: 0.81–0.85 (m, 3H), 1.52–1.56 (m, 2H), 2.94–3.02 (m, 2H), 3.12–3.15 (m, 1H), 3.25 (d, J = 12.8 Hz, 1H), 3.66 (s, 3H), 6.57–6.60 (m, 2H), 6.78 (d, J = 7.6 Hz, 1H), 6.84–6.87 (m, 2H), 6.97–7.00 (m, 2H), 7.19–7.25 (m, 1H), 8.99 (br s, 1H); 13C-NMR (CDCl3) δ: 10.7, 23.2, 43.0, 55.1, 67.3, 83.6, 110.4, 113.1, 122.6, 125.4, 126.2, 127.5, 129.7, 131.7, 141.2, 158.4, 179.3; HRMS (ESI-TOF) m/z: Calcd. for C19H21NNaO3 [M + Na]+: 334.1419; Found: 334.1415.
![Molecules 22 00801 i032]()
3-(4-Methylbenzyl)-3-propoxyindolin-2-one (3cc). Light orange solid, m.p. 75.1–76.8 °C; yield 83%; 1H-NMR (CDCl3) δ: 0.76–0.80 (m, 3H), 1.46–1.53 (m, 2H), 2.15 (s, 3H), 2.89–2.93 (m, 1H), 2.98 (d, J = 12.8 Hz, 1H), 3.08–3.11 (m, 1H), 3.21 (d, J = 12.8 Hz, 1H), 6.68 (d, J = 7.6 Hz, 1H), 6.76–6.82 (m, 4H), 6.92–6.97 (m, 2H), 7.12–7.19 (m, 1H), 8.41 (br s, 1H); 13C-NMR (CDCl3) δ: 10.5, 21.0, 23.1, 43.4, 67.2, 83.3, 110.1, 122.4, 125.3, 127.4, 128.3, 129.5, 130.5, 130.9, 136.1, 140.9, 178.7; HRMS (ESI-TOF) m/z: Calcd. for C19H21NNaO2 [M + Na]+: 318.1470; Found: 318.1472.
![Molecules 22 00801 i033]()
3-(4-Bromobenzyl)-3-propoxyindolin-2-one (3dc). Light orange solid, m.p. 143.3–144.8 °C; yield 82%; 1H-NMR (CDCl3) δ: 0.84–0.87 (m, 3H), 1.53–1.59 (m, 2H), 2.95–3.02 (m, 2H), 3.14–3.19 (m, 1H), 3.27 (d, J = 12.8 Hz, 1H), 6.81–6.86 (m, 3H), 6.94–6.96 (m, 1H), 7.01–7.04 (m, 1H), 7.20–7.24 (m, 3H), 8.88 (br s, 1H); 13C-NMR (CDCl3) δ: 10.5, 23.0, 43.1, 67.2, 83.0, 110.4, 120.9, 122.6, 125.2, 126.9, 129.8, 130.7, 132.4, 133.2, 140.8, 178.7; HRMS (ESI-TOF) m/z: Calcd. for C18H18BrNNaO2 [M + Na]+: 382.0419; Found: 382.0417.
![Molecules 22 00801 i034]()
3-(2-Bromobenzyl)-3-propoxyindolin-2-one (3ec). Light orange solid, m.p. 153.1–154.9 °C; yield 81%; 1H-NMR (CDCl3) δ: 0.85–0.88 (m, 3H), 1.54–1.62 (m, 2H), 2.95 (d, J = 6.0 Hz, 1H), 3.21 (d, J = 6.4 Hz, 1H), 3.32 (d, J = 11.2 Hz, 1H), 3.48 (d, J = 11.2 Hz, 1H), 6.59 (d, J = 6.0 Hz, 1H), 6.89–6.92 (m, 2H), 7.07–7.09 (m, 1H), 7.21–7.26 (m, 2H), 7.41 (d, J = 6.4 Hz, 1H), 7.56 (d, J = 6.0 Hz, 1H), 9.22 (br s, 1H); 13C-NMR (CDCl3) δ: 10.6, 23.1, 42.1, 67.0, 82.3, 110.3, 122.5, 125.5, 126.5, 126.6, 126.7, 128.4, 129.6, 132.3, 133.0, 134.7, 140.7, 179.2; HRMS (ESI-TOF) m/z: Calcd. for C18H18BrNNaO2 [M + Na]+: 382.0419; Found: 382.0421.
![Molecules 22 00801 i035]()
3-(2-Chlorobenzyl)-3-propoxyindolin-2-one (3fc). Light orange solid, m.p. 160.3–161.9 °C; yield 80%; 1H-NMR (CDCl3) δ: 0.85–0.88 (m, 3H), 1.56–1.62 (m, 2H), 2.94–2.98 (m, 1H), 3.18–3.22 (m, 1H), 3.33 (d, J = 13.6 Hz, 1H), 3.46 (d, J = 13.6 Hz, 1H), 6.69 (d, J = 7.2 Hz, 1H), 6.86–6.94 (m, 2H), 7.13–7.26 (m, 4H), 7.51 (d, J = 4.0 Hz, 1H), 9.15 (br s, 1H); 13C-NMR (CDCl3) δ: 10.6, 23.1, 39.6, 67.1, 82.4, 110.2, 122.5, 125.4, 126.0, 126.7, 128.2, 129.0, 129.6, 132.8, 135.3, 140.8, 179.2; HRMS (ESI-TOF) m/z: Calcd. for C18H18ClNNaO2 [M + Na]+: 338.0924; Found: 338.0924.
![Molecules 22 00801 i036]()
3-(4-Chlorobenzyl)-3-propoxyindolin-2-one (3gc). Light orange solid, m.p. 158.1–161.2 °C; yield 84%; 1H-NMR (CDCl3) δ: 0.84–0.87 (m, 3H), 1.53–1.60 (m, 2H), 2.96–3.00 (m, 1H), 3.03 (d, J = 12.8 Hz, 1H), 3.14–3.18 (m, 1H), 3.29 (d, J = 12.8 Hz, 1H), 6.81 (d, J = 7.6 Hz, 1H), 6.89–6.97 (m, 3H), 7.01–7.07 (m, 3H), 7.22–7.26 (m, 1H), 8.90 (br s, 1H); 13C-NMR (CDCl3) δ: 10.5, 23.0, 43.1, 67.3, 83.0, 110.3, 122.6, 125.2, 126.9, 127.7, 129.8, 132.0, 132.7, 140.8, 178.7; HRMS (ESI-TOF) m/z: Calcd. for C18H18ClNNaO2 [M + Na]+: 338.0924; Found: 338.0927.
![Molecules 22 00801 i037]()
3-(4-Bromobenzyl)-3-isopropoxyindolin-2-one (3dd). Light orange solid, m.p. 148.1–150.1 °C; yield 77%; 1H-NMR (CDCl3, 500 MHz) δ: 1.01 (d, J = 6.5 Hz, 3H), 1.10 (d, J = 6.0 Hz, 3H), 2.97 (d, J = 13.0 Hz, 1H), 3.23 (d, J = 13.0 Hz, 1H), 6.78–6.83 (m, 3H), 6.98–7.02 (m, 2H), 7.19–7.27 (m, 3H), 8.57 (br s, 1H); 13C-NMR (CDCl3, 125 MHz) δ: 23.1, 24.1, 43.8, 69.5, 82.7, 110.3, 120.9, 122.4, 125.6, 127.3, 129.8, 130.6, 132.4, 133.2, 140.6, 179.3; HRMS (ESI-TOF) m/z: Calcd. for C18H18BrNNaO2 [M + Na]+: 382.0419; Found: 382.0419.
![Molecules 22 00801 i038]()
3-(2-Bromobenzyl)-3-isopropoxyindolin-2-one (3ed). Light orange solid, m.p. 182.0–183.3 °C; yield 74%; 1H-NMR (CDCl3) δ: 0.99 (d, J = 6.0 Hz, 3H), 1.10 (d, J = 6.0 Hz, 3H), 3.24 (d, J = 14.0 Hz, 1H), 3.40–3.44 (m, 2H), 6.53 (d, J = 7.2 Hz, 1H), 6.84–6.89 (m, 2H), 7.03–7.08 (m, 1H), 7.18–7.25 (m, 2H), 7.37–7.41 (m, 1H), 7.55–7.57 (m, 1H), 9.16 (br s, 1H); 13C-NMR (CDCl3) δ: 23.2, 24.0, 42.7, 69.3, 82.1, 110.5, 122.4, 126.1, 126.7, 126.8, 126.9, 128.5, 129.7, 132.3, 133.1, 134.9, 140.6, 180.4; HRMS (ESI-TOF) m/z: Calcd. for C18H18BrNNaO2 [M + Na]+: 382.0419; Found: 382.0422.
![Molecules 22 00801 i039]()
3-(2-Chlorobenzyl)-3-isopropoxyindolin-2-one (3fd). Light orange solid, m.p. 197.2–198.7 °C; yield 70%; 1H-NMR (CDCl3) δ: 1.01 (d, J = 6.0 Hz, 3H), 1.12 (d, J = 6.0 Hz, 3H), 3.27 (d, J = 13.6 Hz, 1H), 3.40–3.45 (m, 2H), 6.66 (d, J = 7.2 Hz, 1H), 6.87–6.92 (m, 2H), 7.12–7.26 (m, 4H), 7.49–7.52 (m, 1H), 9.16 (br s, 1H); 13C-NMR (CDCl3) δ: 23.1, 24.0, 40.1, 69.2, 82.1, 110.3, 122.2, 125.9, 126.0, 127.0, 128.1, 128.9, 129.6, 132.9, 135.4, 140.5, 180.1; HRMS (ESI-TOF) m/z: Calcd. for C18H18ClNNaO2 [M + Na]+: 338.0924; Found: 338.0925.
![Molecules 22 00801 i040]()
3-(4-Chlorobenzyl)-3-isopropoxyindolin-2-one (3gd). Light orange solid, m.p. 198.2–201.3 °C; yield 72%; 1H-NMR (CDCl3) δ: 1.01 (d, J = 6.4 Hz, 3H), 1.08 (d, J = 6.0 Hz, 3H), 2.98 (d, J = 12.8 Hz, 1H), 3.25 (d, J = 12.8 Hz, 1H), 3.38–3.45 (m, 1H), 6.81 (d, J = 7.6 Hz, 1H), 6.86–6.89 (m, 2H), 6.97–7.04 (m, 4H), 7.22–7.27 (m, 1H), 8.97 (br s, 1H); 13C-NMR (CDCl3) δ: 23.1, 24.1, 43.7, 69.5, 82.8, 110.4, 122.4, 125.6, 127.3, 127.7, 129.8, 132.0, 132.6, 132.7, 140.6, 179.6; HRMS (ESI-TOF) m/z: Calcd. for C18H18ClNNaO2 [M + Na]+: 338.0924; Found: 338.0926.
![Molecules 22 00801 i041]()
3-Benzyl-3-butoxyindolin-2-one (3ae). Light orange solid, m.p. 125.5–126.7 °C; yield 80%; 1H-NMR (CDCl3) δ: 0.82–0.85 (m, 3H), 1.28–1.36 (m, 2H), 1.49–1.54 (m, 2H), 3.01–3.09 (m, 2H), 3.16–3.20 (m, 1H), 3.32 (d, J = 12.8 Hz, 1H), 6.78–6.81 (m, 1H), 6.95–7.11 (m, 7H), 7.19–7.26 (m, 1H), 9.01 (br s, 1H); 13C-NMR (CDCl3) δ: 13.8, 19.1, 31.8, 43.8, 65.3, 83.4, 110.3, 122.4, 125.2, 126.7, 127.2, 127.5, 129.6, 130.6, 134.1, 141.0, 179.1; HRMS (ESI-TOF) m/z: Calcd. for C19H21NNaO2 [M + Na]+: 318.1470; Found: 318.1473.
![Molecules 22 00801 i042]()
3-Butoxy-3-(4-methoxybenzyl)indolin-2-one (3be). Light orange solid, m.p. 139.5–141.7 °C; yield 83%; 1H-NMR (CDCl3) δ: 0.81–0.85 (m, 3H), 1.28–1.34 (m, 2H), 1.48–1.52 (m, 2H), 6.59 (d, J = 8.4 Hz, 2H), 6.80 (d, J = 7.6 Hz, 1H), 6.87 (d, J = 8.8 Hz, 2H), 6.97–7.04 (m, 2H), 7.20–7.24 (m, 1H), 9.08 (br s, 1H); 13C-NMR (CDCl3) δ: 13.8, 19.1, 31.8, 42.9, 54.9, 65.2, 83.5, 110.3, 112.9, 122.4, 125.2, 126.1, 127.3, 129.5, 131.5, 141.0, 158.2, 179.1; HRMS (ESI-TOF) m/z: Calcd. for C20H23NNaO3 [M + Na]+: 348.1576; Found:348.1576.
![Molecules 22 00801 i043]()
3-Butoxy-3-(4-methylbenzyl)indolin-2-one (3ce). Light orange solid, m.p. 105.1–107.7 °C; yield 85%; 1H-NMR (CDCl3) δ: 0.74–0.78 (m, 3H), 1.22–1.25 (m, 2H), 1.43–1.48 (m, 2H), 2.13 (s, 3H), 2.93–2.98 (m, 2H), 3.10–3.13 (m, 1H), 3.20 (d, J = 13.2 Hz, 1H), 6.71 (d, J = 8.0 Hz, 1H), 6.75–6.81 (m, 4H), 6.90–6.94 (m, 2H), 7.12–7.18 (m, 1H), 8.87 (br s, 1H); 13C-NMR (CDCl3) δ: 13.8, 19.1, 21.0, 31.9, 43.3, 65.2, 83.4, 110.2, 122.4, 125.2, 127.3, 128.2, 129.5, 130.5, 130.9, 136.1, 141.0, 179.0; HRMS (ESI-TOF) m/z: Calcd. for C20H23NNaO2 [M + Na]+: 332.1626; Found:332.1629.
![Molecules 22 00801 i044]()
3-(4-Bromobenzyl)-3-butoxyindolin-2-one (3de). Light orange solid, m.p. 136.0–138.1 °C; yield 84%; 1H-NMR (CDCl3) δ: 0.82–0.85 (m, 3H), 1.30–1.35 (m, 2H), 1.48–1.54 (m, 2H), 2.99–3.03 (m, 2H), 3.17–3.19 (m, 1H), 3.27 (d, J = 13.0 Hz, 1H), 6.80–6.86 (m, 3H), 6.94–6.96 (m, 1H), 7.01–7.04 (m, 1H), 7.20–7.27 (m, 3H), 8.93 (br s, 1H); 13C-NMR (CDCl3) δ: 13.8, 19.1, 31.8, 43.1, 65.3, 83.0, 110.4, 120.9, 122.6, 125.2, 126.9, 129.8, 130.7, 132.4, 133.2, 140.8, 178.7; HRMS (ESI-TOF) m/z: Calcd. for C19H20BrNNaO2 [M + Na]+: 396.0575; Found: 396.0578.
![Molecules 22 00801 i045]()
3-(2-Bromobenzyl)-3-butoxyindolin-2-one (3ee). Light orange solid, m.p. 98.9–99.3 °C; yield 80%; 1H-NMR (CDCl3) δ: 0.82–0.85 (m, 3H), 1.25–1.36 (m, 2H), 1.45–1.51 (m, 2H), 3.00–3.01 (m, 1H), 3.22–3.24 (m, 1H), 3.32 (d, J = 11.2 Hz, 1H), 3.48 (d, J = 11.2 Hz, 1H), 6.59 (d, J = 6.0 Hz, 1H), 6.89–6.92 (m, 2H), 7.07–7.08 (m, 1H), 7.20–7.25 (m, 2H), 7.40–7.42 (m, 1H), 7.55 (d, J = 6.0 Hz, 1H), 9.55 (br s, 1H); 13C-NMR (CDCl3) δ: 13.8, 19.2, 31.8, 42.1, 65.1, 82.4, 110.4, 122.4, 125.4, 126.5, 126.6, 126.7, 128.4, 129.5, 132.3, 132.9, 134.7, 140.8, 179.4; HRMS (ESI-TOF) m/z: Calcd. for C19H20BrNNaO2 [M + Na]+: 396.0575; Found: 396.0575.
![Molecules 22 00801 i046]()
3-Butoxy-3-(2-chlorobenzyl)indolin-2-one (3fe). Light orange solid, m.p. 142.1–144.0 °C; yield 81%; 1H-NMR (CDCl3) δ: 0.82–0.86 (m, 3H), 1.33–1.38 (m, 2H), 1.49–1.57 (m, 2H), 2.97–3.03 (m, 1H), 3.20–3.25 (m, 1H), 3.32 (d, J = 13.6 Hz, 1H), 3.46 (d, J = 13.6 Hz, 1H), 6.69 (d, J = 7.6 Hz, 1H), 6.87–6.94 (m, 2H), 7.13–7.26 (m, 4H), 7.47–7.50 (m, 1H), 9.29 (br s, 1H); 13C-NMR (CDCl3) δ: 13.8, 19.2, 31.8, 39.6, 65.1, 82.5, 110.3, 122.5, 125.4, 126.0, 126.7, 128.2, 129.0, 129.6, 132.8, 132.9, 135.3, 140.7, 179.2; HRMS (ESI-TOF) m/z: Calcd. for C19H20ClNNaO2 [M + Na]+: 352.1080; Found:352.1082.
![Molecules 22 00801 i047]()
3-Butoxy-3-(4-chlorobenzyl)indolin-2-one (3ge). Light orange solid, m.p. 142.1–144.3 °C; yield 83%; 1H-NMR (CDCl3) δ: 0.81–0.85 (m, 3H), 1.30–1.34 (m, 2H), 1.50–1.54 (m, 2H), 3.00–3.05 (m, 2H), 3.16–3.21 (m, 1H), 3.28 (d, J = 12.8 Hz, 1H), 6.80–6.83 (m, 1H), 6.89–6.96 (m, 3H), 7.01–7.06 (m, 3H), 7.21–7.27 (m, 1H), 9.09 (br s, 1H); 13C-NMR (CDCl3) δ: 13.7, 19.1, 31.8, 43.1, 65.3, 83.1, 110.4, 122.6, 125.1, 126.9, 127.7, 129.7, 132.0, 132.7, 140.9, 178.8; HRMS (ESI-TOF) m/z: Calcd. for C19H20ClNNaO2 [M + Na]+: 352.1080; Found:352.1083.
![Molecules 22 00801 i048]()
3-Butoxy-3-(3-fluorobenzyl)indolin-2-one (3he). Light orange solid, m.p. 129.0–131.1 °C; yield 74%; 1H-NMR (CDCl3, 500 MHz) δ: 0.82–0.86 (m, 3H), 1.26–1.35 (m, 2H), 1.45–1.57 (m, 2H), 3.02–3.05 (m, 2H), 3.19–3.22 (m, 1H), 3.32 (d, J = 13.5 Hz, 1H), 6.72–6.76 (m, 2H), 6.80–6.84 (m, 2H), 6.91–6.95 (m, 1H), 7.01–7.05 (m, 2H), 7.22–7.26 (m, 1H), 9.18 (br s, 1H); 13C-NMR (CDCl3, 125 MHz) δ: 13.7, 19.1, 31.8, 43.4, 65.3, 83.0, 110.4, 113.6 (d, JCF = 21.1 Hz), 117.4 (d, JCF = 21.2 Hz), 122.6, 125.1, 126.4, 126.9, 128.8, 128.9, 129.8, 136.7, 136.8, 140.9, 162.1 (d, JCF = 243.8 Hz), 178.9; HRMS (ESI-TOF) m/z: Calcd. for C19H20FNNaO2 [M + Na]+: 336.1376; Found:336.1377.
![Molecules 22 00801 i049]()
3-Benzyl-3-(benzyloxy)indolin-2-one (3af). Light orange solid, m.p. 168.8–170.3 °C; yield 84%; 1H-NMR (CDCl3) δ: 3.18 (d, J = 12.8 Hz, 1H), 3.41 (d, J = 12.8 Hz, 1H), 4.12 (d, J = 8.2 Hz, 1H), 4.25 (d, J = 12.8 Hz, 1H), 6.80 (d, J = 8.0 Hz, 1H), 6.97–7.10 (m, 7H), 7.21–7.29 (m, 6H), 8.97 (br s, 1H); 13C-NMR (CDCl3) δ: 43.8, 67.8, 83.8, 110.4, 122.6, 125.4, 126.8, 127.6, 127.7, 127.8, 128.2, 129.9, 130.7, 133.9, 137.5, 141.1, 178.4; HRMS (ESI-TOF) m/z: Calcd. for C22H19NNaO2 [M + Na]+: 352.1313; Found:352.1313.
![Molecules 22 00801 i050]()
3-(Benzyloxy)-3-(4-methoxybenzyl)indolin-2-one (3bf). Light orange solid, m.p. 218.1–219.8 °C; yield 85%; 1H-NMR (CDCl3) δ: 3.12 (d, J = 13.2 Hz, 1H), 3.34 (d, J = 13.2 Hz, 1H), 3.64 (s, 3H), 4.11 (d, J = 10.8 Hz, 1H), 4.23 (d, J = 10.4 Hz, 1H), 6.58–6.61 (m, 2H), 6.79–6.81 (m, 1H), 6.87–6.89 (m, 2H), 7.05–7.10 (m, 2H), 7.22–7.29 (m, 6H), 8.88 (br s, 1H); 13C-NMR (CDCl3) δ: 43.0, 55.1, 68.0, 84.0, 110.6, 113.2, 122.8, 125.5, 126.0, 127.1, 127.8, 128.0, 128.3, 130.0, 131.8, 137.7, 141.2, 158.5, 178.7; HRMS (ESI-TOF) m/z: Calcd. for C23H21NNaO3 [M + Na]+: 382.1419; Found:382.1421.
![Molecules 22 00801 i051]()
3-(Benzyloxy)-3-(4-methylbenzyl)indolin-2-one (3cf). Light orange solid, m.p. 132.2–134.3 °C; yield 80%; 1H-NMR (CDCl3) δ: 2.10 (s, 3H), 3.06 (d, J = 13.2 Hz, 1H), 3.28 (d, J = 12.8 Hz, 1H), 4.03 (d, J = 10.4 Hz, 1H), 4.16 (d, J = 10.8 Hz, 1H), 6.72 (d, J = 8.0 Hz, 1H), 6.78 (s, 4H), 6.96–7.02 (m, 2H), 7.14–7.20 (m, 6H), 8.93 (br s, 1H); 13C-NMR (CDCl3) δ: 21.0, 43.3, 67.8, 83.8, 110.4, 122.6, 125.3, 126.9, 127.6, 127.8, 128.2, 128.4, 129.8, 130.5, 130.7, 136.2, 137.6, 141.1, 178.5; HRMS (ESI-TOF) m/z: Calcd. for C23H21NNaO2 [M + Na]+: 366.1470; Found:366.1474.
![Molecules 22 00801 i052]()
3-(Benzyloxy)-3-(4-bromobenzyl)indolin-2-one (3df). Light orange solid, m.p. 201.2–203.2 °C; yield 82%; 1H-NMR (DMSO-d6) δ: 3.08 (d, J = 10.0 Hz, 1H), 3.27 (d, J = 10.0 Hz, 1H), 3.99 (d, J = 8.4 Hz, 1H), 4.12 (d, J = 8.4 Hz, 1H), 6.71 (d, J = 6.0 Hz, 1H), 6.86 (d, J = 6.4 Hz, 1H), 7.19–7.22 (m, 1H), 7.26–7.31 (m, 9H); 13C-NMR (DMSO-d6) δ: 41.4, 66.1, 82.3, 109.5, 119.5, 121.4, 124.5, 125.6, 127.0, 127.1, 127.7, 129.5, 130.0, 132.0, 133.2, 137.2, 141.8, 175.6; HRMS (ESI-TOF) m/z: Calcd. for C22H18BrNNaO2 [M + Na]+: 430.0419; Found:430.0423.
![Molecules 22 00801 i053]()
3-(Benzyloxy)-3-(2-bromobenzyl)indolin-2-one (3ef). Light orange solid, m.p. 158.8–160.1 °C; yield 81%; 1H-NMR (CDCl3) δ: 3.42 (d, J = 14.0 Hz, 1H), 3.58 (d, J = 14.0 Hz, 1H), 4.12 (d, J = 10.8 Hz, 1H), 4.30 (d, J = 10.8 Hz, 1H), 6.70 (d, J = 7.6 Hz, 1H), 6.91–6.95 (m, 2H), 7.07–7.09 (m, 1H), 7.21–7.30 (m, 7H), 7.42 (d, J = 8.1 Hz, 1H), 7.56 (d, J = 8.0 Hz, 1H), 9.35 (br s, 1H); 13C-NMR (CDCl3) δ: 42.2, 67.8, 82.9, 110.7, 122.8, 125.8, 126.3, 126.6, 127.0, 127.7, 127.8, 128.3, 128.7, 130.0, 132.5, 133.1, 134.6, 137.8, 141.0, 179.0; HRMS (ESI-TOF) m/z: Calcd. for C22H18BrNNaO2 [M + Na]+: 430.0419; Found:430.0418.
![Molecules 22 00801 i054]()
3-(Benzyloxy)-3-(2-chlorobenzyl)indolin-2-one (3ff). Light orange solid, m.p. 145.3–147.2 °C; yield 82%; 1H-NMR (CDCl3) δ: 3.42 (d, J = 14.0 Hz, 1H), 3.56 (d, J = 13.6 Hz, 1H), 4.12 (d, J = 10.8 Hz, 1H), 4.29 (d, J = 10.8 Hz, 1H), 6.80 (d, J = 7.2 Hz, 1H), 6.89–6.96 (m, 2H), 7.12–7.15 (m, 2H), 7.20–7.28 (m, 7H), 7.48–7.50 (m, 1H), 9.34 (br s, 1H); 13C-NMR (CDCl3) δ: 39.6, 67.7, 82.9, 110.5, 122.6, 125.5, 126.1, 126.3, 127.6, 128.2, 128.3, 129.1, 129.9, 132.6, 132.9, 135.3, 137.6, 140.8, 178.7; HRMS (ESI-TOF) m/z: Calcd. for C22H18ClNNaO2 [M + Na]+: 386.0924; Found:386.0925.
![Molecules 22 00801 i055]()
3-(Benzyloxy)-3-(4-chlorobenzyl)indolin-2-one (3gf). Light orange solid, m.p. 110.3–112.8 °C; yield 83%; 1H-NMR (DMSO–d6) δ: 3.09 (d, J = 12.8 Hz, 1H), 3.29 (d, J = 12.8 Hz, 1H), 3.99 (d, J = 10.8 Hz, 1H), 4.13 (d, J = 10.8 Hz, 1H), 6.71 (d, J = 8.0 Hz, 1H), 6.92 (d, J = 8.4 Hz, 2H), 7.00–7.03 (m, 1H), 7.15–7.30 (m, 9H), 10.5 (br s, 1H); 13C-NMR (DMSO-d6) δ: 41.4, 66.1, 82.4, 109.5, 121.4, 124.5, 125.7, 127.0, 127.1, 127.7, 129.5, 131.0, 131.6, 132.9, 137.2, 141.8, 175.6; HRMS (ESI-TOF) m/z: Calcd. for C22H18ClNNaO2 [M + Na]+: 386.0924; Found:386.0924.
![Molecules 22 00801 i056]()
3-(Benzyloxy)-3-(3-fluorobenzyl)indolin-2-one (3hf). Light orange solid, m.p. 153.2–155.1 °C; yield 75%; 1H-NMR (CDCl3, 500 MHz) δ: 3.16 (d, J = 10.4 Hz, 1H), 3.39 (d, J = 13.4 Hz, 1H), 4.13 (d, J = 11.0 Hz, 1H), 4.27 (d, J = 10.5 Hz, 1H), 6.72–6.74 (m, 1H), 6.76–6.78 (m, 1H), 6.81–6.84 (m, 2H), 7.04–7.06 (m, 3H), 7.23–7.29 (m, 6H); 13C-NMR (CDCl3, 125 MHz) δ: 43.4, 67.9, 83.4, 110.5, 113.7 (d, JCF = 20.1 Hz), 117.4 (d, JCF = 21.3 Hz), 122.8, 125.3, 126.5, 127.7, 127.8, 128.3, 129.0, 129.1, 130.1, 136.5, 137.4, 141.0, 162.1 (d, JCF = 243.8 Hz), 178.2; HRMS (ESI-TOF) m/z: Calcd. for C22H18FNNaO2 [M + Na]+: 370.1219; Found:370.1221.
![Molecules 22 00801 i057]()
3-Benzyl-3-(benzyloxy)-5-chloroindolin-2-one (3if). Light orange solid, m.p. 217.3–220.5 °C; yield 82%; 1H-NMR (DMSO–d6) δ: 3.09 (d, J = 12.4 H, 1H), 3.33 (d, J = 12.8 Hz, 1H), 4.03 (d, J = 10.8 Hz, 1H), 4.16 (d, J = 10.8 Hz, 1H), 6.68 (d, J = 8.8 Hz, 1H), 6.92–6.95 (m, 2H), 7.11–7.13 (m, 3H), 7.22–7.32 (m, 7H), 10.6 (br s, 1H); 13C-NMR (DMSO-d6) δ: 42.4, 66.8, 83.2, 111.3, 125.2, 125.9, 126.8, 127.5, 127.6, 127.7, 128.2, 128.5, 129.7, 130.3, 133.9, 137.6, 141.2, 176.0; HRMS (ESI-TOF) m/z: Calcd. for C22H18ClNNaO2 [M + Na]+: 386.0924; Found:386.0926.
![Molecules 22 00801 i058]()
3-Benzyl-3-(benzyloxy)-6-chloroindolin-2-one (3jf). Light orange solid, m.p. 165.1–168.5 °C; yield 85%; 1H-NMR (CDCl3) δ: 3.04 (d, J = 13.2 Hz, 1H), 3.31 (d, J = 13.2 Hz, 1H), 4.01 (d, J = 10.8 Hz, 1H), 4.16 (d, J = 10.8 Hz, 1H), 6.75–6.76 (m, 1H), 6.84–6.94 (m, 4H), 7.00–7.06 (m, 3H), 7.16–7.23 (m, 5H), 9.04 (br s, 1H); 13C-NMR (CDCl3) δ: 43.6, 67.9, 83.4, 111.2, 122.7, 125.1, 126.4, 127.0, 127.8, 128.3, 130.7, 133.6, 135.5, 137.2, 142.1, 178.5; HRMS (ESI-TOF) m/z: Calcd. for C22H18ClNNaO2 [M + Na]+: 386.0924; Found: 386.0925.
![Molecules 22 00801 i059]()
3-(2-Hydroxyethoxy)-3-(4-methylbenzyl)indolin-2-one (3cg). Light orange solid, m.p. 211.6–213.4 °C; yield 83%; 1H-NMR (DMSO-d6) δ: 2.14 (s, 3H), 2.95–3.03 (m, 2H), 3.08–3.12 (m, 1H), 3.17 (d, J = 10.0 Hz, 1H), 3.38–3.42 (m, 2H), 4.57 (br s, 1H), 6.61 (d, J = 6.4 Hz, 1H), 6.73 (d, J = 6.4 Hz, 2H), 6.86 (d, J = 6.0 Hz, 2H), 6.96–6.99 (m, 1H), 7.14–7.17 (m, 1H), 7.21 (d, J = 5.6 Hz, 1H), 10.3 (br s, 1H); 13C-NMR (DMSO-d6) δ: 20.1, 41.7, 59.6, 65.9, 82.5, 109.2, 121.2, 124.5, 126.3, 127.7, 129.2, 129.6, 130.6, 135.0, 141.8, 175.8; HRMS (ESI-TOF) m/z: Calcd. for C18H19NNaO3 [M + Na]+: 320.1263; Found:320.1267.
![Molecules 22 00801 i060]()
3-(4-Chlorobenzyl)-3-(2-hydroxyethoxy)indolin-2-one (3gg). Light orange solid, m.p. 205.3–207.3 °C; yield 87%; 1H-NMR (CDCl3) δ: 2.93–2.97 (m, 1H), 3.03 (d, J = 6.4 Hz, 1H), 3.07–3.11 (m, 1H), 3.21 (d, J = 6.4 Hz, 1H), 3.39–3.43 (m, 2H), 4.56–4.58 (m, 1H), 6.64–6.65 (m, 1H), 6.88 (d, J = 6.4 Hz, 2H), 6.97–7.00 (m, 1H), 7.13–7.19 (m, 4H), 10.4 (br s, 1H); 13C-NMR (CDCl3) δ: 41.3, 59.5, 66.0, 82.2, 109.3, 121.3, 124.5, 125.9, 127.1, 129.4, 130.9, 131.5, 132.9, 141.7, 175.7; HRMS (ESI-TOF) m/z: Calcd. for C17H16ClNNaO3 [M + Na]+: 340.0716; Found:340.0716.
![Molecules 22 00801 i061]()
3-Benzyl-3-(2,3-dihydroxypropoxy)indolin-2-one (3ah). Light orange oil; yield 51%, 1:1dr; 1H-NMR (CDCl3) δ: 3.03–3.10 (m, 2H), 3.12–3.18 (m, 1H), 3.21–3.32 (m, 2.6 H), 3.45–3.49 (m, 1H), 3.59–3.84 (m, 2.6 H), 6.74–6.77 (m, 1H), 6.92–7.04 (m, 4H), 7.07–7.15 (m, 3H), 7.19–7.23 (m, 1H), 8.65 (br s, 1H); 13C -NMR (CDCl3) δ: 43.6, 63.4, 63.5, 66.7, 67.7, 70.4, 70.7, 83.6, 83.7, 110.6, 110.7, 122.8, 126.9, 127.7, 130.0, 130.5, 130.6, 133.6, 140.8, 178.7, 178.8; HRMS (ESI-TOF) m/z: Calcd. for C18H19NNaO4 [M + Na]+: 336.1212; Found:336.1215.