Quasi-Living Polymerization of Propene with an Isotactic-Specific Zirconocene Catalyst
Abstract
:1. Introduction
2. Results and Discussion
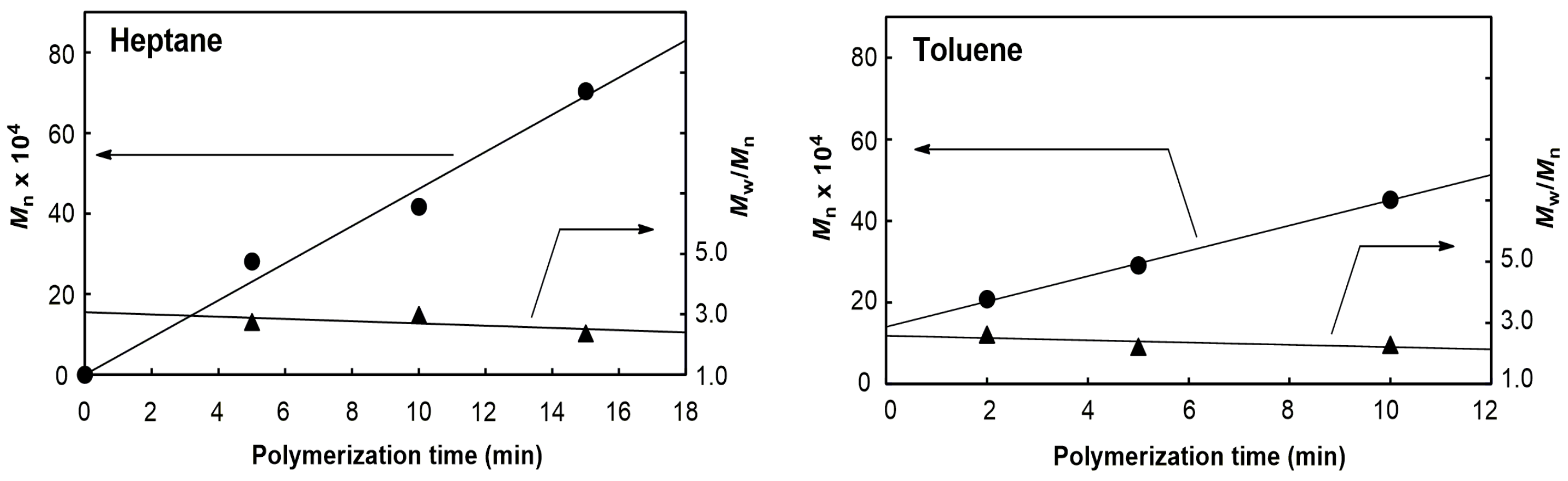
2.1. Polymerization of Propene
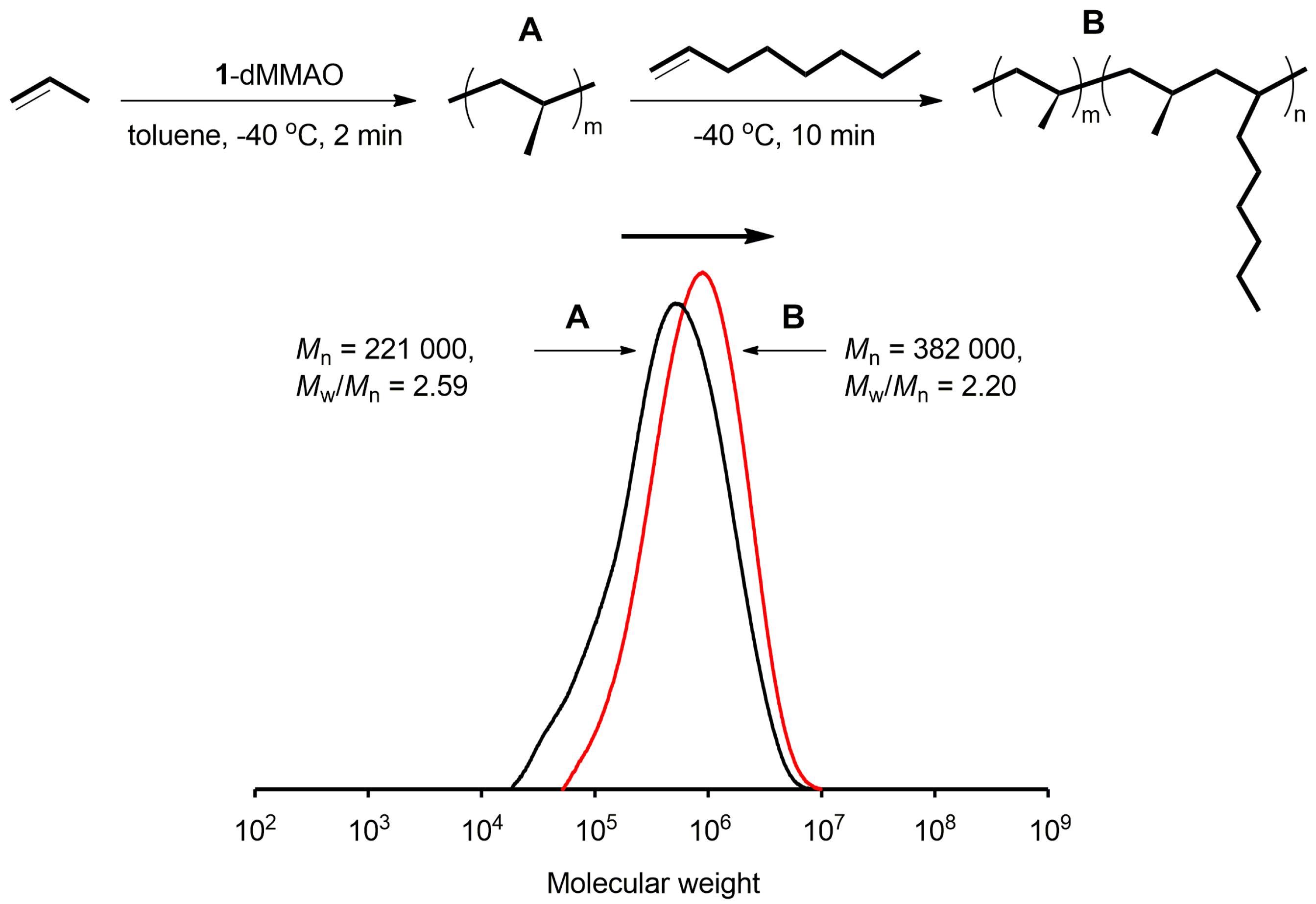
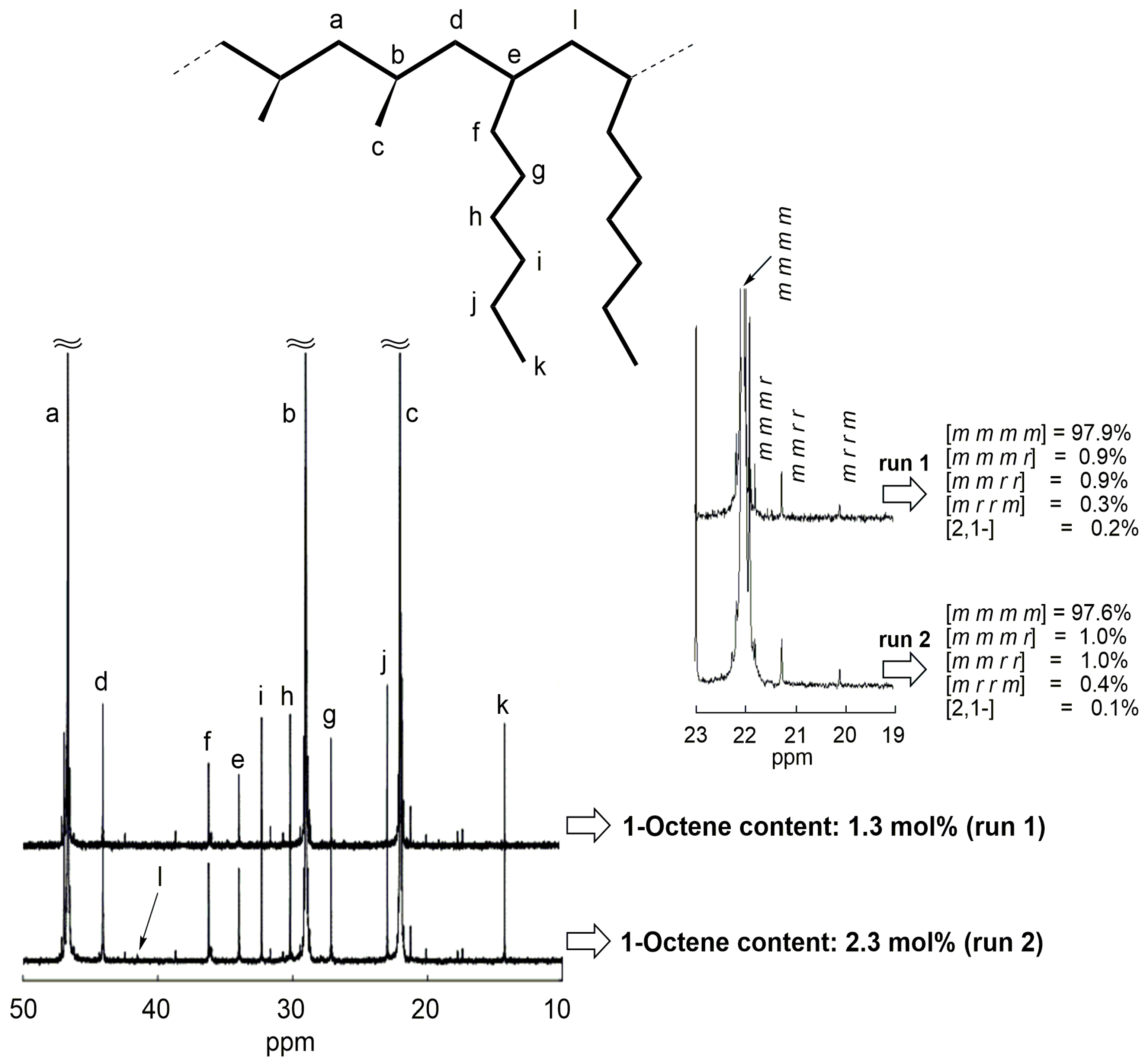
2.2. Block Copolymerization of Propene and 1-Octene
3. Experimental Section
3.1. General Remarks
3.2. Polymerization Procedure
3.3. Analytical Procedure
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Proto, A.; Capacchione, C. Stereoselective Polymerization with Single-Site Catalysts; Baugh, L.S., Canich, J.A.M., Eds.; CRC Press: New York, NY, USA, 2007; pp. 203–231. [Google Scholar]
- Stürzel, M.; Mihan, S.; Mülhaupt, R. From multisite polymerization catalysis to sustainable materials and all-polyolefin composites. Chem. Rev. 2016, 116, 1398–1433. [Google Scholar] [CrossRef] [PubMed]
- Coates, G.W.; Hustad, P.D.; Reinartz, S. Catalysts for the living insertion polymerization of alkenes: Access to new polyolefin architectures using Ziegler–Natta chemistry. Angew. Chem. Int. Ed. 2002, 41, 2236–2257. [Google Scholar] [CrossRef]
- Domski, G.J.; Rose, J.M.; Coates, G.W.; Bolig, A.D.; Brookhart, M. Living alkene polymerization: New methods for the precision synthesis of polyolefins. Prog. Polym. Sci. 2007, 32, 30–92. [Google Scholar] [CrossRef]
- Edson, J.B.; Domski, G.J.; Rose, J.M.; Bolig, A.D.; Brookhart, M.; Coates, G.W. Controlled and Living Polymerizations; Müller, A.H.E., Matyjaszewski, K., Eds.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2009; pp. 167–239. [Google Scholar]
- Sita, L.R. Ex uno plures (“Out of one, many”): New paradigms for expanding the range of polyolefins through reversible group transfers. Angew. Chem. Int. Ed. 2009, 48, 2464–2472. [Google Scholar] [CrossRef] [PubMed]
- Makio, H.; Terao, H.; Iwashita, A.; Fujita, T. FI catalysts for olefin polymerization–A comprehensive treatment. Chem. Rev. 2011, 111, 2363–2449. [Google Scholar] [CrossRef] [PubMed]
- Eagan, J.M.; Xu, J.; Girolamo, R.D.; Thurber, C.M.; Macosko, C.W.; LaPointe, A.M.; Bates, F.S.; Coates, G.W. Combining polyethylene and polypropylene: Enhanced performance with PE/iPP multiblock polymers. Science 2017, 355, 814–816. [Google Scholar] [CrossRef] [PubMed]
- Busico, V.; Cipullo, R.; Fraldi, N.; Ronca, S.; Togrou, M. The first molecularly characterized isotactic polypropylene-block-polyethylene obtained via “Quasi-living” insertion polymerization. Macromolecules 2003, 36, 3806–3808. [Google Scholar] [CrossRef]
- Cipullo, R.; Busico, V.; Fraldi, N.; Pellecchia, R.; Talarico, G. Improving the behavior of bis(phenoxyamine) group 4 metal catalysts for controlled alkene polymerization. Macromolecules 2009, 42, 3869–3872. [Google Scholar] [CrossRef]
- Harney, M.B.; Zhang, Y.; Sita, L.R. Discrete, multiblock isotactic–atactic stereoblock polypropene microstructures of differing block architectures through programmable stereomodulated living Ziegler–Natta polymerization. Angew. Chem. Int. Ed. 2006, 45, 2400–2404. [Google Scholar] [CrossRef] [PubMed]
- Giller, C.; Gururajan, G.; Wei, J.; Zhang, W.; Hwang, W.; Chase, D.B.; Rabolt, J.F.; Sita, L.R. Synthesis, characterization, and electrospinning of architecturally-discrete isotactic–atactic–isotactic triblock stereoblock polypropene elastomers. Macromolecules 2011, 44, 471–482. [Google Scholar] [CrossRef]
- Uozumi, T.; Tsubaki, S.; Jin, J.; Sano, T.; Soga, K. Isospecific propylene polymerization using the [ArN(CH2)3NAr]TiCl2/Al(iBu)3/Ph3CB(C6F5)4 catalyst system in the presence of cyclohexene. Macromol. Chem. Phys. 2001, 202, 3279–3283. [Google Scholar] [CrossRef]
- Li, G.; Lamberti, M.; D′Amora, S.; Pellecchia, C. C1-Symmetric pentacoordinate anilidopyridylpyrrolide zirconium (IV) complexes as highly isospecific olefin polymerization catalysts. Macromolecules 2010, 43, 8887–8891. [Google Scholar] [CrossRef]
- Kiesewetter, E.T.; Randoll, S.; Radlauer, M.; Waymouth, R.M. Stereospecific octahedral group 4 bis(phenolate) ether complexes for olefin polymerization. J. Am. Chem. Soc. 2010, 132, 5566–5567. [Google Scholar] [CrossRef] [PubMed]
- Diamond, G.M.; Hall, K.A.; LaPointe, A.M.; Leclerc, M.K.; Longmire, J.; Shoemaker, J.A.W.; Sun, P. High-throughput discovery and optimization of hafnium heteroaryl-amido catalysts for the isospecific polymerization of propylene. ACS Catal. 2011, 1, 887–900. [Google Scholar] [CrossRef]
- Nakata, N.; Toda, T.; Matsuo, T.; Ishii, A. Controlled isospecific polymerization of α-olefins by hafnium complex incorporating with a trans-cyclooctanediyl-bridged [OSSO]-type bis(phenolate) ligand. Macromolecules 2013, 46, 6758–6764. [Google Scholar] [CrossRef]
- Machat, M.R.; Lanzinger, D.; Pöthig, A.; Rieger, B. Ultrarigid indenyl-based hafnocene complexes for the highly isoselective polymerization of propene: Tunable polymerization performance adopting various sterically demanding 4-aryl substituents. Organometallics 2017, 36, 399–408. [Google Scholar] [CrossRef]
- Machat, M.R.; Jandl, C.; Rieger, B. Titanocenes in olefin polymerization: Sustainable catalyst system or an extinct species? Organometallics 2017. [Google Scholar] [CrossRef]
- Shiono, T. Living polymerization of olefins with ansa-dimethylsilylene(fluorenyl)(amido)dimethyltitanium-based catalysts. Polym. J. 2011, 43, 331–351. [Google Scholar] [CrossRef]
- Hagimoto, H.; Shiono, T.; Ikeda, T. Living polymerization of propene with a chelating diamide complex of titanium using dried methylaluminoxane. Macromol. Rapid Commun. 2002, 23, 73–76. [Google Scholar] [CrossRef]
- Nishii, K.; Matsumae, T.; Dare, E.O.; Shiono, T.; Ikeda, T. Effect of solvents on living polymerization of propylene with [t-BuNSiMe2Flu]TiMe2-MMAO catalyst system. Macromol. Chem. Phys. 2004, 205, 363–369. [Google Scholar] [CrossRef]
- Cai, Z.; Ikeda, T.; Akita, M.; Shiono, T. Substituent effects of tert-butyl groups on fluorenyl ligand in syndiospecific living polymerization of propylene with ansa-fluorenylamidodimethyltitanium complex. Macromolecules 2005, 38, 8135–8139. [Google Scholar] [CrossRef]
- Nishii, K.; Shiono, T. Effect of cyclopentadienyl ligands in propylene polymerization with ansa-monocyclopentadienylamidodimethyltitanium complexes. Koubunshi Ronbunshu 2011, 68, 341–344. [Google Scholar] [CrossRef]
- Nishii, K.; Ikeda, T.; Akita, M.; Shiono, T. Polymerization of propylene with [t-BuNSiMe2Ind]TiMe2-MAO catalyst system. J. Mol. Catal. A Chem. 2005, 231, 241–246. [Google Scholar] [CrossRef]
- Herfert, N.; Fink, G. Elementarprozesse der Ziegler-Katalyse, 6 Ethylen- und propenhomopolymerisation mit den stereorigiden katalysatorsystemen iPr[FluCp]ZrCl2/MAO und Me2Si[Ind]2ZrCl2/MAO. Makromol. Chem. 1992, 193, 773–778. [Google Scholar] [CrossRef]
- Naga, N.; Shiono, T.; Ikeda, T. Copolymerization of propene and nonconjugated diene involving intramolecular cyclization with metallocene/methylaluminoxane. Macromolecules 1999, 32, 1348–1355. [Google Scholar] [CrossRef]
- Schneider, M.J.; Mülhaupt, R. Influence of indenyl ligand substitution pattern on metallocene-catalyzed propene copolymerization with 1-octene. Macromol. Chem. Phys. 1997, 198, 1121–1129. [Google Scholar] [CrossRef]
- Fan, Z.-Q.; Yasin, T.; Feng, L.-X. Copolymerization of propylene with 1-octene catalyzed by rac-Me2Si(2,4,6-Me3-Ind)2ZrCl2/methyl aluminoxane. J. Polym. Sci. Part A Polym. Chem. 2000, 38, 4299–4307. [Google Scholar] [CrossRef]
Sample Availability: Samples are not available. |
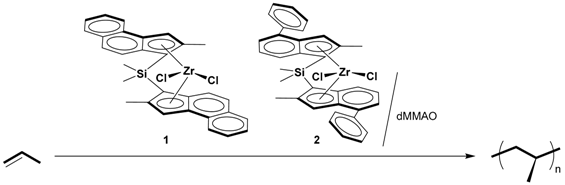
| Run | Complex | Solvent | [Al]/[Zr] | Time (min) | A b | Mn c (× 104) | Mw/Mn c | N d |
|---|---|---|---|---|---|---|---|---|
| 1 | 1 | toluene | 800 | 2 | 5400 | 18.0 | 2.22 | 1.00 |
| 2 | 2 | toluene | 800 | 5 | 3100 | 12.3 | 2.35 | 2.10 |
| 3 | 1 | toluene | 400 | 2 | 5000 | 16.4 | 3.08 | 1.00 |
| 4 | 2 | toluene | 400 | 5 | 2100 | 13.7 | 2.29 | 1.20 |
| 5 | 1 | toluene | 300 | 2 | 5000 | 23.8 | 2.33 | 0.70 |
| 6 | 1 | heptane | 300 | 10 | 300 | 44.9 | 2.71 | 0.11 |
| 7 | 1 | toluene | 100 | 2 | 230 | 36.6 | 2.26 | 0.02 |
| Run | Propylene (g) | Time (min) | Yield (%) | Mn b (×104) | Mw/Mn b | N c (μmol) |
|---|---|---|---|---|---|---|
| 1 | 0.63 | 60 | 93 | 21.3 | 1.91 | 3 |
| 2 | 1.26 | 60 | 91 | 20.7 | 2.19 | 6 |
| 3 | 0.63 + 0.63 | 60 + 60 | 93 | 17.3 | 2.12 | 7 |
| Run | Solvent | Time (min) | Mn b (×104) | Mw/Mn b (×104) |
|---|---|---|---|---|
| 1 | heptane | 5 | 28.1 | 2.74 |
| 2 | heptane | 10 | 41.7 | 2.98 |
| 3 | heptane | 15 | 70.4 | 2.37 |
| 4 | toluene | 2 | 20.8 | 2.62 |
| 5 | toluene | 5 | 29.1 | 2.21 |
| 6 | toluene | 10 | 45.2 | 2.28 |
| Run | 1-Octene (mol %) | Time (min) | Mn b (×104) | Mw/Mn b | OC Cont.c (mol %) | Tm d (°C) |
|---|---|---|---|---|---|---|
| Prepolymer e | - | - | 22.1 | 2.59 | ||
| 1 | 5 | 10 | 38.2 | 2.20 | 1.3 | 150 |
| Prepolymer e | - | - | 26.6 | 2.48 | ||
| 2 | 10 | 10 | 30.5 | 2.40 | 2.3 | 151 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nishii, K.; Murase, M.; Shiono, T. Quasi-Living Polymerization of Propene with an Isotactic-Specific Zirconocene Catalyst. Molecules 2017, 22, 725. https://doi.org/10.3390/molecules22050725
Nishii K, Murase M, Shiono T. Quasi-Living Polymerization of Propene with an Isotactic-Specific Zirconocene Catalyst. Molecules. 2017; 22(5):725. https://doi.org/10.3390/molecules22050725
Chicago/Turabian StyleNishii, Kei, Miyuki Murase, and Takeshi Shiono. 2017. "Quasi-Living Polymerization of Propene with an Isotactic-Specific Zirconocene Catalyst" Molecules 22, no. 5: 725. https://doi.org/10.3390/molecules22050725






