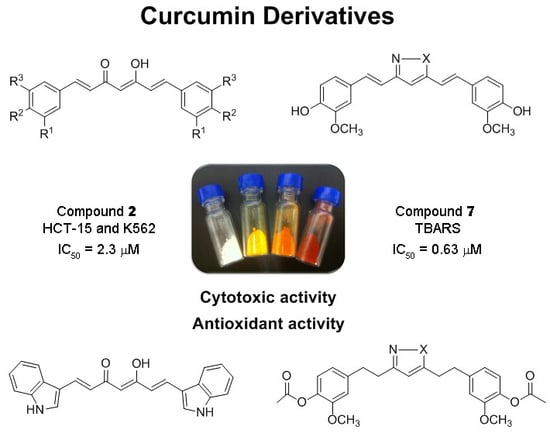
Synthesis of Curcuminoids and Evaluation of Their Cytotoxic and Antioxidant Properties
Abstract
:1. Introduction
2. Results and Discussion
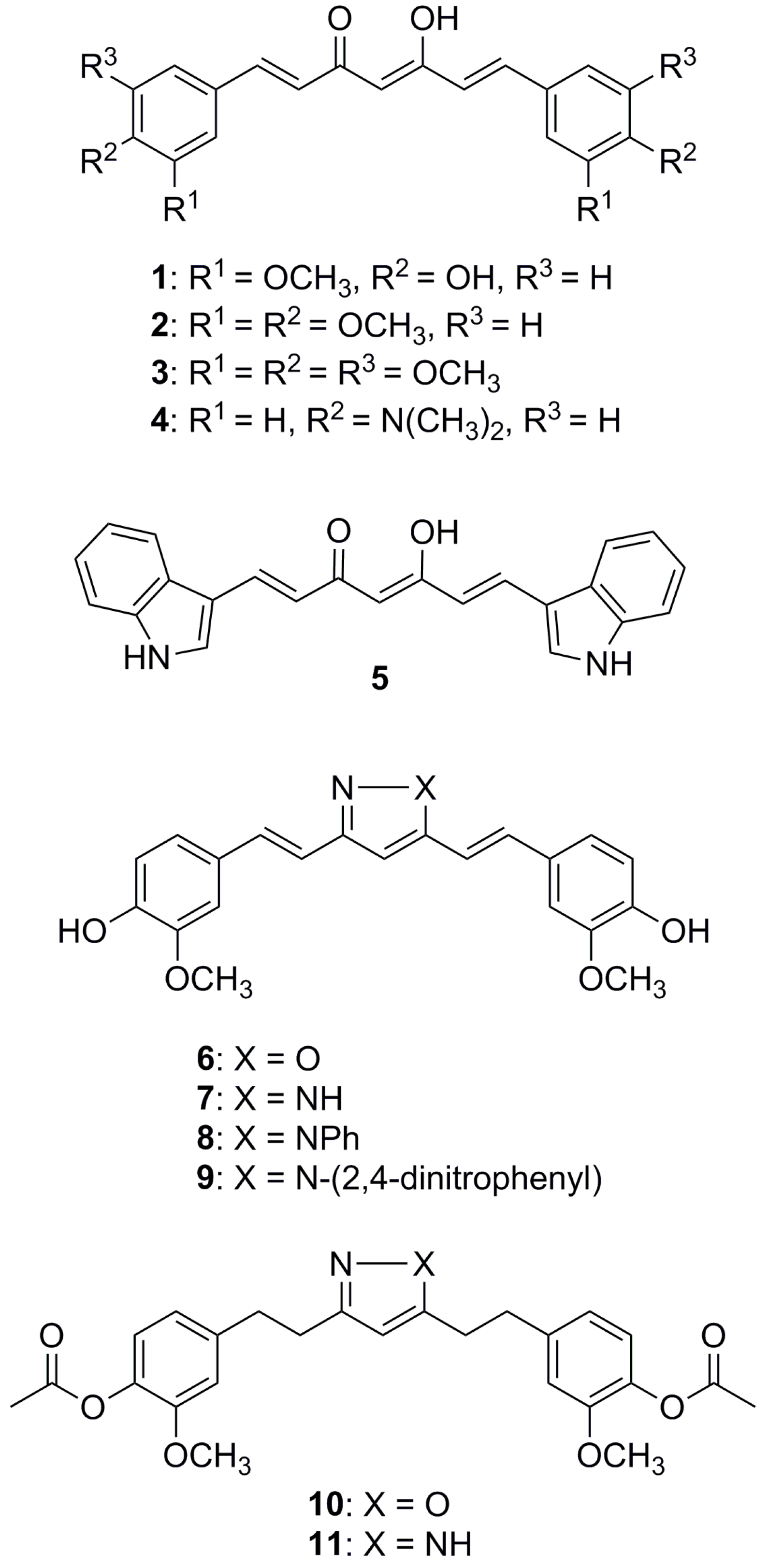
2.1. Synthesis of Curcumin Derivatives
2.2. Biological Assays
2.2.1. Cytotoxic Activity against Human Cancer Cell Lines
2.2.2. Antioxidant Activity in the Thiobarbituric Acid Reactive Substances and 2,2-Diphenyl-1-picrylhydrazyl Assays
3. Experiment Sections
3.1. Chemistry
3.1.1. General Considerations
3.1.2. General Procedure for the Preparation of 1, 2, 4, and 5
3.1.3. Procedure for the Synthesis of Isoxazole Derivative 6
3.1.4. General Procedure for the Preparation of Pyrazole Analogues of Curcumin (7–9)
3.1.5. Synthesis of Compounds 10 and 11
3.2. Biological Assays
3.2.1. Cytotoxic Activity
3.2.2. Antioxidant Activity
Scavenging Activity on Free Radical DPPH
Lipid Peroxidation Inhibition (TBARS)
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Goel, A.; Kunnumakkara, A.B.; Aggarwal, B.B. Curcumin as “Curecumin”: From kitchen to clinic. Biochem. Pharmacol. 2008, 75, 787–809. [Google Scholar] [CrossRef] [PubMed]
- Maheshwari, R.K.; Singh, A.K.; Gaddipati, J.; Srimal, R.C. Multiple biological activities of curcumin: A short review. Life Sci. 2006, 78, 2081–2087. [Google Scholar] [CrossRef] [PubMed]
- Prasad, S.; Gupta, S.C.; Tyagi, A.K.; Aggarwal, B.B. Curcumin, a component of golden spice: From bedside to bench and back. Biotechnol. Adv. 2014, 32, 1053–1064. [Google Scholar] [CrossRef] [PubMed]
- Matchanickal, R.A.; Rafi, M.M. Curcumin: Potential health benefits, molecular mechanism of action, and its anticancer properties in vitro and in vivo. ACS Symp. Ser. 2006, 925, 92–107. [Google Scholar]
- Fu, S.; Kurzrock, R. Development of curcumin as an epigenetic agent. Cancer 2010, 116, 4670–4676. [Google Scholar] [CrossRef] [PubMed]
- Chakraborti, S.; Dhar, G.; Dwivedi, V.; Das, A.; Poddar, A.; Chakraborti, G.; Basu, G.; Chakrabarti, P.; Surolia, A.; Bhattacharyya, B. Stable and potent analogues derived from the modification of the dicarbonyl moiety of curcumin. Biochemistry 2013, 52, 7449–7460. [Google Scholar] [CrossRef] [PubMed]
- Shaikh, J.; Ankola, D.D.; Beniwal, V.; Singh, D.; Kumar, M.N.V.R. Nanoparticle encapsulation improves oral bioavailability of curcumin by at least 9-fold when compared to curcumin administered with piperine as absorption enhancer. Eur. J. Pharm. Sci. 2009, 37, 223–230. [Google Scholar] [CrossRef] [PubMed]
- Anand, P.; Kunnumakkara, A.B.; Newman, R.A.; Aggarwal, B.B. Bioavailability of curcumin: Problems and promises. Mol. Pharm. 2007, 4, 807–818. [Google Scholar] [CrossRef] [PubMed]
- Yadav, V.R.; Prasad, S.; Kannappan, R.; Ravindran, J.; Chaturvedi, M.M.; Vaahtera, L.; Parkkinen, J.; Aggarwal, B.B. Cyclodextrin-complexed curcumin exhibits anti-inflammatory and antiproliferative activities superior to those of curcumin through higher cellular uptake. Biochem. Pharm. 2010, 80, 102–1032. [Google Scholar] [CrossRef] [PubMed]
- Mandal, S.; Ghosh, S.; Banik, D.; Banerjee, C.; Kuchlyan, J.; Sarkar, N. An investigation into the effect of the structure of bile salt aggregates on the binding interactions and ESIHT dynamics of curcumin: A photophysical approach to probe bile salt aggregates as a potential drug carrier. J. Phys. Chem. B 2013, 117, 13795–13807. [Google Scholar] [CrossRef] [PubMed]
- Caldarelli, A.; Penucchini, E.; Caprioglio, D.; Genazzani, A.A.; Minassi, A. Synthesis and tubulin-binding properties of non-symmetrical click C5-curcuminoids. Bioorg. Med. Chem. 2013, 21, 5510–5517. [Google Scholar] [CrossRef] [PubMed]
- Anto, R.J.; Kuttan, G.; Babu, K.V.D.; Rajasekharan, K.N.; Kuttan, R. Anti-tumour and free radical scavenging activity of synthetic curcuminoids. Int. J. Pharm. 1996, 131, 1–7. [Google Scholar] [CrossRef]
- Ferrari, E.; Pignedoli, F.; Imbriano, C.; Marverti, G.; Basile, V.; Venturi, E.; Saladini, M. Newly synthesized curcumin derivatives: Crosstalk between chemico-physical properties and biological activity. J. Med. Chem. 2011, 54, 8066–8077. [Google Scholar] [CrossRef] [PubMed]
- Fuchs, J.R.; Pandit, B.; Bhasin, D.; Etter, J.P.; Regan, N.; Abdelhamid, D.; Li, C.; Lin, J.; Li, P.-K. Structure-activity relationship studies of curcumin analogues. Bioorg. Med. Chem. Lett. 2009, 19, 2065–2069. [Google Scholar] [CrossRef] [PubMed]
- Liang, G.; Yang, S.; Zhou, H.; Shao, L.; Huang, K.; Xiao, J.; Huang, Z.; Li, X. Synthesis, crystal structure and anti-inflammatory properties of curcumin analogues. Eur. J. Med. Chem. 2009, 44, 915–919. [Google Scholar] [CrossRef] [PubMed]
- Simoni, D.; Rizzi, M.; Rondanin, R.; Baruchello, R.; Marchetti, P.; Invidiata, F.P.; Labbozzetta, M.; Poma, P.; Carina, V.; Notarbartolo, M.; et al. Antitumor effects of curcumin and structurally β-diketone modified analogs on multidrug resistant cancer cells. Bioorg. Med. Chem. Lett. 2008, 18, 845–849. [Google Scholar] [CrossRef] [PubMed]
- Pae, H.-O.; Jeong, S.-O.; Kim, H.S.; Kim, S.H.; Song, Y.S.; Kim, S.-K.; Chai, K.-Y.; Chung, H.-T. Dimethoxycurcumin, a synthetic curcumin analogue with higher metabolic stability, inhibits NO production, inducible NO synthase expression and NF-κB activation in RAW264.7 macrophages activated with LPS. Mol. Nutr. Food Res. 2008, 52, 1082–1091. [Google Scholar] [CrossRef] [PubMed]
- Dutta, S.; Padhye, S.; Priyadarsini, K.I.; Newton, C. Antioxidant and antiproliferative activity of curcumin semicarbazone. Bioorg. Med. Chem. Lett. 2005, 15, 2738–2744. [Google Scholar] [CrossRef] [PubMed]
- Kunwar, A.; Jayakumar, S.; Srivastava, A.K.; Priyadarsini, K.I. Dimethoxycurcumin-induced cell death in human breast carcinoma MCF7 cells: Evidence for pro-oxidant activity, mitochondrial dysfunction, and apoptosis. Arch. Toxicol. 2012, 86, 603–614. [Google Scholar] [CrossRef] [PubMed]
- Ravindran, J.; Subbaraju, G.V.; Ramani, M.V.; Sung, B.; Aggarwal, B.B. Bisdemethylcurcumin and structurally related hispolon analogues of curcumin exhibit enhanced prooxidant, anti-proliferative and anti-inflammatory activities in vitro. Biochem. Pharmacol. 2010, 79, 1658–1666. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.K.; Rai, D.; Yadav, D.; Bhargava, A.; Balzarini, J.; De Clercq, E. Synthesis, antibacterial and antiviral properties of curcumin bioconjugates bearing dipeptide, fatty acids and folic acid. Eur. J. Med. Chem. 2010, 45, 1078–1086. [Google Scholar] [CrossRef] [PubMed]
- Kumar, D.; Mishra, P.K.; Anand, A.V.K.; Agrawal, P.K.; Mohapatra, R. Isolation, synthesis and pharmacological evaluation of some novel curcumin derivatives as anticancer agents. J. Med. Plants Res. 2012, 6, 2880–2884. [Google Scholar]
- Amolins, M.W.; Peterson, L.B.; Blagg, B.S.J. Synthesis and evaluation of electron-rich curcumin analogues. Bioorg. Med. Chem. 2009, 17, 360–367. [Google Scholar] [CrossRef] [PubMed]
- Weber, W.M.; Hunsaker, L.A.; Abcouwer, S.F.; Deck, L.M.; Vander Jagt, D.L. Anti-oxidant activities of curcumin and related enones. Bioorg. Med. Chem. 2005, 13, 3811–3820. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.-Y.; Liu, Z.-Q. Phenolic and enolic hydroxyl groups in curcumin: Which plays the major role in scavenging radicals? J. Agric. Food. Chem. 2009, 57, 11041–11046. [Google Scholar] [CrossRef] [PubMed]
- Pabon, H.J.J. Synthesis of curcumin and related compounds. Recl. Trav. Chim. Pays-Bas. 1964, 83, 379–386. [Google Scholar] [CrossRef]
- Selvkumar, B.; Venkataraman, R. Microwave assisted synthesis of novel aryl and heteroaryl hydrazinocurcumins. Pharm. Chem. 2011, 3, 84–88. [Google Scholar]
- Mishra, S.; Karmodiya, K.; Surolia, N.; Surolia, A. Synthesis and exploration of novel curcumin analogues as anti-malarial agents. Bioorg. Med. Chem. 2008, 16, 2894–2902. [Google Scholar] [CrossRef] [PubMed]
- Lozada, M.C.; Enriquez, R.G.; Lobato, C.E.; Ortiz, B.; Soriano, M.; Gnecco, D.; Reynolds, W.F. Synthesis and structure of new heterocyclic derivatives of curcumin. Heterocycles 2005, 65, 49–58. [Google Scholar]
- Yim-im, W.; Sawatdichaikul, O.; Semsri, S.; Horata, N.; Mokmak, W.; Tongsima, S.; Suksamrarn, A.; Choowongkomon, K. Computational analyses of curcuminoid analogs against kinase domain of HER2. BMC Bioinf. 2014, 15, 261. [Google Scholar] [CrossRef] [PubMed]
- Lozada, M.C.; Soria-Arteche, O.; Apan, M.T.R.; Nieto-Camacho, A.; Enriquez, R.G.; Izquierdo, T.; Jimenez-Corona, A. Synthesis, cytotoxic and antioxidant evaluations of amino derivatives from perezone. Bioorg. Med. Chem. 2012, 20, 5077–5084. [Google Scholar] [CrossRef] [PubMed]
- Venkatesan, P.; Rao, M.N.A. Structure-activity relationships for the inhibition of lipid peroxidation and the scavenging of free radicals by synthetic symmetrical curcumin analogues. J. Pharm. Pharmacol. 2000, 52, 1123–1128. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.K.; Jeong, W.; Kang, J.; Chong, Y. Significant enhancement in radical-scavenging activity of curcuminoids conferred by acetoxy substituent at the central methylene carbon. Bioorg. Med. Chem. 2011, 19, 3793–3800. [Google Scholar] [CrossRef] [PubMed]
- Sherin, D.R.; Rajasekharan, K.N. Mechanochemical Synthesis and Antioxidant Activity of Curcumin-Templated Azoles. Arch. Pharm. 2015, 348, 908–914. [Google Scholar] [CrossRef] [PubMed]
- Ohtsu, H.; Itokawa, H.; Xiao, Z.; Su, C.-Y.; Shih, C.C.Y.; Chiang, T.; Chang, E.; Lee, Y.; Chiu, S.-Y.; Chang, C.; et al. Antitumor agents 222. Synthesis and anti-androgen activity of new diarylheptanoids. Bioorg. Med. Chem. 2003, 11, 5083–5090. [Google Scholar] [CrossRef] [PubMed]
- Venkateswarlu, S.; Ramachandra, M.S.; Subbaraju, G.V. Synthesis and biological evaluation of polyhydroxycurcuminoids. Bioorg. Med. Chem. 2005, 13, 6374–6380. [Google Scholar] [CrossRef] [PubMed]
- Sherin, D.R.; Thomas, S.G.; Rajasekharan, K.N. Mechanochemical synthesis of 2,2-difluoro-4, 6-bis(β-styryl)-1,3,2-dioxaborines and their use in cyanide ion sensing. Heterocycl. Commun. 2015, 21, 381–385. [Google Scholar] [CrossRef]
- Fadda, A.A.; Badria, F.A.; El-Attar, K.M. Synthesis and evaluation of curcumin analogues as cytotoxic agents. Med. Chem. Res. 2010, 19, 413–430. [Google Scholar] [CrossRef]
- Shioorkar, M.G.; Ubale, M.B. Simple and efficient microwave assisted PEG mediated synthesis of pyrazole analogues of curcumin. Pharm. Chem. 2015, 7, 274–277. [Google Scholar]
- Monks, A.; Scudiero, D.; Skehan, P.; Shoemaker, R.; Paull, K.; Vistica, D.; Hose, C.; Langley, J.; Cronise, P.; Vaigro-Wolff, A.; et al. Feasibility of a high-flux anticancer drug screen using a diverse panel of cultured human tumor cell lines. J. Natl. Cancer Inst. 1991, 83, 757–766. [Google Scholar] [CrossRef] [PubMed]
- Mellors, A.; Tappel, A.L. The inhibition of mitochondrial peroxidation by ubiquinone and ubiquinol. J. Biol. Chem. 1966, 241, 4353–4356. [Google Scholar] [PubMed]
- Rossato, J.I.; Ketzer, L.A.; Centuriao, F.B.; Silva, S.J.N.; Ludtke, D.S.; Zeni, G.; Braga, A.L.; Rubin, M.A.; Rocha, J.B.T. Antioxidant properties of new chalcogenides against lipid peroxidation in rat brain. Neurochem. Res. 2002, 27, 297–303. [Google Scholar] [CrossRef] [PubMed]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [PubMed]
- Ohkawa, H.; Ohishi, N.; Yagi, K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal. Biochem. 1979, 95, 351–358. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are available from the authors. |

| Compound a | U-251 MG d | PC-3 e | HCT-15 f | K562 g | SKLU-1 h |
|---|---|---|---|---|---|
| 1 b | 59.1 ± 4.9 | 22.0 ± 2.4 | 81.2 ± 0.7 | 59.9 ± 5.0 | 72.4 ± 2.8 |
| 2 c | 89.7 ± 4.0 | 78.0 ± 2.5 | 93.0 ± 2.3 | 93.0 ± 3.5 | 86.8 ± 2.4 |
| 3 | 41.1 ± 6.2 | 28.2 ± 1.4 | 33.8 ± 8.3 | 43.7 ± 6.2 | 24.2 ± 10.6 |
| 4 | 18.0 ± 3.5 | 26.3 ± 4.6 | 35.3 ± 11.9 | 27.8 ± 2.8 | 18.9 ± 2.1 |
| 5 c | 73.7 ± 4.4 | 83.0 ± 6.2 | 94.1 ± 2.8 | 93.7 ± 5.8 | 91.4 ± 1.0 |
| 6 c | 50.6 ± 5.4 | 55.8 ± 1.2 | 78.9 ± 7.0 | 84.5 ± 8.6 | 80.1 ± 10.8 |
| 7 c | 63.3 ± 5.1 | 64.5 ± 1.7 | 77.0 ± 3.6 | 87.5 ± 10.9 | 88.6 ± 1.2 |
| 8 | 90.6 ± 5.1 | 91.5 ± 4.5 | 95.7 ± 4.2 | 97.3 ± 2.5 | 98.9 ± 1.0 |
| 9 | 15.2 ± 0.7 | 19.9 ± 0.7 | 33.5 ± 8.8 | 39.2 ± 5.7 | 5.2 ± 1.8 |
| 10 | 19.7 ± 4.2 | 63.4 ± 12.1 | 83.7 ± 7.4 | 71.2 ± 6.4 | 18.9 ± 5.1 |
| 11 | 34.0 ± 5.5 | 57.9 ± 3.7 | 69.3 ± 5.0 | 76.8 ± 3.5 | 51.2 ± 2.5 |
| Compounds | IC50 µM | |
|---|---|---|
| HCT-15 | K562 | |
| 1 | 13.9 ± 0.6 | 9.2 ± 0.4 |
| 2 | 2.3 ± 0.1 | 2.3 ± 0.3 |
| 5 | 4.8 ± 0.4 | 13.3 ± 0.6 |
| 6 | 5.8 ± 0.7 | 22.1 ± 0.9 |
| 7 | 6.2 ± 0.9 | 6.3 ± 0.6 |
| 8 | 5.0 ± 0.4 | 4.5 ± 0.2 |
| 10 | 2.8 ± 0.1 | 3.0 ± 0.4 |
| 11 | 12.7 ± 0.1 | 8.8 ± 0.3 |
| Adriamycin | 0.0050 ± 0.0009 | 0.0140 ± 0.0009 |
| Compound | IC50 (µM) TBARS a | IC50 (µM) DPPH b |
|---|---|---|
| 1 | 3.22 ± 0.32 | 23.52 ± 1.20 |
| 2 | 18.67 ± 2.28 | Inactive |
| 6 | 1.31 ± 0.15 | 25.92 ± 1.73 |
| 7 | 0.63 ± 0.02 | 25.81 ± 2.97 |
| 10 | 15.30 ± 0.46 | Inactive |
| Quercetin | 1.49 ± 0.03 | 10.89 ± 0.47 |
| α-Tocopherol | 6.78 ± 2.16 | 31.74 ± 1.04 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lozada-García, M.C.; Enríquez, R.G.; Ramírez-Apán, T.O.; Nieto-Camacho, A.; Palacios-Espinosa, J.F.; Custodio-Galván, Z.; Soria-Arteche, O.; Pérez-Villanueva, J. Synthesis of Curcuminoids and Evaluation of Their Cytotoxic and Antioxidant Properties. Molecules 2017, 22, 633. https://doi.org/10.3390/molecules22040633
Lozada-García MC, Enríquez RG, Ramírez-Apán TO, Nieto-Camacho A, Palacios-Espinosa JF, Custodio-Galván Z, Soria-Arteche O, Pérez-Villanueva J. Synthesis of Curcuminoids and Evaluation of Their Cytotoxic and Antioxidant Properties. Molecules. 2017; 22(4):633. https://doi.org/10.3390/molecules22040633
Chicago/Turabian StyleLozada-García, María Concepción, Raúl G. Enríquez, Teresa O. Ramírez-Apán, Antonio Nieto-Camacho, Juan Francisco Palacios-Espinosa, Zeltzin Custodio-Galván, Olivia Soria-Arteche, and Jaime Pérez-Villanueva. 2017. "Synthesis of Curcuminoids and Evaluation of Their Cytotoxic and Antioxidant Properties" Molecules 22, no. 4: 633. https://doi.org/10.3390/molecules22040633