2.1. The Structural Modification—Glycation of Serum Albumin
Glycation as a result of the metabolic changes that occur during diabetes causes a number of structural and functional modification in serum albumin. The extent of modification that occurs in protein depends mainly on the time of protein incubation with reducing sugar and a type of reducing sugar. Fructose, as compared with glucose, reacts faster with proteins and produces more protein-bound fluorescence than glucose [
14,
18].
In the present study, fructose has been used as a bovine serum albumin (BSA) initiator of glycation. In order to focus on the main albumin binding sites, Sudlow’s site I located in subdomain IIA and Sudlow’s site II located in subdomain IIIA, we used λ
ex = 275 nm and λ
ex = 295 nm excitation wavelengths. λ
ex = 275 nm excites both tyrosyl and tryprophanyl residues while λ
ex = 295 nm almost exclusively tryptophanyl residue. BSA contains two tryptophanyl residues, Trp-214 (subdomain IIA) and Trp-135 (subdomain IB), whereas BSA tyrosyl residues are located in subdomain IA (Tyr-30), IB (Tyr-138, -140, -148, -150, -156, -157, and -161), IIA (Tyr-263), IIB (Tyr-319, -332, -334, -341, -353, and -370), IIIA (Tyr-401, -411, -452, and -497) [
19]. According to the literature, sensitive to in vivo and in vitro glycation are albumin amino acid residues with high nucleophile properties (lysine, Lys and arginine (Arg)) and also a free thiol group of cysteine (Cys-34) located both around and inside of subdomains IIA, IIIA, IB and IA [
20]. It means that the glycation process can have an influence on the main Sudlow’s site I and II, and the using of fluorescence quenching method with two excitation wavelengths (275 nm and 295 nm) allows for the analysis of these sites. Moreover, due to the BSA second Trp-135 residue (subdomain IB) the study of glycation impact on the environment of subdomain IB is possible. The influence of glycation process on albumin conformation was investigated by the comparison of synchronous and emission spectra recorded for non-glycated (BSA) and glycated bovine serum albumin (gBSA). Furthermore, the second derivative of fluorescence spectroscopy and spectral parameter A were first used for the identification of subtle changes in the tertiary conformation of proteins. Because the wavelength of λ
ex = 295 nm excites almost entirely tryptophanyl residue while λ
ex = 275 nm excites both tryptophanyl and tyrosyl residues, and it is impossible to observe separately the fluorescence of tyrosyl residues [
21], synchronous fluorescence spectroscopy allows for the separation of the emission spectra originating from the Trp and Tyr residues and more specific information concerning the structure of protein than derived from conventional fluorescence spectroscopy can be obtained. Spectral parameter A has been used in order to monitor the structural changes in tryptophanyl residues microenvironment. According to literature data [
22], the synchronous fluorescence spectra were obtained considering the wavelength intervals Δλ = 60 nm and Δλ = 15 nm to evidence the tryptophanyl and tyrosyl residues, respectively (Δλ = λ
em − λ
ex). The synchronous fluorescence spectra of both types of fluorophores in BSA and gBSA at the selected temperature of 309 K have been presented in
Figure 1.
Synchronous fluorescence spectra of BSA and gBSA Trp and Tyr residues show single emission maxima at λ
max = 340 nm and λ
max = 300 nm, respectively (
Figure 1). It is noteworthy that the synchronous fluorescence spectrum of glycated bovine serum albumin allowed observing an additional signal in the region between λ
em = 370 nm and 470 nm with λ
max = 405 nm (for the wavelength intervals Δλ = 60 nm) (
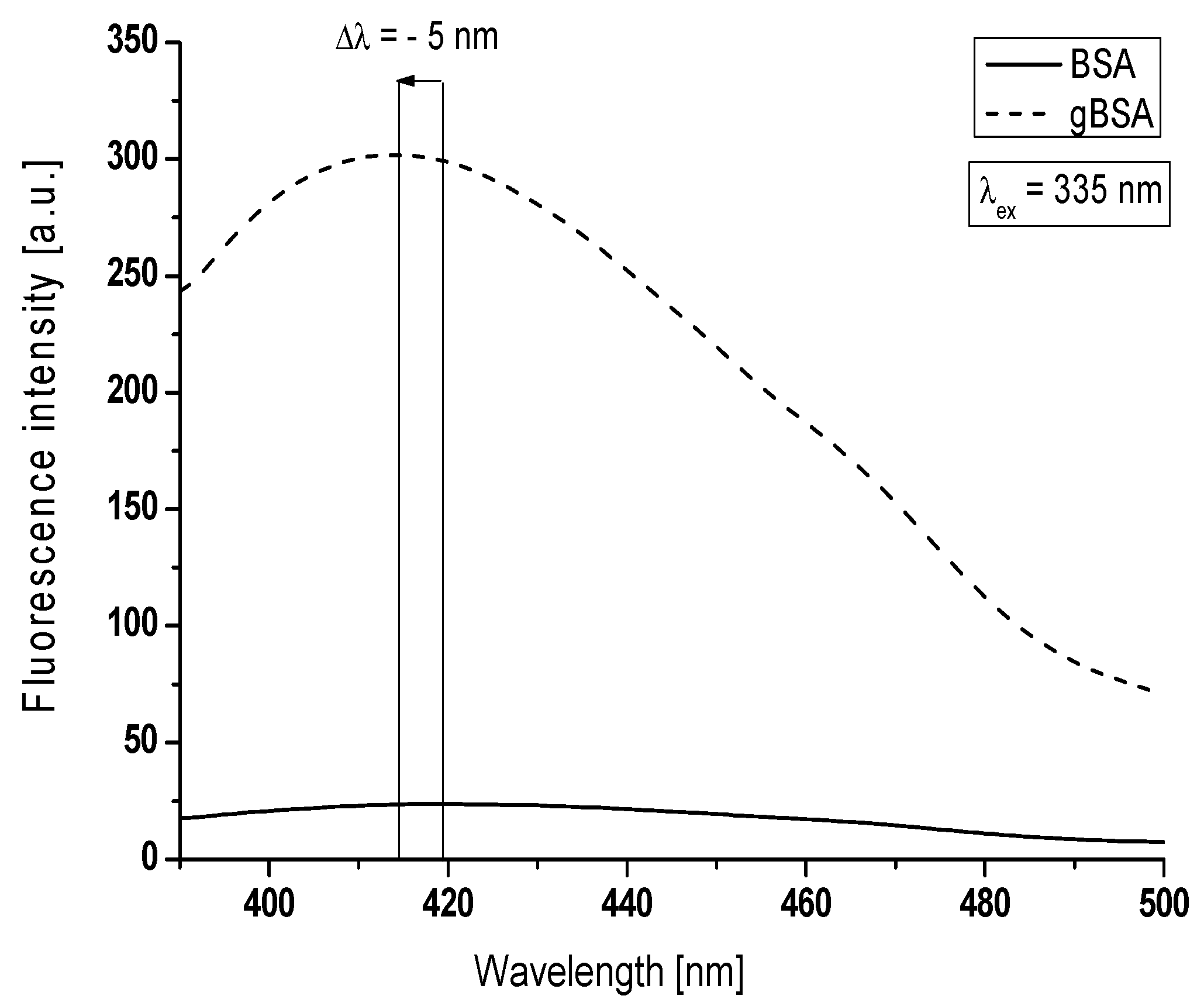
Figure 1). It can be concluded that this effect is caused by the formation of BSA fluorescence Advanced Glycation End-products (AGEs) and an additional signal derives from the fluorescent AGEs. To prove the impact of glycation by fructose on the formation of AGEs in bovine serum albumin, fluorescence emission spectra of BSA and gBSA were recorded at excitation wavelength of λ
ex = 335 nm (absorbance maximum wavelength of AGEs) (
Figure 2).
Figure 2 summarizes a typical experiment comparing changes between emission florescence spectra of Advanced Glycation End-products coming from non-glycated and created in glycated albumin. Greater values of maximum fluorescence (F
max) of AGEs were obtained for glycated albumin (F
414nm = 302) than non-glycated (F
419nm = 24). It is important to note that a widely accepted assumption is that greater fluorescence intensity is, the more significant protein modification occurs [
23]. Argpyrimidine shows fluorescence maximum at about λ
em = 400 nm and pentosidine at λ
em = 375–385 nm [
22]. In opposite to our results, S. Vetter and V. Indurthi reported that Advanced Glycation End-products of human serum albumin (HSA) glycated by glucose do not show typical for pentosidine or argpyrimidine fluorescence but red-shifted fluorescence with maxima about λ
em = 420 nm and λ
em = 435 nm (excitation at λ
ex = 330 nm or λ
ex = 365 nm), respectively [
24]. The increase in the AGEs fluorescence intensities is accompanied by a blue-shift (Δλ = 5 nm) of maximum fluorescence at λ
ex = 335 nm. This blue-shift of BSA maximum fluorescence under the glycation process by fructose makes the changes that AGEs environment becomes more hydrophobic. Rondeau et al. analyzed AGE-products of modified by glucose bovine serum albumin [
25]. Using λ
ex = 360 nm, they also have observed blue-shifted fluorescence indicating the reduction in the polarity of AGEs environment.
Due to the glycation process fluorescence intensity decreased about 6 times for Trp residues (Δλ = 60 nm) and 2.5 times for Tyr residues (Δλ = 15 nm). The same tendency was also observed at T = 311 K and T = 313 K. These results may suggest a modification of the structure of bovine serum albumin by glycation, especially in tryptophanyl and tyrosyl residues environment, which can affect the main binding sites of albumin in subdomain IIA and/or IIIA (Sudlow’s site I and/or II), and also in subdomains IB and IIB. It is conventional in the fluorescence that the shift of position at maximum emission wavelength (λ
max) corresponds to changes in polarity around the chromophores molecule. A blue-shift of λ
max indicates the increase in hydrophobicity around chromophores while a red-shift of λ
max implies increase in hydrophilicity and the chromophores are more exposed to the solvent molecules [
22]. Using Δλ = 60 nm and Δλ = 15 nm, no changes in the maximum emission wavelengths on synchronous spectra of BSA and gBSA tryptophanyl and tyrosyl residues were observed (
Figure 1) and no changes in the polarity around the indole group of Trp residues have been registered. It seems surprising especially that the maximum emission of Trp is highly sensitive to the local environment.
In order to monitor the structural changes in the Trp microenvironment of BSA (
Figure 3a) and gBSA (
Figure 3b) tryptophanyl residues (Trp-135 and Trp-214), λ
ex from 290 nm to 305 nm has been used and the spectral parameter
A for BSA and gBSA has been calculated in the range temperature between 309 K and 313 K (
Table 1,
Figure 3 in the inserts). In order to determine spectral parameter
A it is necessary to use two wavelengths from the opposite edges of bandwidth, where steepness of the band is large. This causes that for the small change of wavelength the change in the fluorescence intensity is large. In the literature and in the present study the 320 nm and 365 nm wavelengths for proteins containing Trp residues have been used because they correspond to approximately half of the length of band steepness (
. The spectral parameter
A is the most sensitive indicator of spectral shifts which is less sensitive to experimental errors. As a consequence parameter
A provides more accurate position of fluorescence spectra in comparison with a position of the maximum fluorescence (λ
max) [
26].
Emission fluorescence spectra of gBSA Trp-135 and -214 residues differ from the Trp residue of non-glycated albumin for all excitation wavelengths (
Figure 3). The change in the gBSA spectrum in comparison with BSA is significant especially at λ
ex = 305 nm. With the increase of excitation wavelength from 290 nm to 305 nm spectral parameter
A decreases 1.2 times at all temperatures (
Table 1). It probably means that fluorescent spectra of Trp residues shift towards long wavelengths (red-shift). This phenomenon called red-edge excitation shift is caused by microheterogeneity in the tryptophan (Trp) environment and electronic coupling between the tryptophan indole and neighboring dipoles which result in a distribution of electronic transition energies of the Trp [
27]. On the other hand, spectral parameter
A decreases five times the increase of excitation wavelength, obtained for glycated albumin at all temperatures. However, the shape of gBSA Trp residue spectrum (
Figure 3b) points out the phenomenon that at the excitation wavelength 365 nm the fluorescence comes probably from AGEs due to the BSA glycation by fructose. It can be concluded that spectral parameter
A is a tool that confirms the presence of AGEs in BSA-fructose complex regardless of the temperature. At the excitation wavelength λ
ex = 305 nm, spectral parameter
A does not change with the change of temperature (0.21–0.22). This phenomenon means that the increase of temperature does not influence on AGEs concentration.
Another evidence for modification of BSA structure was the analysis of the second derivative spectra. Derivative spectroscopy can extract more information contained within a spectral distribution usually not accessible through the direct spectroscopic measurements and reduce the effect of much spectral interference [
28].
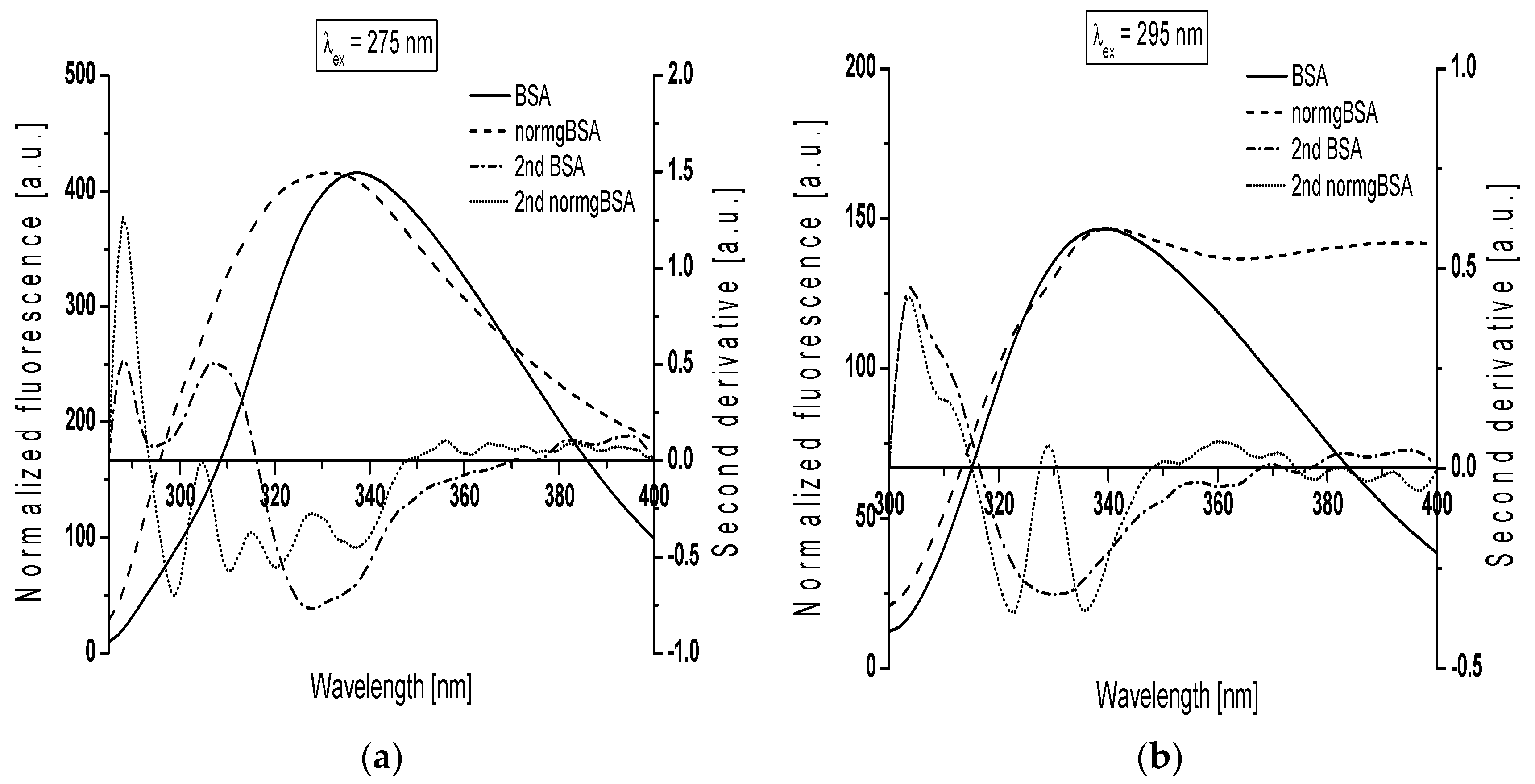
Figure 4 presents gBSA emission spectrum (normgBSA) normalized to BSA and their second derivative fluorescence spectra for: (a) λ
ex = 275 nm; and (b) λ
ex = 295 nm. Before the determination of the second derivative spectra, the fluorescence spectra of gBSA was normalized to respective maxima fluorescence spectra of BSA, λ
em = 337 nm and λ
em = 339 nm for λ
ex = 275 nm and λ
ex = 295 nm, respectively.
The changes in the second derivative spectra in the wavelength range 370 nm–400 nm and the range below 320 nm indicate the structure reorganization around tryptophanyl (Trp-214 and Trp-135) and tyrosyl residues (Tyr) in serum albumin, respectively [
29,
30]. At λ
ex = 275 nm, the second derivative fluorescence spectra of non-glycated bovine serum albumin exhibits two peaks maximum (at wavelengths 288 nm and 307 nm) and marked valleys at 295 nm and 328 nm, while the second derivative spectra of normalized glycated bovine serum albumin (normgBSA) has four peaks maximum (288 nm, 305 nm, 315 nm and 328 nm) and four marked valleys (299 nm, 311 nm, 320 nm and 337 nm) (
Figure 4a). At λ
ex = 295 nm the second derivative fluorescence spectra of BSA exhibits only one peak maximum (at wavelengths 304 nm) with marked valley at 329 nm and small shoulder from the red side of the peak, while the second derivative spectra of normgBSA has two peaks maximum (304 nm and 329 nm), two valleys (322 nm and 336 nm) and also one shoulder from the red side of the peak at wavelengths 304 nm (
Figure 4b). Glycation of BSA causes changes in the second derivative fluorescence spectrum at both λ
ex = 275 nm and λ
ex = 295 nm (
Figure 4a,b) indicating that Trp-214, Trp-135 and nineteen tyrosyl residues (Tyr) in bovine serum albumin participate in the process of glycation. The values of the second derivative fluorescence spectra for BSA and normgBSA were determined by peak to peak method (the distance from the positive peak maximum to the negative peak minimum) as an empirical parameter
H (relative peak composition) defined by Mozo-Villarias [
29,
30]. The empirical parameter
H is an indicator of the polarity changes around aromatic residues of proteins. The values of parameter
H are collected in
Table 2.
Glycation of BSA causes the increase in polarity around the tryptophanyl (Trp-214 and Trp-135) (λ
ex = 275 nm) and tyrosyl (Tyr) residues (λ
ex = 295 nm) that was shown as an increase in the value of parameter
H (
Table 2). Kumar et al. compared the second derivative fluorescence spectra of somatostatin and serum albumin incubated with guanidine hydrochloride. They suggested that first derivative of a signal from the blue-wave spectrum is more sensitive to structural changes [
29]. Qualitative analysis indicated that glycation of bovine serum albumin changes (reorganizes) the structure of macromolecule around both tryptophanyl and tyrosyl residues. The main glycation sites are located in the vicinity of known drug binding sites. Wa et al. have characterized the glycation adducts on human serum albumin by matrix-assisted laser desorption/ionization time-of-flight mass spectrometry and found numerous residues of lysines, Lys-12, Lys-51, Lys-199, Lys-205, Lys-439 and Lys-538, to be modified through the formation of fructose-lysine [
11]. In addition, some lysine residues of BSA glycated in vitro with glucose have been shown to be involved in the formation of versperlysine products [
31]. Other studies revealed Lys-524 as the major glycation site in BSA [
32,
33] and also Lys-275, Lys-232 and Lys-396-constituted amino acids susceptible to glycated [
32,
34]. Though less abundant in the amino acid sequence of albumin than lysine residues (23 for 59 lysine residues), arginine residues can also be involved in glycation. The tryptic peptide mapping of modified human serum albumin, in vitro and in vivo, by methylglyoxal, indicated the major modification at Arg-410, which is located in drug binding site II. Minor arginine sites involved in glycation, such as Arg-114, Arg-160, Arg-186, Arg-218 and Arg-428, have also been identified [
10]. It is noteworthy that the positions and architecture of fatty acids (FAs) binding sites on serum albumin have been also identified for these sites, in subdomains IB, IIIA, and IIIB [
35,
36]. Native bovine albumin exhibits two main binding sites for lipoic acid, an effective antioxidant, whereas methylglyoxal-modified protein shows three sequential binding sites with a reduction in affinity for the main one [
37] therefore the influence of FAs on glycation process should be taken into account. Yamazaki et al. described strong affinity of fatty acids for albumin via several lysine and arginine sites. They observed the slight effect of oleate, laurate, caproate and linoleate binding on the glycation process [
38]. Binding of fatty acids to albumin has an influence on its structure also in terms of binding parameters. Bojko et al. have analysed the influence of fatty acids and on drug-albumin binding [
39]. They investigated that the presence of myristic acid alters the affinity between bovine serum albumin and drug.
2.2. Effect of Glycation on Binding of Tolbutamide to Bovine Serum Albumin
Hereby, we used BSA in order to prove the glycation of albumin mainly in subdomain IB. The complex between tolbutamide (TB) and non-glycated (BSA) and glycated (gBSA) bovine serum albumin has been studied using the fluorescence emission spectra recorded at the excitation wavelengths λ
ex = 275 nm and λ
ex = 295 nm, respectively. The wavelength λ
ex = 295 nm causes the excitation of two tryptophanyl residues in BSA, i.e., Trp-135 (localized in subdomain IB) and Trp-214 (localized in subdomain IIA) while wavelength λ
ex = 275 nm excites not only tryptophanyl residues (Trp-135 and Trp-214), but also tyrosine residues mainly located in subdomains IB (Tyr-138, Tyr-140, Tyr-148, Tyr-150, Tyr-156, Tyr-157, and Tyr-161), IIA (Tyr-263) and IIIA (Tyr-401, Tyr-411, Tyr-452, and Tyr-497) [
19]. Consequently, both types of fluorophores and/or their microenvironment may participate in ligand-albumin interaction. Intramolecular interactions of TB with albumin (BSA and gBSA) were studied using the quenching fluorescence method.
Based on the Stern-Volmer plot (Equation (1)), the mode of the interaction between TB and both BSA and gBSA was analyzed (
Figure 5).
dependence on TB concentration when only tryptophanyl (Trp-135 and Trp-214) residues have been excited (
Figure 5b) displays negative deviation from linearity for both BSA and gBSA. It does mean that there are fluorophores accessible to the quencher (TB) with different value of the Stern-Volmer constant K
SV [
40]. Trp-135 and Trp-214 are located in different microenvironment therefore the accessibility of TB to these fluorophores also is not the same. Negative deviation of Stern-Volmer plot from linearity allows to estimate that subdomain IIA and IB are involved in TB-serum albumin interaction. However, the linear course of Stern-Volmer plot obtained for gBSA (λ
ex = 275 nm) results from the weak quenching of gBSA (~4%) by TB (
Figure 5a).
Stern-Volmer curve modified by Lehrer (Equation (2)) was plotted to determine the effective Stern-Volmer constants K
SV (L∙mol
−1) for tolbutamide complexes with BSA and gBSA. The data have been collected in
Table 3. The K
SV constant determined for TB-gBSA system is higher than that for the TB-BSA system excited at 295 nm for each applied temperature. This phenomenon shows that fructose glycation of BSA changes the fluorophores conformation and makes the tolbutamide diffusion to Trp-135 and Trp-214 easier. The K
SV constant determined for TB-gBSA system is smaller than for the TB-BSA system excited at 275 nm in 309 K, 311 K temperature and higher in 313 K. This effect is difficult to explain because using λ
ex = 275 nm excitation wavelength excites 22 fluorophores of BSA (20 Tyr and 2 Trp residues).
The analysis of TB interactions with BSA and gBSA excited at λ
ex = 275 nm (
Figure 6a) and λ
ex = 295 nm (
Figure 6b) have been presented with the use of a binding isotherm.
Nonlinear shape of the binding isotherms of BSA and gBSA at both exciting wavelengths (λ
ex = 275 nm and λ
ex = 295 nm) in the presence of tolbutamide (
Figure 6a,b) indicated mixed (specific and nonspecific) nature of TB interaction with both albumins [
41].
The quantitative analysis of binding parameters describes the affinity of TB to the binding site. The Klotz Equation (3) allows determining the association constants K
a (L∙mol
−1) and the number of binding sites (
) for the independent classes of TB binding sites in non-glycated and glycated albumin molecule. Only one class of binding site was found for TB in both BSA and gBSA structures (λ
ex = 275 nm and λ
ex = 295 nm) with the following K
a constants values have been calculated: K
a(275 nm) (7.88 ± 0.11) × 10
4 L∙mol
−1, K
a(295 nm) (1.64 ± 0.04) × 10
4 L∙mol
−1 and K
a(275 nm) (0.42 ± 0.02) × 10
4 L∙mol
−1, K
a(295 nm) (6.61 ± 0.08) × 10
4 L∙mol
−1, respectively. Based on the obtained association constants we can conclude that glycation with fructose changes TB affinity towards bovine albumin. TB has a greater affinity for subdomain IIA and/or IB of glycated albumin than non-glycated protein (
Table 3, λ
ex = 295 nm). In addition, TB forms stronger complex with non-glycated than glycated albumin (
Table 3, λ
ex = 275 nm). The differences between the constants calculated for 275 nm and 295 nm are the results of the participation of different excited fluorophores, originating from different subdomains, in the formation of complexes. Moreover, the discrepancy in the values of binding constants calculated at λ
ex = 275 nm and λ
ex = 295 nm may be explained by greater participation of tyrosyl (λ
ex = 275 nm) or only tryptophanyl (λ
ex = 295 nm) residues in the complex with tolbutamide. Binding of tolbutamide to non-glycated and glycated serum albumin has been also analyzed by Joseph et al. [
42]. The main goal of their study was to examine the binding of tolbutamide to human serum albumin (HSA) at various levels of glycation by the use of high-performance affinity chromatography. They investigated that the binding of tolbutamide to all of tested preparates of glycated human serum albumin (gHSA) could be described by a two site model involving both strong and weak affinity interactions. Joseph et al. reported that the association equilibrium constants
for TB at high affinity sites on gHSA were in the range of 0.8–1.2 × 10
5 L∙mol
−1 and increased by 1.4-fold in going from normal HSA to mildly gHSA. Moreover, the K
a for TB increased by 1.1- to 1.4-fold and by 1.2- to 1.3-fold in going from normal HSA to the gHSA samples at Sudow’s site II and I, respectively. It is noteworthy that we have registered the K
a values of the same order (10
4) despite the use of technique having different fundamentals like fluorescence spectroscopy. Michalcová and Glatz studied the binding of the first generation of sulfonylurea antidiabetics to HSA and its glycated form by capillary electrophoresis-frontal analysis [
43]. They reported that the binding constants decreased in the sequence acetohexamide > tolbutamide > chlorpropamide > carbutamide both for normal and glycated human serum albumins, with glycated giving lower values.
2.3. The Effect of Temperature on Stability of the TB-BSA and TB-gBSA Complexes
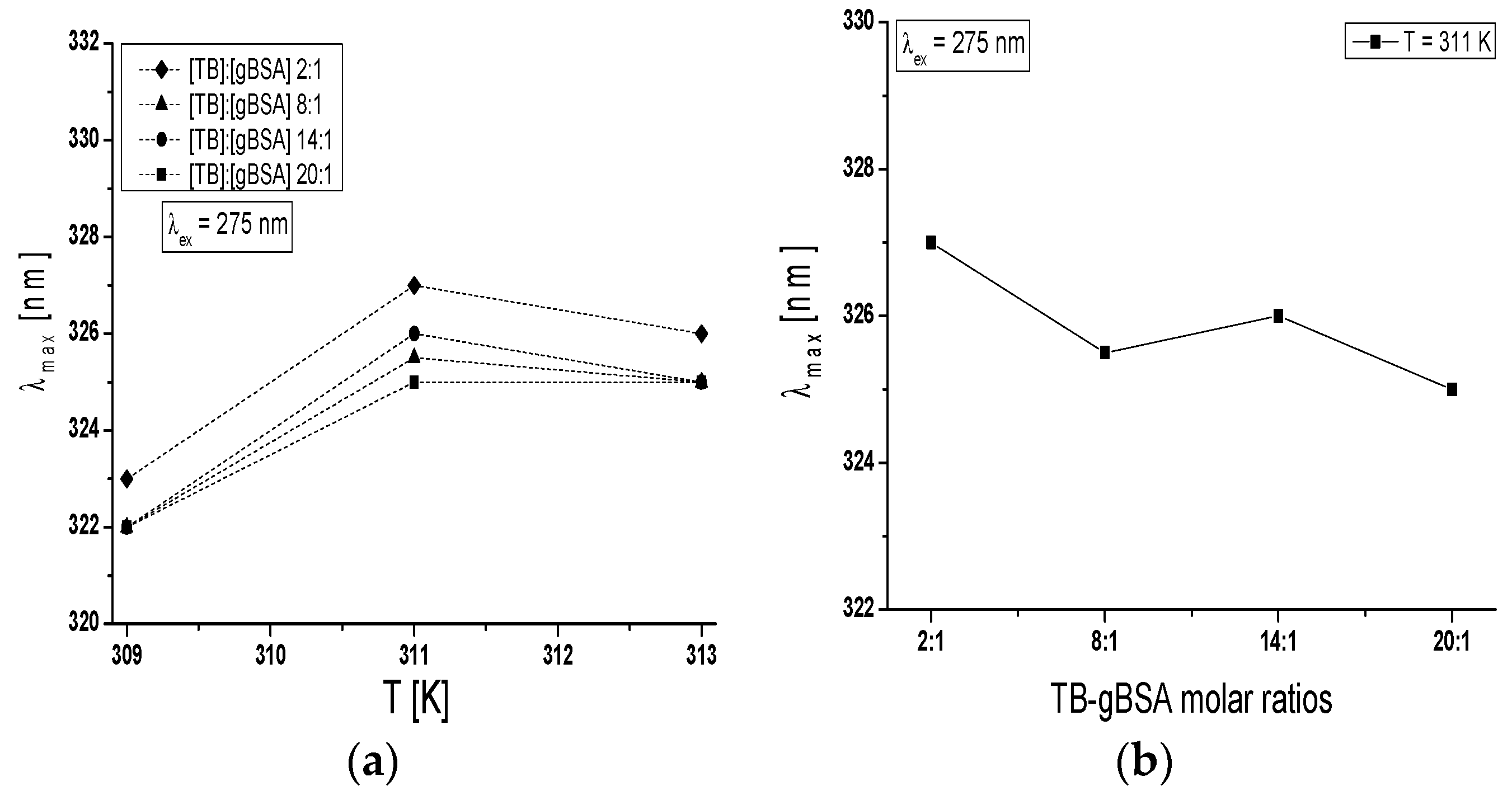
Maximum wavelength λ
max of TB-BSA spectrum at TB:BSA = 2:1; 8:1; 14:1 and 20:1 molar ratios does not depend on temperature in the range between 309 K and 313 K (data not shown). However, the rise of temperature from 309 K to 313 K caused the red-shift of TB-gBSA system maximum wavelength at all studied TB:gBSA molar ratios by 3–4 nm at 275 nm wavelength excitation, when all Tyr and Trp residues potentially fluoresce (
Figure 7a) and by 2–3 nm towards blue-shift at 295 nm wavelength excitation, when only Trp residues fluoresce (data not shown). The red-shift of maximum wavelength λ
max of TB-gBSA spectrum indicates the increase in fluorophore environment polarity of bovine serum albumin, while the blue-shift of λ
max means that amino acid residues (Trps) are located in a more hydrophobic environment and are less exposed to the solvent due to the glycation. Changes in polarity around the fluorophore molecule with the increase in temperature range may be due to the destabilization of the tertiary structure of albumin. Moreover, the formation of TB-BSA and TB-gBSA complexes may affect the microenvironment of binding sites. This could result in the change of non-glycated and glycated bovine albumin sensitivity to temperature. Furthermore, it is interesting to note that at T = 311 K the maximum wavelength (λ
max) is different for the majority of molar ratios and decreases with the increase of TB:gBSA molar ratio (
Figure 7b).
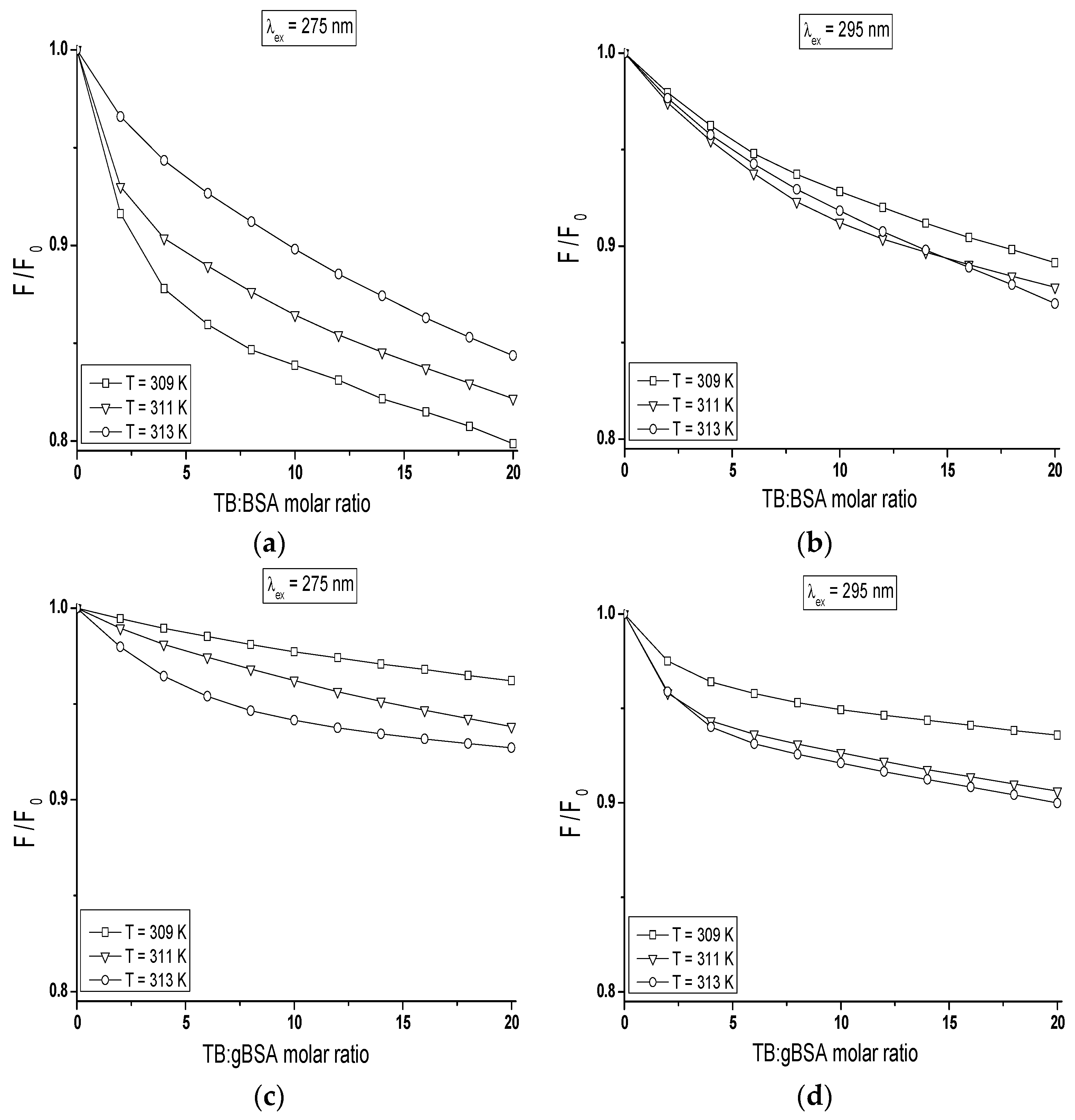
The quenching curves of BSA and gBSA fluorescence excited at λ
ex = 275 nm and λ
ex = 295 nm in the presence of tolbutamide at three different temperatures have been presented in
Figure 8.
The course of these curves is temperature dependent and confirms the ability of tolbutamide molecule to receive the energy from excited fluorophores. As it is shown in
Figure 8, the fluorescence of BSA and gBSA excited at λ
ex = 275 nm is quenched by tolbutamide by 20.1%, 17.8%, 15.6% (
Figure 8a) and 3.8%, 6.2%, 7.3% (
Figure 8c) for 309 K, 311 K and 313 K, respectively (TB:albumin 20:1 molar ratio). At the same molar ratio, the fluorescence of these two albumins excited at λ
ex = 295 nm is quenched by tolbutamide with a lower for TB:BSA and higher for TB:gBSA efficiency, i.e., 10.9%, 12.1%, and 13% (
Figure 8b); and 6.4%, 9.4%, and 10% (
Figure 8d), respectively. This quenching phenomenon in the temperature range between 309 K and 313 K, more significant for BSA than gBSA (λ
ex = 275 nm and λ
ex = 295 nm), shows that the energy transfer from non-glycated to tolbutamide is easier than from glycated albumin and confirms the fact of TB-BSA complex formation in close proximity to excited fluorophores. The comparison of the quenching fluorescence curves of both proteins excited at λ
ex = 275 nm and λ
ex = 295 nm at all applied temperatures shows that fluorescence of BSA is quenched by TB to a greater extend at λ
ex = 275 nm than λ
ex = 295 nm. For glycated albumin the opposite effect was observed. The fluorescence of gBSA is quenched by TB to a greater extend at λ
ex = 295 nm than λ
ex = 275 nm. As these wavelengths excite different fluorophores in the albumin, more distinct decrease in fluorescence of protein excited at λ
ex = 295 nm indicates that fructose glycated BSA molecule involves preferentially subdomain IIA and/or IB in binding process of tolbutamide. Moreover, the quenching effect of BSA and gBSA fluorescence in the presence of tolbutamide increases (
Figure 8b–d) while for BSA excited at λ
ex = 275 nm decreases (
Figure 8a) with the increase of temperature from 309 K to 313 K. This effect suggests that structural modification of bovine albumin due to glycation causes the changes in the excited fluorophores environment, mainly in the region of tyrosine residues.
Stern-Volmer analysis is a useful tool for estimation of the accessibility of albumin fluorophores (Trp and Tyr residues) to the drug molecules and for understanding the involved quenching mechanism (dynamic and/or static).
Figure 9a,d presents the Stern-Volmer plots for TB-BSA (
Figure 9a,b) and TB-gBSA (
Figure 9c,d) complexes at λ
ex = 275 nm (
Figure 9a,c) and λ
ex = 295 nm (
Figure 9b,d) excitation wavelengths, respectively.
The course of Stern-Volmer curves obtained for BSA and gBSA in the presence of tolbutamide varies depending on the excitation wavelength (λ
ex = 275 nm and λ
ex = 295 nm) in the temperature range between 309 K and 313 K. It is noteworthy that below TB concentration 3 × 10
−5 mol∙L
−1 at all temperatures and at 295 nm excitation wavelength the Stern-Volmer plot showed a linear relationship between quenching effect
of non-glycated BSA and TB concentration (
Figure 9b). According to Efting and Ghiron’s theory, it means that Trp-214 and Trp-135 excited state is quenched both by the collisional mechanism and complex formation [
40]. A negative deviation from a straight course of Stern-Volmer curves were found for TB-glycated BSA complexes at all applied TB concentration range (
Figure 9d). The negative deviation in the Stern-Volmer curve course above TB:BSA and TB:gBSA 4:1 molar ratio points out that both Trp-135 and Trp-214 microenvironment interacts with TB while below TB:BSA and TB:gBSA 4:1 molar ratio a linear course of Stern-Volmer plots (λ
ex = 275 nm, T = 309 K and T = 313 K) has been registered (
Figure 9a). As for the molar ratio TB:gBSA <6:1 molar ratio a linear course of Stern-Volmer plot at the temperature T = 311 K was observed (
Figure 9c). Akbay et al. explained that, during dynamic quenching, the ligand may penetrate the surrounding of albumin molecule and then the fluorescence quenching is caused by the collision of the quencher molecule and fluorophores [
44]. However static quenching proves that all excited amino acids residues are equally available for ligand or alternatively selected amino acid residue dominates the fluorescence [
45]. The fluorescence quenching process depends on the nature of the fluorophores and the quencher molecule. Our study suggests that glycation of BSA by fructose influences on the tertiary structure of albumin and modifies the fluorescence quenching mechanism of the TB-BSA complex.
Due to general deviation of Stern-Volmer plot from linearity the quenching process was analyzed according to the Stern-Volmer equation modified by Lehrer (Equation (2)) and can be determined by the value of quenching rate constants k
q (L∙mol
−1∙s
−1) (Equation (1)). The Stern-Volmer constant K
SV (L∙mol
−1) obtained based on the Equation (2) allows to estimate the distance between the excited fluorophore/s and ligand molecule. Reduction in K
SV value means the increase in a distance between albumin tryptophanyl and tyrosyl groups and ligand molecule. The Stern-Volmer constant values calculated for TB-BSA and TB-gBSA system at T = 309 K, T = 311 K and T = 313 K temperatures have been collected in
Table 3. The decrease in K
SV values with the rise of the temperature (from 309 K to 313 K) shows the weakening of tolbutamide accessibility to the tryptophanyl and tyrosyl residues of non-glycated and glycated bovine serum albumin (except TB-gBSA at λ
ex = 275 nm). This phenomenon suggests the changes in the protein structure due to the temperature effect, more significant observed at λ
ex = 275 nm than at λ
ex = 295 nm. Considering the Stern-Volmer constant K
SV and the fluorescence lifetime of albumins τ
0 in the absence of quencher of the order 10
4 L∙mol
−1 and 10
−9 s, respectively, the quenching rate constant k
q was of the order 10
13 L∙mol
−1∙s
−1, which largely exceeds the accepted limit of the rate constant of the diffusional quenching implying biopolymers k
q = 2 × 10
10 L∙mol
−1∙s
−1 [
46]. This observation supports the fact that the experimental quenching of BSA and gBSA fluorescence is due to a predominantly static process resulting in the formation of TB-BSA and TB-gBSA complex.
The association constants K
a (L∙mol
−1) and the number of binding sites
for the independent class of drug binding sites in the bovine albumin have been determined using Klotz Equation (3). The binding parameters (K
a and
n) for TB-BSA and TB-gBSA (λ
ex = 275 nm, λ
ex = 295 nm) system for specified temperatures were determined and collected in
Table 3. Due to the value of determination coefficients larger than 0.99 we concluded that that the interaction between TB and BSA/gBSA is consistent with the site-binding model defined by Klotz Equation (3). The linear Klotz plots (data not shown) at both λ
ex = 275 nm and λ
ex = 295 nm, at all analyzed temperatures (T = 309 K, T = 311 K and T = 313 K) show that TB occupies in non-glycated albumin only one class of binding sites in BSA subdomain IB or IIA. We observed that glycation of bovine serum albumin did not change the shape of Klotz plots. It means that TB binds to structural altered albumin (glycated BSA) first class of binding sites, similarly as to non-glycated. Based on the variations in association constants K
a values (
Table 3), the changes in BSA binding ability towards tolbutamide under the glycation by fructose process have been confirmed. Subdomain IIA and IB of fructose glycated BSA has enhanced affinity towards tolbutamide at all applied temperatures than non-glycated BSA. Mentioned subdomains of fructose glycated BSA (λ
ex = 275 nm) diminish affinity for tolbutamide. With the increase of temperature, the association constants
values drop for TB-BSA (λ
ex = 275 nm and λ
ex = 295 nm) and TB-gBSA (λ
ex = 295 nm) complexes and this phenomenon is typical for complex formation. For the TB-gBSA system excited at λ
ex = 275 nm, not only K
a but also K
SV values increase with the increase of temperature. This phenomenon suggests that collisional quenching of gBSA fluorescence dominates the fluorescence quenching than complex formation.