Anti-Cancer Activity of Resveratrol and Derivatives Produced by Grapevine Cell Suspensions in a 14 L Stirred Bioreactor
Abstract
:1. Introduction
2. Results and Discussion
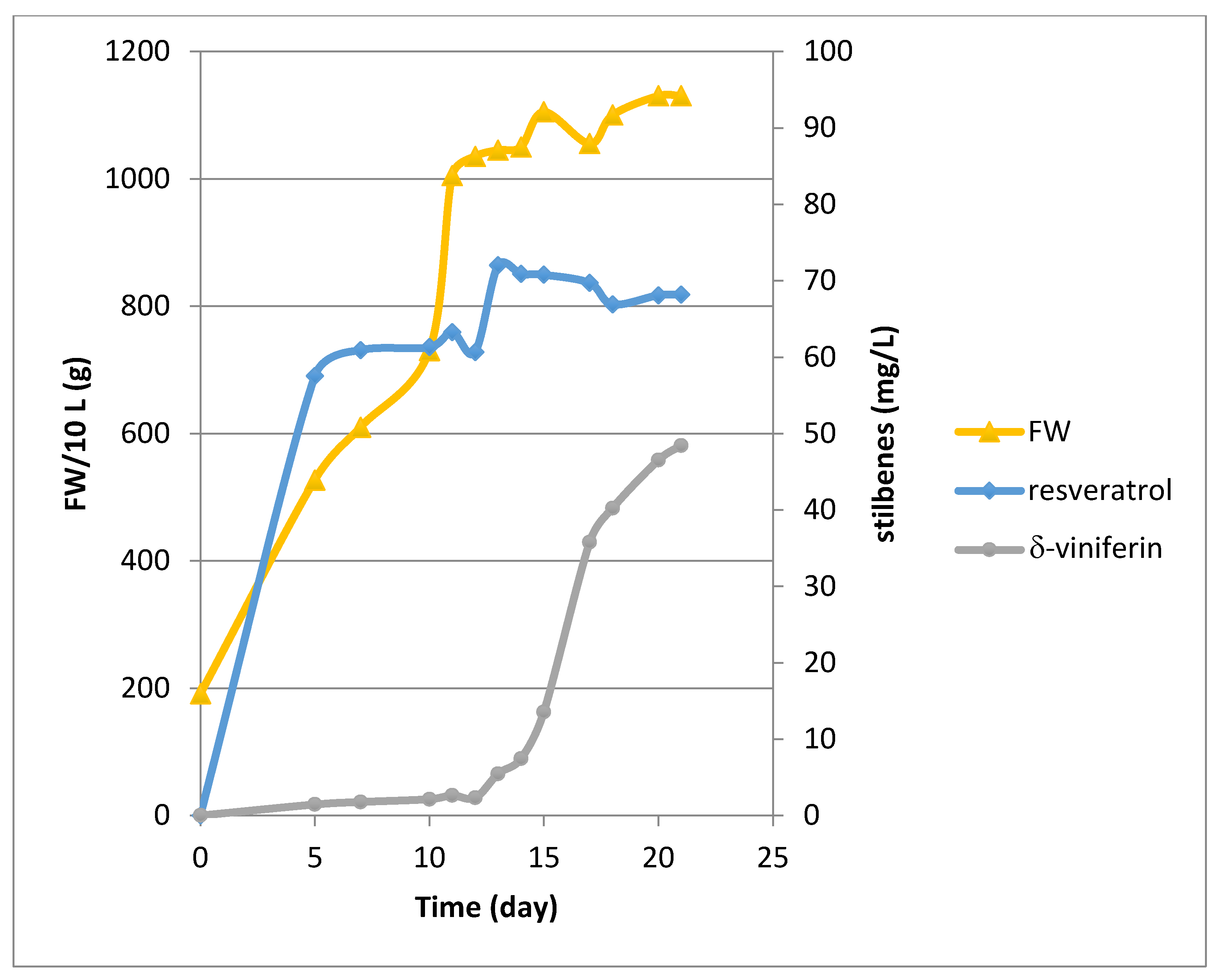
2.1. Growth and Bioproduction of Phytostilbenes in the 14 L Bioreactor
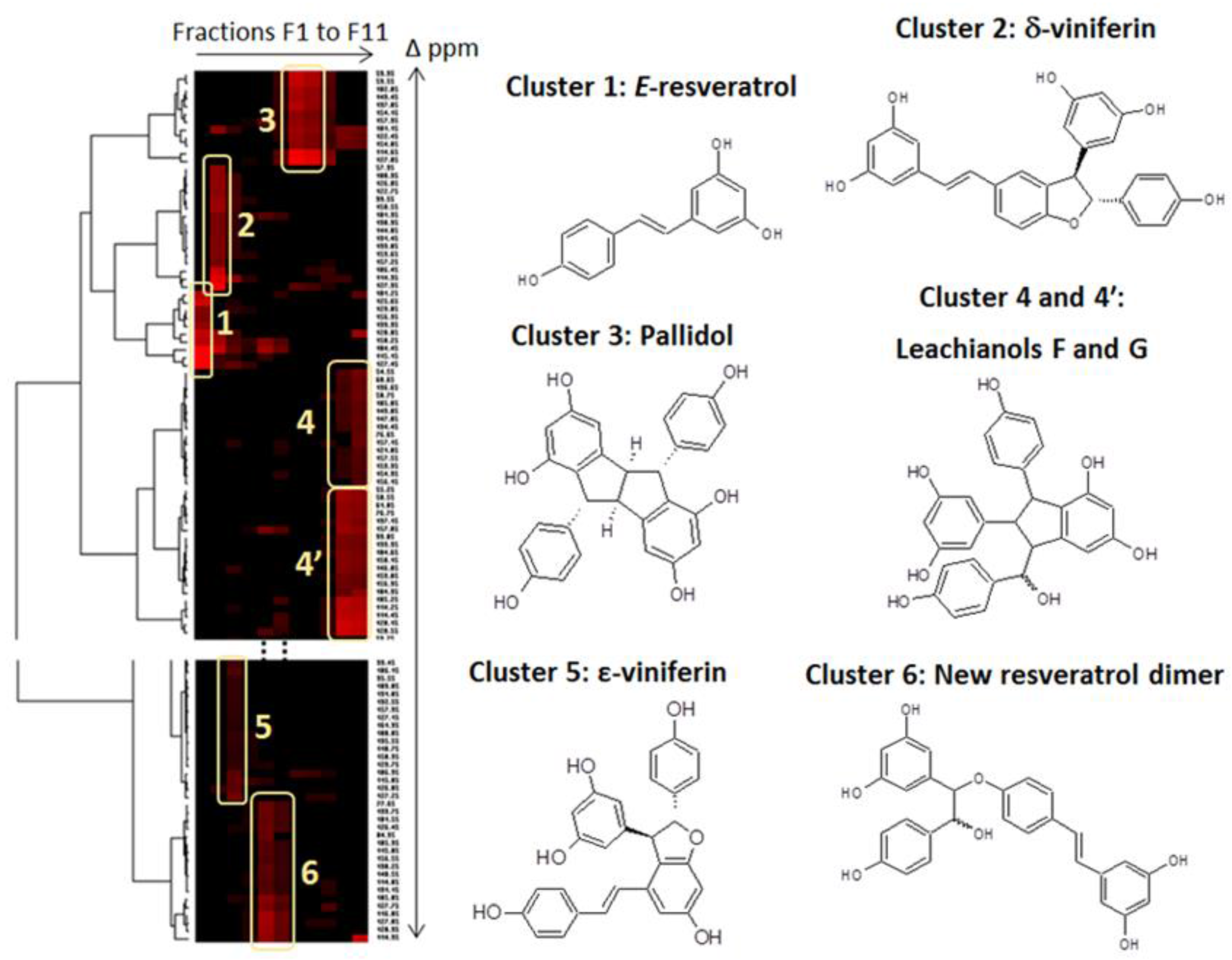
2.2. CPC Separation and NMR Identification of Resveratrol and Its Bio-Produced Derivatives
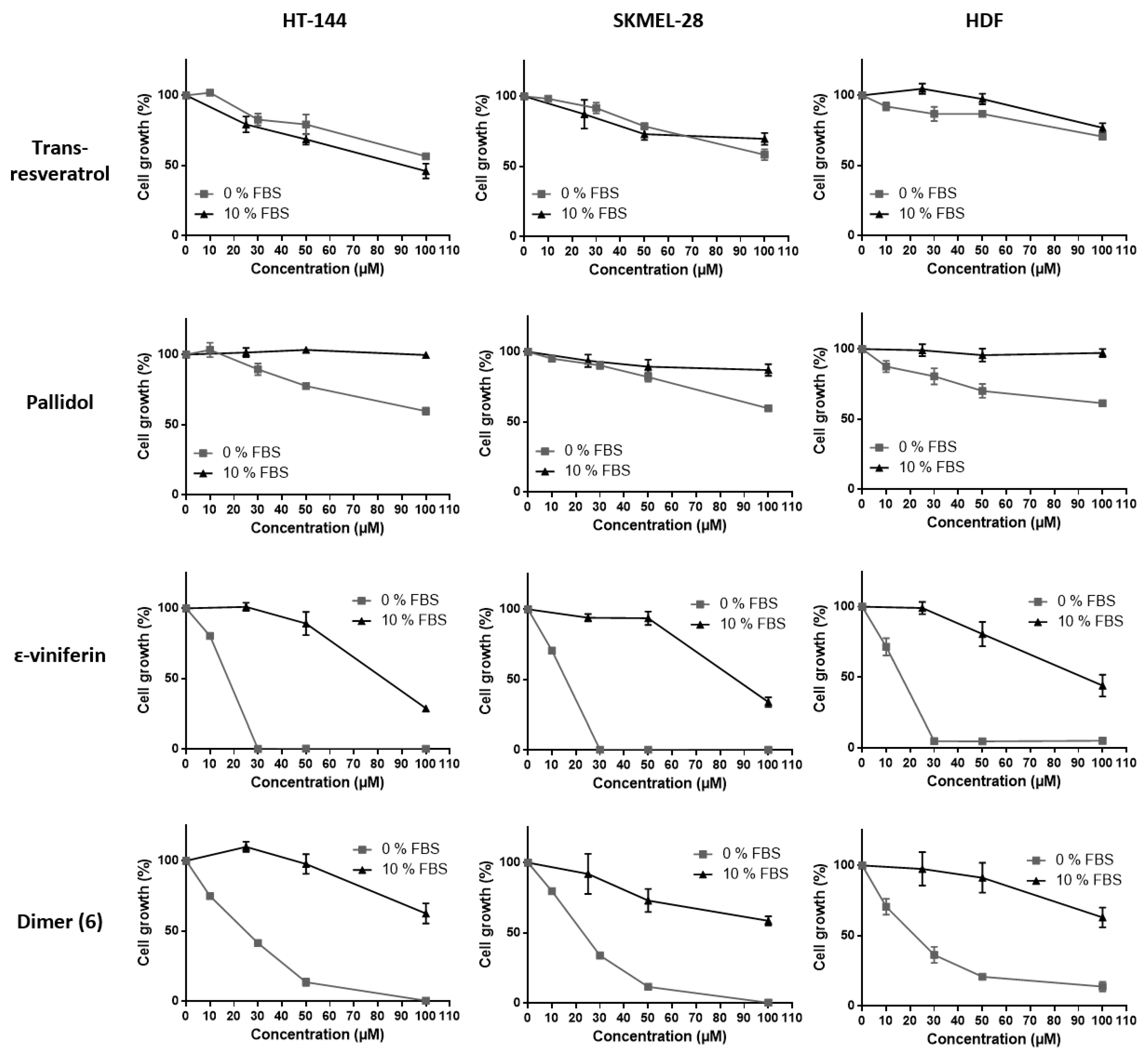
2.3. Effects of Resveratrol and Bio-Produced Stilbenes on Cancer Lines and Healthy Human Dermal Fibroblasts
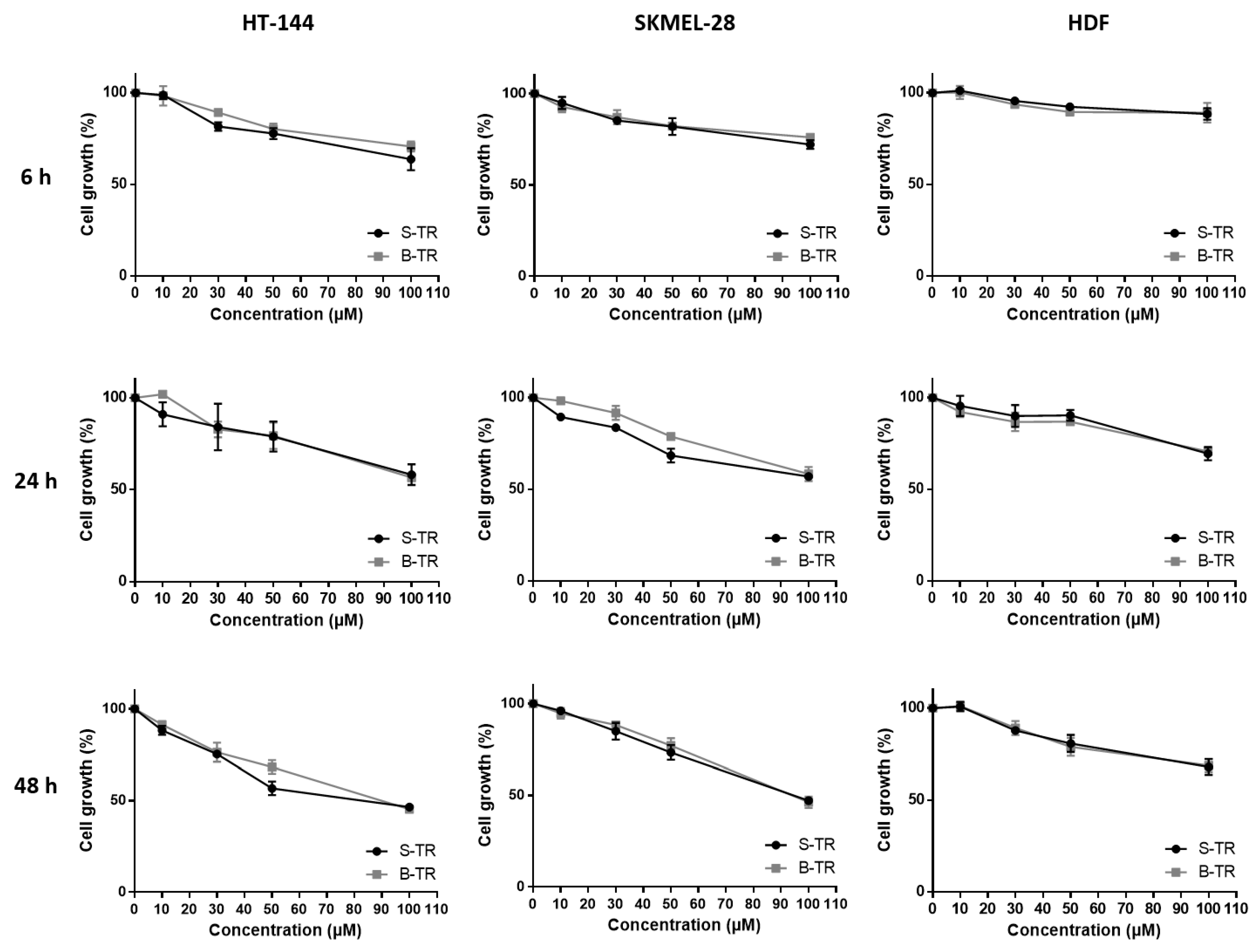
2.3.1. Comparison of the Inhibition Activity of Bio-Produced and Synthetic Resveratrol on Cell Viability
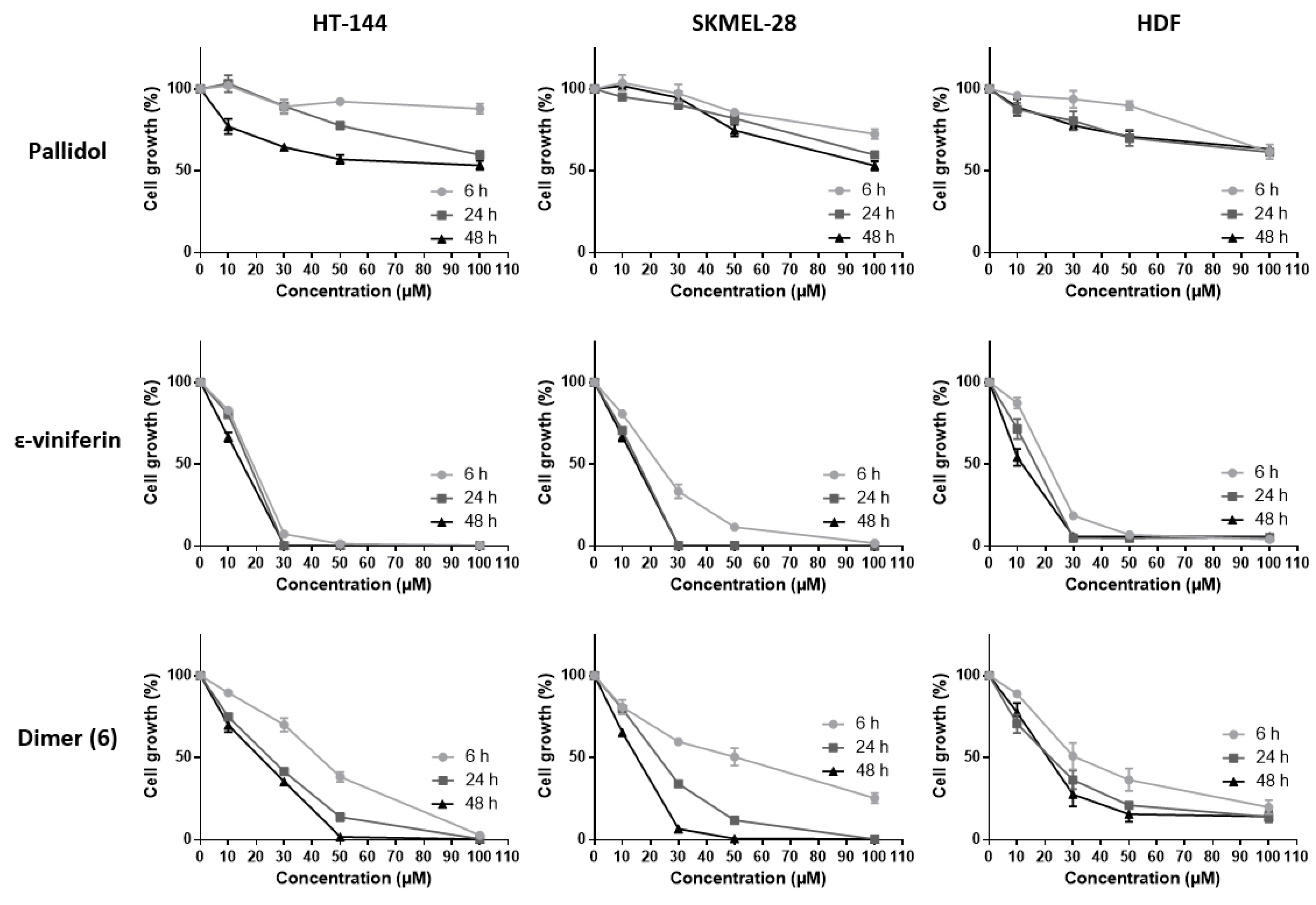
2.3.2. Inhibition of Cell Viability by Bio-Produced Resveratrol Oligomers
2.3.3. Effect of Fetal Bovine Serum on the Biological Activity of the Bio-Produced Stilbenes
3. Materials and Methods
3.1. Chemicals, Reagents and Materials
3.2. Bioreactor Culture and Elicitation Process
3.3. Extraction of Total Stilbenes from the Culture Medium and CPC Fractionation
3.4. NMR Analyses of the CPC Fractions and Identification of the Major Bio-Produced Stilbenes
3.5. UPLC Analyses
3.6. Biological Tests
3.6.1. Cell Cultures
3.6.2. Experimental Treatments
3.6.3. Cell Viability Assay
3.7. Statistical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Delaunois, B.; Cordelier, S.; Conreux, A.; Clément, C.; Jeandet, P. Molecular engineering of resveratrol in plants. Plant Biotechnol. J. 2009, 7, 2–12. [Google Scholar] [CrossRef] [PubMed]
- Jeandet, P.; Delaunois, B.; Conreux, A.; Donnez, D.; Nuzzo, V.; Cordelier, S.; Clément, C.; Courot, E. Biosynthesis, metabolism, molecular engineering, and biological functions of stilbene phytoalexins in plants. BioFactors 2010, 36, 331–341. [Google Scholar] [CrossRef] [PubMed]
- Jeandet, P.; Delaunois, B.; Aziz, A.; Donnez, D.; Vasserot, Y.; Cordelier, S.; Courot, E. Metabolic engineering of yeast and plants for the production of the biologically active hydroxystilbene, resveratrol. J. Biomed. Biotechnol. 2012, 2012, 579089. [Google Scholar] [CrossRef] [PubMed]
- Jeandet, P.; Clément, C.; Courot, E.; Cordelier, S. Modulation of phytoalexin biosynthesis in engineered plants for disease resistance. Int. J. Mol. Sci. 2013, 14, 14136–14170. [Google Scholar] [CrossRef] [PubMed]
- Jeandet, P.; Hébrard, C.; Deville, M.-A.; Cordelier, S.; Dorey, S.; Aziz, A.; Crouzet, J. Deciphering the role of phytoalexins in plant-microorganism interactions and human health. Molecules 2014, 19, 18033–18056. [Google Scholar] [CrossRef] [PubMed]
- Bishayee, A.; Barnes, K.F.; Bhatia, D.; Darvesh, A.S.; Carroll, R.T. Resveratrol suppresses oxidative stress and inflammatory response in diethylnitrosamine-initiated rat hepatocarcinogenesis. Cancer Prev. Res. 2010, 3, 753–763. [Google Scholar] [CrossRef] [PubMed]
- Gülçin, İ. Antioxidant properties of resveratrol: A structure-activity insight. Innov. Food Sci. Emerg. Technol. 2010, 11, 210–218. [Google Scholar] [CrossRef]
- Wu, J.M.; Hsieh, T. Resveratrol: A cardioprotective substance. Ann. N. Y. Acad. Sci. 2011, 1215, 16–21. [Google Scholar] [CrossRef] [PubMed]
- Szkudelski, T.; Szkudelska, K. Resveratrol and diabetes: From animal to human studies. Biochim. Biophys. Acta 2015, 1852, 1145–1154. [Google Scholar] [CrossRef] [PubMed]
- Baxter, R.A. Anti-aging properties of resveratrol: Review and report of a potent new antioxidant skin care formulation. J. Cosmet. Dermatol. 2008, 7, 2–7. [Google Scholar] [CrossRef] [PubMed]
- Aluyen, J.K.; Ton, Q.N.; Tran, T.; Yang, A.E.; Gottlieb, H.B.; Bellanger, R.A. Resveratrol: Potential as anticancer agent. J. Diet. Suppl. 2012, 9, 45–56. [Google Scholar] [CrossRef] [PubMed]
- Pezzuto, J.M. Resveratrol as an Inhibitor of Carcinogenesis. Pharm. Biol. 2008, 46, 443–573. [Google Scholar] [CrossRef]
- Fang, J.-G.; Lu, M.; Chen, Z.-H.; Zhu, H.-H.; Li, Y.; Yang, L.; Wu, L.-M.; Liu, Z.-L. Antioxidant effects of resveratrol and its analogues against the free-radical-induced peroxidation of linoleic acid in micelles. Chemistry 2002, 8, 4191–4198. [Google Scholar] [CrossRef]
- Stivala, L.A.; Savio, M.; Carafoli, F.; Perucca, P.; Bianchi, L.; Maga, G.; Forti, L.; Pagnoni, U.M.; Albini, A.; Prosperi, E.; et al. Specific structural determinants are responsible for the antioxidant activity and the cell cycle effects of resveratrol. J. Biol. Chem. 2001, 276, 22586–22594. [Google Scholar] [CrossRef] [PubMed]
- Baur, J.A.; Sinclair, D.A. Therapeutic potential of resveratrol: The in vivo evidence. Nat. Rev. Drug Discov. 2006, 5, 493–506. [Google Scholar] [CrossRef] [PubMed]
- Fulda, S. Resveratrol and derivatives for the prevention and treatment of cancer. Drug Discov. Today 2010, 15, 757–765. [Google Scholar] [CrossRef] [PubMed]
- Piotrowska, H.; Kucinska, M.; Murias, M. Biological activity of piceatannol: Leaving the shadow of resveratrol. Mutat. Res. 2012, 750, 60–82. [Google Scholar] [CrossRef] [PubMed]
- McCormack, D.; McFadden, D. Pterostilbene and cancer: Current review. J. Surg. Res. 2012, 173, e53–e61. [Google Scholar] [CrossRef] [PubMed]
- Morales, M.; Bru, R.; García-Carmona, F.; Ros Barceló, A.; Pedreño, M.A. Effect of dimethyl-β-cyclodextrins on resveratrol metabolism in Gamay grapevine cell cultures before and after inoculation with shape Xylophilus ampelinus. Plant Cell Tissue Org. Cult. 1998, 53, 179–187. [Google Scholar] [CrossRef]
- Bru, R.; Sellés, S.; Casado-Vela, J.; Belchí-Navarro, S.; Pedreño, M.A. Modified cyclodextrins are chemically defined glucan inducers of defense responses in grapevine cell cultures. J. Agric. Food Chem. 2006, 54, 65–71. [Google Scholar] [CrossRef] [PubMed]
- Lijavetzky, D.; Almagro, L.; Belchi-Navarro, S.; Martinez-Zapater, J.M.; Bru, R.; Pedreno, M.A. Synergistic effect of methyljasmonate and cyclodextrin on stilbene biosynthesis pathway gene expression and resveratrol production in Monastrell grapevine cell cultures. BMC Res. Notes 2008, 1, 132. [Google Scholar] [CrossRef] [PubMed]
- Jeandet, P.; Clément, C.; Courot, E. Resveratrol production at large scale using plant cell suspensions. Eng. Life Sci. 2014, 14, 622–632. [Google Scholar] [CrossRef]
- Jeandet, P.; Clément, C.; Tisserant, L.-P.; Crouzet, J.; Courot, E. Use of grapevine cell cultures for the production of phytostilbenes of cosmetic interest. C. R. Chim. 2016, 19, 1062–1070. [Google Scholar] [CrossRef]
- Hubert, J.; Nuzillard, J.-M.; Purson, S.; Hamzaoui, M.; Borie, N.; Reynaud, R.; Renault, J.-H. Identification of natural metabolites in mixture: A pattern recognition strategy based on 13C-NMR. Anal. Chem. 2014, 86, 2955–2962. [Google Scholar] [CrossRef] [PubMed]
- Tisserant, L.-P.; Hubert, J.; Lequart, M.; Borie, N.; Maurin, N.; Pilard, S.; Jeandet, P.; Aziz, A.; Renault, J.-H.; Nuzillard, J.-M.; et al. 13C-NMR and LC-MS profiling of stilbenes from elicited grapevine hairy root cultures. J. Nat. Prod. 2016, 79, 2846–2855. [Google Scholar] [CrossRef] [PubMed]
- Ponzoni, C.; Beneventi, E.; Cramarossa, M.R.; Raimondi, S.; Trevisi, G.; Pagnoni, U.M.; Riva, S.; Forti, L. Laccase-catalyzed dimerization of hydroxystilbenes. Adv. Synth. Catal. 2007, 349, 1497–1506. [Google Scholar] [CrossRef]
- Marel, A.-K.; Lizard, G.; Izard, J.-C.; Latruffe, N.; Delmas, D. Inhibitory effects of trans-resveratrol analogs molecules on the proliferation and the cell cycle progression of human colon tumoral cells. Mol. Nutr. Food Res. 2008, 52, 538–548. [Google Scholar] [CrossRef] [PubMed]
- Vingtdeux, V.; Giliberto, L.; Zhao, H.; Chandakkar, P.; Wu, Q.; Simon, J.E.; Janle, E.M.; Lobo, J.; Ferruzzi, M.G.; Davies, P.; et al. AMP-activated protein kinase signaling activation by resveratrol modulates amyloid-β peptide metabolism. J. Biol. Chem. 2010, 285, 9100–9113. [Google Scholar] [CrossRef] [PubMed]
- Aziz, M.H.; Nihal, M.; Fu, V.X.; Jarrard, D.F.; Ahmad, N. Resveratrol-caused apoptosis of human prostate carcinoma LNCaP cells is mediated via modulation of phosphatidylinositol 3′-kinase/Akt pathway and Bcl-2 family proteins. Mol. Cancer Ther. 2006, 5, 1335–1341. [Google Scholar] [CrossRef] [PubMed]
- Olas, B.; Wachowicz, B.; Majsterek, I.; Blasiak, J. Resveratrol may reduce oxidative stress induced by platinum compounds in human plasma, blood platelets and lymphocytes. Anticancer Drugs 2005, 16, 659–665. [Google Scholar] [CrossRef] [PubMed]
- Sebastià, N.; Montoro, A.; Montoro, A.; Almonacid, M.; Villaescusa, J.I.; Cervera, J.; Such, E.; Silla, M.A.; Soriano, J.M. Assessment in vitro of radioprotective efficacy of curcumin and resveratrol. Radiat. Meas. 2011, 46, 962–966. [Google Scholar] [CrossRef]
- Chowdhury, S.A.; Kishino, K.; Satoh, R.; Hashimoto, K.; Kikuchi, H.; Nishikawa, H.; Shirataki, Y.; Sakagami, H. Tumor-specificity and apoptosis-inducing activity of stilbenes and flavonoids. Anticancer Res. 2005, 25, 2055–2063. [Google Scholar] [PubMed]
- González-Sarrías, A.; Gromek, S.; Niesen, D.; Seeram, N.P.; Henry, G.E. Resveratrol oligomers isolated from Carex species inhibit growth of human colon tumorigenic cells mediated by cell cycle arrest. J. Agric. Food Chem. 2011, 59, 8632–8638. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; He, C.; Ran, R.; Zhang, D.; Li, D.; Xiao, P.-G.; Altman, E. The resveratrol oligomers, cis- and trans-gnetin H, from Paeonia suffruticosa seeds inhibit the growth of several human cancer cell lines. J. Ethnopharmacol. 2015, 169, 24–33. [Google Scholar] [CrossRef] [PubMed]
- Muhtadi; Hakim, E.H.; Juliawaty, L.D.; Syah, Y.M.; Achmad, S.A.; Latip, J.; Ghisalberti, E.L. Cytotoxic resveratrol oligomers from the tree bark of Dipterocarpus hasseltii. Fitoterapia 2006, 77, 550–555. [Google Scholar] [CrossRef] [PubMed]
- Barjot, C.; Tournaire, M.; Castagnino, C.; Vigor, C.; Vercauteren, J.; Rossi, J.-F. Evaluation of antitumor effects of two vine stalk oligomers of resveratrol on a panel of lymphoid and myeloid cell lines: Comparison with resveratrol. Life Sci. 2007, 81, 1565–1574. [Google Scholar] [CrossRef] [PubMed]
- Zawawi, N.K.N.A.; Ahmat, N.; Mazatulikhma, M.Z.; Shafiq, R.M.; Wahid, N.H.; Sufian, A.S. Bioactive oligostilbenoids from Shorea maxwelliana King and their chemotaxonomic significance. Nat. Prod. Res. 2013, 27, 1589–1593. [Google Scholar] [CrossRef] [PubMed]
- Rohaiza, S.; Yaacob, W.A.; Din, L.B.; Nazlina, I. Cytotoxic oligostilbenes from Shorea hopeifolia. Afr. J. Pharm. Pharmacol. 2011, 5, 1272–1277. [Google Scholar] [CrossRef]
- Latip, J.; Zain, W.Z.W.M.; Ahmat, N.; Yamin, B.M.; Yusof, N.I.N.; Syah, Y.M.; Achmad, S.A. Cytotoxic oligostilbenoids from Vatica odorata. Aust. J. Basic Appl. Sci. 2011, 5, 113–118. [Google Scholar]
- Delmas, D.; Aires, V.; Limagne, E.; Dutartre, P.; Mazué, F.; Ghiringhelli, F.; Latruffe, N. Transport, stability, and biological activity of resveratrol. Ann. N. Y. Acad. Sci. 2011, 1215, 48–59. [Google Scholar] [CrossRef] [PubMed]
- Bourassa, P.; Kanakis, C.D.; Tarantilis, P.; Pollissiou, M.G.; Tajmir-Riahi, H.A. Resveratrol, genistein, and curcumin bind bovine serum albumin. J. Phys. Chem. B 2010, 114, 3348–3354. [Google Scholar] [CrossRef] [PubMed]
- Gamborg, O.L.; Miller, R.A.; Ojima, K. Nutrient requirements of suspension cultures of soybean root cells. Exp Cell Res. 1968, 50, 151–158. [Google Scholar] [CrossRef]
- Morgan, D.M. Tetrazolium (MTT) assay for cellular viability and activity. Methods Mol. Biol. 1998, 79, 179–183. [Google Scholar] [PubMed]
- Billard, C.; Izard, J.-C.; Roman, V.; Kern, C.; Mathiot, C.; Mentz, F.; Kolb, J.-P. Comparative antiproliferative and apoptotic effects of resveratrol, e-viniferin and vine-shots derived polyphenols (vineatrols) on chronic B lymphocytic leukemia cells and normal human lymphocytes. Leuk. Lymphoma 2002, 43, 1991–2002. [Google Scholar] [CrossRef] [PubMed]
- Colin, D.; Lancon, A.; Delmas, D.; Lizard, G.; Abrossinow, J.; Kahn, E.; Jannin, B.; Latruffe, N. Antiproliferative activities of resveratrol and related compounds in human hepatocyte derived HepG2 cells are associated with biochemical cell disturbance revealed by fluorescence analyses. Biochimie 2008, 90, 1674–1684. [Google Scholar] [CrossRef] [PubMed]
- Giovannelli, L.; Innocenti, M.; Santamaria, A.R.; Bigagli, E.; Pasqua, G.; Mulinacci, N. Antitumoural activity of viniferin-enriched extracts from Vitis vinifera L. cell cultures. Nat. Prod. Res. 2014, 28, 2006–2016. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Samples of the compounds are not available from the authors.
| Stilbene Bioproducts | IC50 (µM) | |||||
|---|---|---|---|---|---|---|
| HT-144 | SKMEL-28 | HDF | ||||
| with FBS | without FBS | with FBS | without FBS | with FBS | without FBS | |
| Trans-resveratrol | 93.32 ± 7.60 | > 100 | > 100 | > 100 | > 100 | > 100 |
| Pallidol | > 100 | > 100 | > 100 | > 100 | > 100 | > 100 |
| ɛ-viniferin | 82.31 ± 3.23 | 17.58 ± 0.09 | 86.62 ± 2.59 | 15.88 ± 0.44 | 91.94 ± 11.34 | 16.41 ± 1.19 |
| Dimer (6) | > 100 | 24.94 ± 0.62 | > 100 | 23.01 ± 0.30 | > 100 | 22.21 ± 2.61 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nivelle, L.; Hubert, J.; Courot, E.; Jeandet, P.; Aziz, A.; Nuzillard, J.-M.; Renault, J.-H.; Clément, C.; Martiny, L.; Delmas, D.; et al. Anti-Cancer Activity of Resveratrol and Derivatives Produced by Grapevine Cell Suspensions in a 14 L Stirred Bioreactor. Molecules 2017, 22, 474. https://doi.org/10.3390/molecules22030474
Nivelle L, Hubert J, Courot E, Jeandet P, Aziz A, Nuzillard J-M, Renault J-H, Clément C, Martiny L, Delmas D, et al. Anti-Cancer Activity of Resveratrol and Derivatives Produced by Grapevine Cell Suspensions in a 14 L Stirred Bioreactor. Molecules. 2017; 22(3):474. https://doi.org/10.3390/molecules22030474
Chicago/Turabian StyleNivelle, Laetitia, Jane Hubert, Eric Courot, Philippe Jeandet, Aziz Aziz, Jean-Marc Nuzillard, Jean-Hugues Renault, Christophe Clément, Laurent Martiny, Dominique Delmas, and et al. 2017. "Anti-Cancer Activity of Resveratrol and Derivatives Produced by Grapevine Cell Suspensions in a 14 L Stirred Bioreactor" Molecules 22, no. 3: 474. https://doi.org/10.3390/molecules22030474








