Alkoxy and Enediyne Derivatives Containing 1,4-Benzoquinone Subunits—Synthesis and Antitumor Activity
Abstract
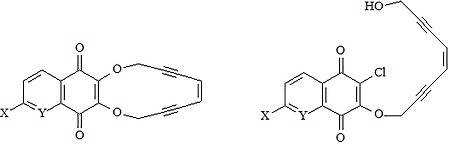
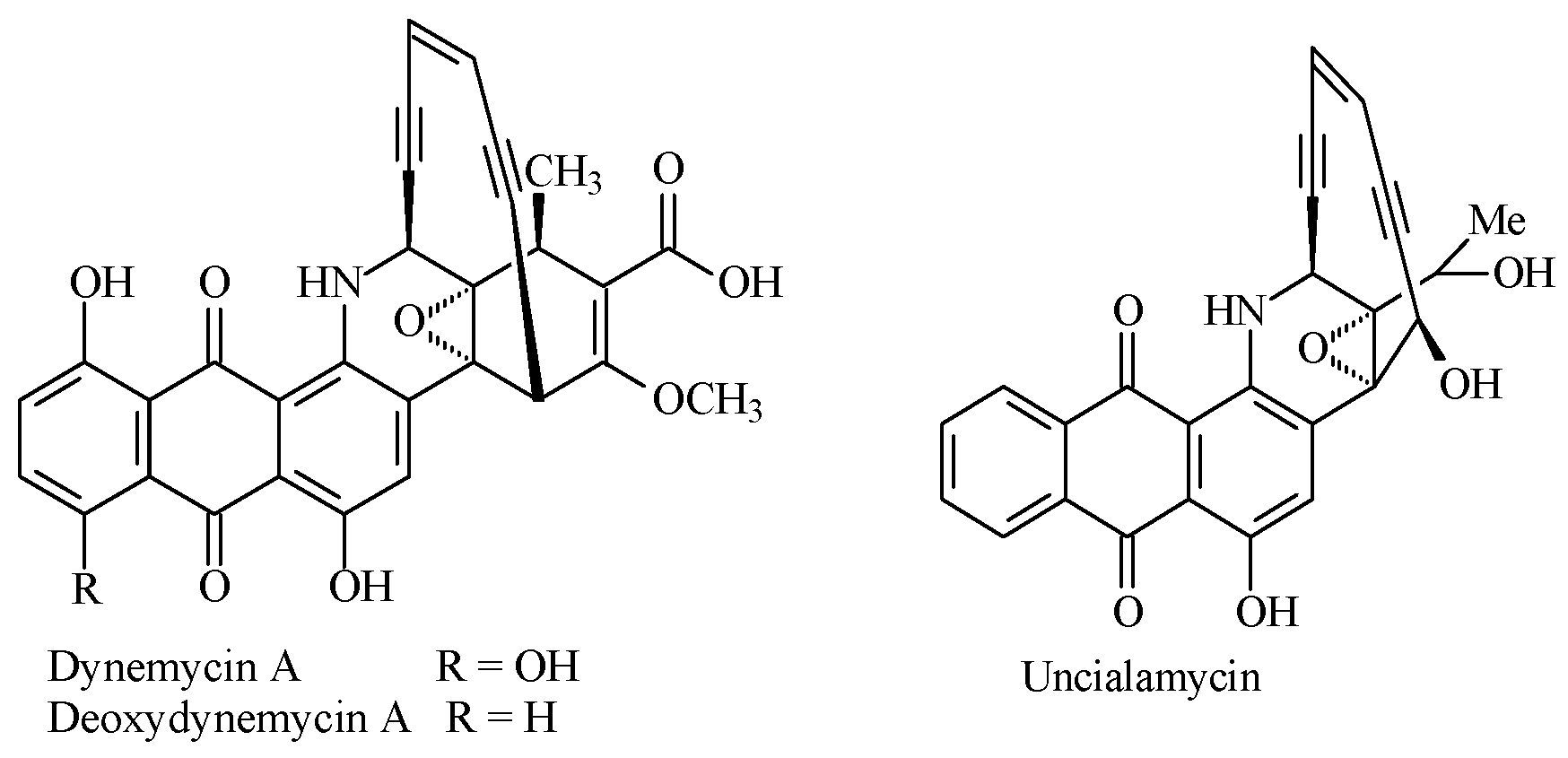
:1. Introduction
2. Results and Discussion
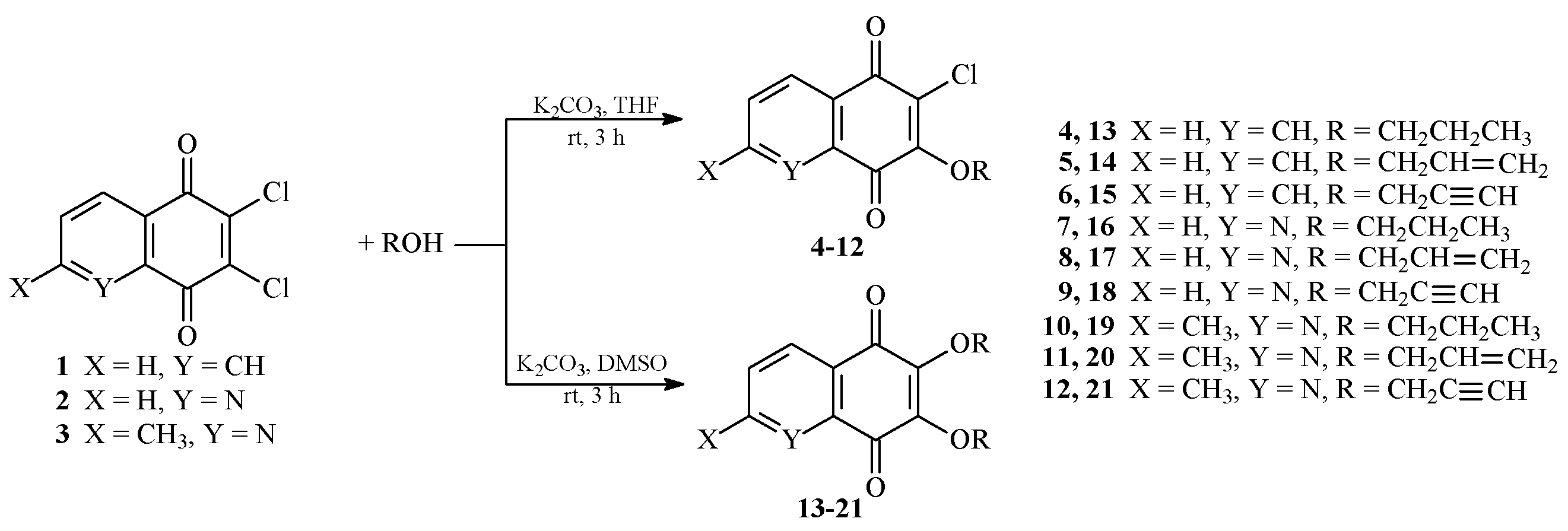
2.1. Chemistry
2.2. Antiproliferative Activity
3. Materials and Methods
3.1. Chemistry
3.1.1. General Procedure for the Synthesis of Monoalkoxy Derivatives 4–12
3.1.2. General Procedure for the Synthesis of Dialkoxy Derivatives 13–21
3.1.3. General Procedure for the Synthesis of Acyclic Enediyne Derivatives 23–25
3.1.4. General Procedure for the Synthesis of Cyclic Enediyne Derivatives 26–28
3.2. Biological Activity
3.2.1. Cell Culture
3.2.2. Effect of Compounds on Number and Viability of Cells
3.2.3. WST-1 Test
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Abraham, I.; Joshi, R.; Pardasani, P.; Pardasani, R. Recent advances in 1,4-benzoquinone chemistry. J. Braz. Chem. Soc. 2011, 22, 385–421. [Google Scholar] [CrossRef]
- Dembitsky, V.M.; Levitsky, D.O.; Gloriozovac, T.A.; Poroikovc, V.V. Acetylenic aquatic anticancer agents and related compounds. Nat. Prod. Commun. 2006, 1, 773–812. [Google Scholar]
- Siddiq, A.; Dembitsky, V.M. Acetylenic anticancer agents. Anti. Canc. Agents Med. Chem. 2008, 8, 132–170. [Google Scholar] [CrossRef]
- Kuklev, D.V.; Dembitsky, V.M. Epoxy acetylenic lipids: Their analogues and derivatives. Prog. Lipid Res. 2014, 56, 67–91. [Google Scholar] [CrossRef] [PubMed]
- Kuklev, D.V.; Domb, A.; Dembitsky, V.M. Bioactive acetylenic metabolites. Phytomedicine 2013, 20, 1145–1159. [Google Scholar] [CrossRef] [PubMed]
- Colegate, S.; Molyneux, R. Bioactive Natural Products. Detection, Isolation, and Structural Determination, 2nd ed.; Taylor & Francis Group: London, UK, 2008; pp. 1–9. [Google Scholar]
- Bhakuni, D.; Rawat, D. Bioactive Marine Natural Products, 1st ed.; Springer: New York, NY, USA, 2005; pp. 81–90. [Google Scholar]
- Babula, P.; Mikelova, R.; Adam, V.; Kizek, R.; Havel, L.; Sladky, Z. Naphthoquinones—Biosynthesis, occurrence and metabolism in plants. Ceska Slov. Farm. 2006, 55, 151–159. [Google Scholar] [PubMed]
- Babula, P.; Adam, V.; Havel, L.; Kizek, R. Naphthoquinones and their pharmacological properties. Ceska Slov. Farm. 2007, 56, 114–120. [Google Scholar] [PubMed]
- Boger, D.; Yasuda, M.; Mitscher, L.; Drake, S.; Kitos, P.; Thompson, S. Streptonigrin and lavendamycin partial structures. Probes for the minimum, potent pharmacophore of streptonigrin, lavendamycin, and synthetic quinoline-5,8-diones. J. Med. Chem. 1987, 30, 1913–1928. [Google Scholar] [CrossRef]
- Lown, J. The mechanism of action of quinone antibiotics. Mol. Cell. Biochem. 1983, 55, 17–40. [Google Scholar] [CrossRef] [PubMed]
- Cheung-Ong, K.; Giaever, G.; Nislow, C. DNA-damaging agents in cancer chemotherapy: Serendipity and chemical biology. Chem. Biol. 2013, 20, 648–659. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, Y.; Lin, L.; Tsai, T. Determination and identification of plumbagin from the roots of Plumbago zeylanica L. by liquid chromatography with tandem mass spectrometry. J. Chromatogr. A 2005, 1083, 141–145. [Google Scholar] [CrossRef] [PubMed]
- Bolzan, A.D.; Bianchi, M.S. Genotoxicity of streptonigrin: A rewiew. Mut. Res. 2001, 488, 25–37. [Google Scholar] [CrossRef]
- Avendaño, C.; Menéndez, J. Medicinal Chemistry of Anticancer Drugs; Elsevier: Amsterdam, The Netherlands, 2008. [Google Scholar]
- Miyata, Y. Hsp90 inhibitor geldanamycin and its derivatives as novel cancer chemotherapeutic agents. Curr. Pharm. Des. 2005, 11, 1131–1138. [Google Scholar] [CrossRef] [PubMed]
- Gredicak, M.; Jerić, I. Enediyne compounds—New promises in anticancer therapy. Acta Pharm. 2007, 57, 133–150. [Google Scholar] [CrossRef] [PubMed]
- Sugiura, Y.; Shiraki, T.; Konishi, M.; Oki, T. DNA intercalation and cleavage of an antitumor antibiotic dynemicin that contains anthracycline and enediyne cores. Proc. Natl. Acad. Sci. USA 1990, 87, 3831–3835. [Google Scholar] [CrossRef] [PubMed]
- Semmelhack, M.F.; Gallagher, J.; Cohen, D. Bioreductive alkylation as a trigger for toxic effects of dynemicin. Tetrahedron Lett. 1990, 31, 1521–1522. [Google Scholar] [CrossRef]
- Sugiura, Y.; Arakawa, T.; Uesugi, M.; Shiraki, T.; Ohkuma, H.; Konishi, M. Reductive and nucleophilic activation products of dynemicin A with methyl thioglycolate. A rational mechanism for DNA cleavage of the thiol-activated dynemicin A. Biochemistry 1991, 30, 2989–2992. [Google Scholar] [CrossRef] [PubMed]
- Davies, J.; Wang, H.; Taylor, T.; Warabi, K.; Huang, X.-H.; Andersen, R.J. Uncialamycin, a new enediyne antibiotic. Org. Lett. 2005, 7, 5233–5236. [Google Scholar] [CrossRef] [PubMed]
- Konishi, M.; Ohkuma, H.; Tsuno, T.; Oki, T.; VanDuyne, G.D.; Clardy, J. Crystal and molecular structure of Dynemicin A: A novel 1,5-diyn-3-ene antitumor antibiotic. J. Am. Chem. Soc. 1990, 112, 3715–3716. [Google Scholar] [CrossRef]
- Magnus, P.; Eisenbeis, S.A.; Fairhurst, R.; Iliadis, T.; Magnus, N.A.; Parry, D. Synthetic and mechanistic studies on the azabicyclo[7.3.1]enediyne core and naphtho[2,3-h]quinoline portions of Dynemicin A. J. Am. Chem. Soc. 1997, 119, 5591–5605. [Google Scholar] [CrossRef]
- Kadela, M.; Jastrzębska, M.; Bębenek, E.; Chrobak, E.; Latocha, M.; Kusz, J.; Książek, M.; Boryczka, S. Synthesis, structure and cytotoxic activity of mono- and dialkoxy derivatives of 5,8-quinolinedione. Molecules 2016, 21, 156. [Google Scholar] [CrossRef] [PubMed]
- Kadela-Tomanek, M.; Jastrzębska, M.; Pawełczak, B.; Bębenek, E.; Chrobak, E.; Latocha, M.; Książek, M.; Kusz, J.; Boryczka, S. Alkynyloxy derivatives of 5,8-quinolinedione: Synthesis, in vitro cytotoxicity studies and computational molecular modeling with NAD(P)H:Quinone oxidoreductase 1. Eur. J. Med. Chem. 2017, 126, 969–982. [Google Scholar] [CrossRef] [PubMed]
- Benites, P.J.; Rawat, D.S.; Zaleski, J.M. Metalloenediynes: Ligand field control of thermal Bergman cyclization reactions. J. Am. Chem. Soc. 2000, 122, 7208–7217. [Google Scholar] [CrossRef]
- Mladenova, M.; Alami, M.; Linstrumelle, G. An efficient stereocontrolled synthesis of di-and triunsaturated carbonyl compounds. Synth. Commun. 2006, 26, 2831–2842. [Google Scholar] [CrossRef]
- Mälkiä, A.; Murtomäki, L.; Urtti, A.; Kontturi, K. Drug permeation in biomembranes in vitro and in silico prediction and influence of physicochemical properties. Eur. J. Pharm. Sci. 2004, 23, 13–47. [Google Scholar] [CrossRef] [PubMed]
- Alavijeh, M.S.; Chishty, M.; Qaiser, M.Z.; Palmer, A.M. Drug metabolism and pharmacokinetics, the blood–brain barrier, and central nervous system drug discovery. NeuroRx 2005, 2, 554–571. [Google Scholar] [CrossRef] [PubMed]
- Duchowicz, P.; Castro, E. QSPR studies on aqueous solubilities of drug-like compounds. Int. J. Mol. Sci. 2009, 10, 2558–2577. [Google Scholar] [CrossRef] [PubMed]
- Kadela-Tomanek, M.; Jastrzębska, M.; Bębenek, E.; Chrobak, E.; Latocha, M.; Kusz, J.; Tarnawska, D.; Boryczka, S. New acetylenic amine derivatives of 5,8-quinolinediones: synthesis, crystal structure and antiproliferative activity. Crystals 2017, 7, 15. [Google Scholar] [CrossRef]
- Jastrzebska, M.; Boryczka, S.; Kadela, M.; Wrzalik, R.; Kusz, J.; Nowak, M. Synthesis, crystal structure and infrared spectra of new 6- and 7-propylamine-5,8-quinolinediones. J. Mol. Struct. 2014, 1067, 160–168. [Google Scholar] [CrossRef]
- Veber, D.; Johnson, S.; Cheng, H.; Smith, B.; Ward, K.; Kopple, K. Molecular properties that influence the oral bioavailability of drug candidate. J. Med. Chem. 2002, 45, 2615–2623. [Google Scholar] [CrossRef] [PubMed]
- Clark, D.E. Rapid calculation of polar molecular surface area and its application to the prediction of transport phenomena. 2. Prediction of blood–brain barrier penetration. J. Pharm. Sci. 1999, 88, 815–821. [Google Scholar] [CrossRef] [PubMed]
- Hutter, M.C. Prediction of blood–brain barrier permeation using quantum chemically derived information. J. Comput. Aided Mol. Des. 2003, 17, 415–433. [Google Scholar] [CrossRef] [PubMed]
- Mulchina, B.J.; Newtona, C.G.; Batya, J.W.; Grassoa, C.H.; Martina, W.J.; Waltona, M.C.; Dangerfielda, E.M.; Plunketta, C.H.; Berridgea, M.V.; Harpera, J.L.; et al. The anti-cancer, anti-inflammatory and tuberculostatic activities of a series of 6,7-substituted-5,8-quinolinequinones. Bioorg. Med. Chem. 2010, 18, 3238–3251. [Google Scholar] [CrossRef] [PubMed]
- Lien, J.C.; Huang, L.J.; Teng, Ch.M.; Wang, J.P.; Kuo, S.C. Synthesis of 2-alkoxy 1,4-naphthoquinone derivatives as antiplatelet, anti-inflammatory, and anti-allergic agents. Chem. Pharm. Bull. 2002, 50, 672–674. [Google Scholar] [CrossRef] [PubMed]
- Flaten, V.M.; Santos, J.G.; Valderrama, J.A. Kinetics and mechanism of the reaction of 2,3-dimethoxy-1,4-naphthoquinone with alkoxide ions in alcoholic solvents. J. Chem. Soc. Perkin Trans. 2 1988, 4, 451–455. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the compounds 1–28 are available from the authors.

| Compound | Human Cell Line/IC50 ± SD (µM) | |||
|---|---|---|---|---|
| C-32 | SNB-19 | MDA-MB-231 | HFF-1 | |
| 1 | 25.0 ± 0.3 | 26.6 ± 0.5 | 31.9 ± 2.7 | 15.6 ± 0.3 |
| 2 a | 22.8 ± 0.7 | 26.2 ± 0.8 | 25.1 ± 3.1 | 15.7 ± 0.5 |
| 3 | 10.5 ± 0.8 | 15.9 ± 0.3 | 23.3 ± 0.3 | 10.0 ± 1.4 |
| 4 | 16.8 ± 0.3 | 18.4 ± 1.7 | 1.4 ± 0.3 | 14.8 ± 0.9 |
| 5 | 4.0 ± 0.1 | 3.8 ± 0.1 | 6.9 ± 1.9 | 3.8 ± 0.8 |
| 6 | 3.2 ± 0.6 | 8.4 ± 1.2 | 21.0 ± 1.5 | 3.9 ± 0.5 |
| 7 a | 0.20 ± 0.1 | 0.4 ± 0.1 | 22.4 ± 2.7 | Neg |
| 8 a | 0.28 ± 0.1 | 0.3 ± 0.1 | 17.2 ± 2.8 | Neg |
| 9 b | 0.32 ± 0.1 | 20.4 ± 0.2 | 21.4 ± 3.4 | 3.3 ± 0.3 |
| 10 | 26.8 ± 1.9 | 19.8 ± 1.4 | 22.2 ± 1.4 | 15.8 ± 1.8 |
| 11 | 168.0 ± 6.2 | 33.8 ± 1.3 | 24.6 ± 2.9 | 20.1 ± 1.2 |
| 12 | 207.6 ± 4.6 | 19.5 ± 1.0 | 17.2 ± 2.5 | 14.0 ± 2.4 |
| 13 | 22.6 ± 0.8 | 19.9 ± 1.4 | 22.6 ± 2.3 | 10.8 ± 0.5 |
| 14 | 10.6 ± 0.6 | 19.6 ± 1.0 | 17.9 ± 0.8 | 4.4 ± 0.9 |
| 15 | 21.7 ± 0.6 | 18.6 ± 0.7 | 26.1 ± 0.3 | 13.5 ± 1.3 |
| 16 a | 1.1 ± 0.1 | 0.7 ± 0.02 | 3.0 ± 0.3 | Neg |
| 17 a | 1.5 ± 0.1 | 0.2 ± 0.1 | 12.6 ± 2.7 | Neg |
| 18 b | 0.3 ± 0.1 | 21.7 ± 1.1 | 1.8 ± 0.1 | 12.6 ± 0.9 |
| 19 | 131.4 ± 2.4 | 25.7 ± 1.4 | 71.3 ± 3.9 | 17.8 ± 1.8 |
| 20 | 18.9 ± 1.7 | 16.6 ± 3.0 | 26.8 ± 1.2 | 0.6 ± 0.2 |
| 21 | 21.1 ± 1.2 | 24.5 ± 1.1 | 23.2 ± 1.5 | 14.5 ± 2.6 |
| cisplatin | 16.4 ± 1.2 | 18.9 ± 0.4 | 25.5 ± 0.2 | 9.1 ± 1.8 |
| Compound | Human Cell Line/IC50 ± SD (µM) | |||
|---|---|---|---|---|
| C-32 | SNB-19 | MDA-MB-231 | HFF-1 | |
| 22 | 158.7 ± 4.6 | 250.4 ± 4.3 | Neg | 260.0 ± 5.6 |
| 23 | 144.6 ± 4.8 | 186.0 ± 5.0 | 22.5 ± 2.4 | 97.6 ± 4.0 |
| 24 | 3.1 ± 0.1 | 2.4 ± 0.1 | 110.2 ± 8.1 | Neg |
| 25 | 115.6 ± 2.9 | 168.7 ± 4.8 | 16.5 ± 1.9 | 27.1 ± 1.1 |
| 26 | 30.3 ± 3.1 | 22.8 ± 1.4 | 2.0 ± 0.2 | 17.5 ± 1.3 |
| 27 | 48.4 ± 8.9 | 31.5 ± 2.5 | 25.1 ± 2.4 | 13.0 ± 3.4 |
| 28 | 155.4 ± 3.7 | 157.1 ± 5.7 | 173.5 ± 5.9 | 27.8 ± 1.2 |
| cisplatin | 16.4 ± 1.2 | 18.9 ± 0.4 | 25.5 ± 0.2 | 9.1 ± 1.8 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kadela-Tomanek, M.; Bębenek, E.; Chrobak, E.; Latocha, M.; Boryczka, S. Alkoxy and Enediyne Derivatives Containing 1,4-Benzoquinone Subunits—Synthesis and Antitumor Activity. Molecules 2017, 22, 447. https://doi.org/10.3390/molecules22030447
Kadela-Tomanek M, Bębenek E, Chrobak E, Latocha M, Boryczka S. Alkoxy and Enediyne Derivatives Containing 1,4-Benzoquinone Subunits—Synthesis and Antitumor Activity. Molecules. 2017; 22(3):447. https://doi.org/10.3390/molecules22030447
Chicago/Turabian StyleKadela-Tomanek, Monika, Ewa Bębenek, Elwira Chrobak, Małgorzata Latocha, and Stanisław Boryczka. 2017. "Alkoxy and Enediyne Derivatives Containing 1,4-Benzoquinone Subunits—Synthesis and Antitumor Activity" Molecules 22, no. 3: 447. https://doi.org/10.3390/molecules22030447
APA StyleKadela-Tomanek, M., Bębenek, E., Chrobak, E., Latocha, M., & Boryczka, S. (2017). Alkoxy and Enediyne Derivatives Containing 1,4-Benzoquinone Subunits—Synthesis and Antitumor Activity. Molecules, 22(3), 447. https://doi.org/10.3390/molecules22030447