Substitution at the C-3 Position of Catechins Has an Influence on the Binding Affinities against Serum Albumin
Abstract
:1. Introduction
2. Results
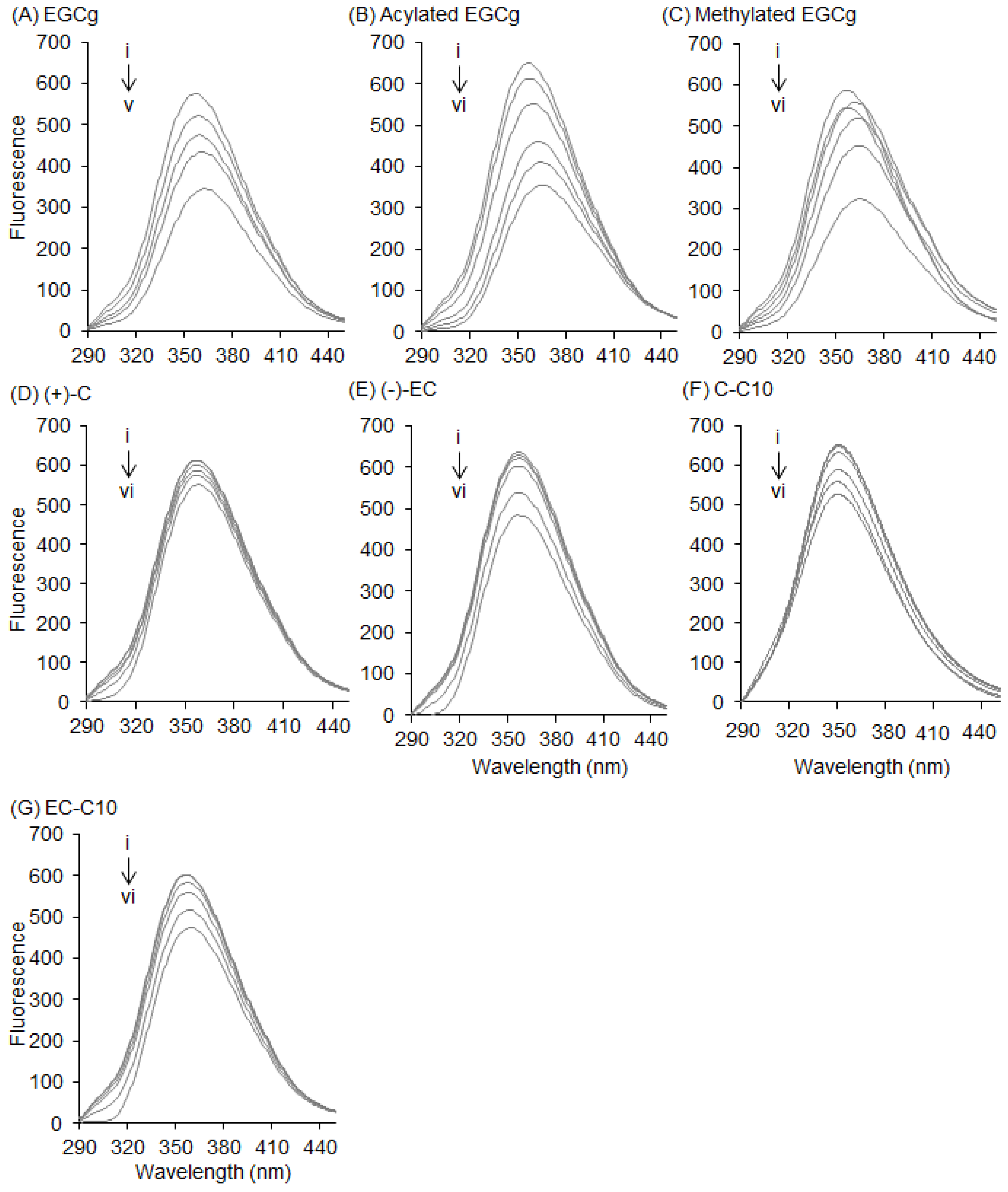
2.1. Interaction between BSA and EGCg and Its Derivatives
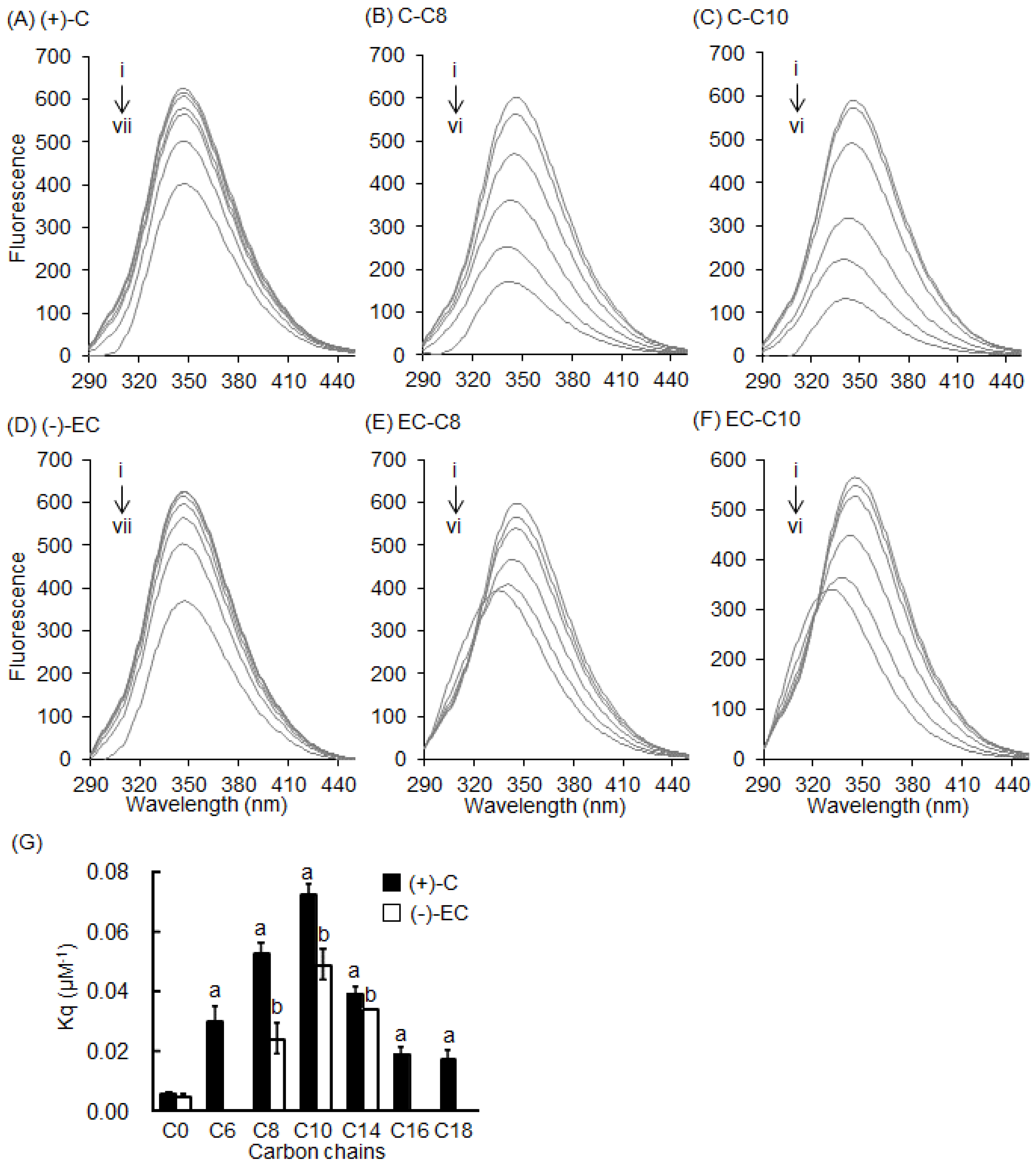
2.2. Stern–Volmer Quenching Constants of Catechins and EGCg Derivatives
2.3. Interaction between BSA and 3-O-acyl-Catechins
2.4. Interaction between HSA and Catechins and Their Derivatives
3. Discussion
4. Material and Methods
4.1. Chemicals
4.2. Docking Simulation between EGCg and BSA
4.3. Analysis of Fluorescence Quenching of BSA and HSA
4.4. Statistical Analysis
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| BSA | bovine serum albumin |
| HSA | human serum albumin |
| EGCg | epigallocatechin gallate |
| (+)-C | catechin |
| (−)-EC | epicatechin |
| Trp | tryptophan |
References
- Shahidi, F. Antioxidants in food and food antioxidants. Nahrung 2000, 44, 158–163. [Google Scholar] [CrossRef]
- Maeda-Yamamoto, M.; Ema, K.; Shibuichi, I. In vitro and in vivo anti-allergic effects of ‘Benifuuki’ green tea containing O-methylated catechin and ginger extract enhancement. Cytotechnology 2007, 55, 135–142. [Google Scholar] [CrossRef]
- Intra, J.; Kuo, S.M. Physiological levels of tea catechins increase cellular lipid antioxidant activity of vitamin C and vitamin E in human intestinal Caco-2 cells. Chem. Biol. Interact. 2007, 169, 91–99. [Google Scholar] [CrossRef] [PubMed]
- Cavet, M.; Harrington, K.; Vollmer, T.; Ward, K.; Zhang, J.Z. Anti-inflammatory and anti-oxidative effects of the green tea polyphenol epigallocatechin gallate in human corneal epithelial cells. Mol. Vis. 2011, 17, 533–542. [Google Scholar] [PubMed]
- Yang, C.; Ju, J.; Lu, G.; Xiao, A. Cancer prevention by tea and tea polyphenols. Asia Pac. J. Clin. Nutr. 2008, 17, 245–248. [Google Scholar] [PubMed]
- Wolfram, S.; Wang, Y.; Thielecke, F. Anti-obesity effects of green tea: From bedside to bench. Mol. Nutr. Food Res. 2006, 50, 176–187. [Google Scholar] [CrossRef] [PubMed]
- Ueda, M.; Nishiumi, S.; Nagayasu, H.; Fukuda, I.; Yoshida, K.; Ashida, H. Epigallocatechin gallate promotes GLUT4 translocation in skeletal muscle. Biochem. Biophys. Res. Commun. 2008, 377, 286–290. [Google Scholar] [CrossRef] [PubMed]
- Ueda-Wakagi, M.; Mukai, R.; Fuse, N.; Mizushina, Y.; Ashida, H. 3-O-Acyl-epicatechins Increase Glucose Uptake Activity and GLUT4 Translocation through Activation of PI3K Signaling in Skeletal Muscle Cells. Int. J. Mol. Sci. 2015, 6, 16288–16299. [Google Scholar] [CrossRef]
- Ueda, M.; Furuyashiki, T.; Yamada, K.; Aoki, Y.; Sakane, I.; Fukuda, I.; Yoshida, K.; Ashida, H. Tea catechins modulate the glucose transport system in 3T3-L1 adipocytes. Food Funct. 2010, 1, 167–173. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.Q.; Zhang, H.M.; Zhang, G.C.; Tao, W.H.; Fei, Z.H.; Liu, Z.T. Spectroscopic studies on the interaction between silicotungstic acid and bovine serum albumin. J. Pharmaceut. Biomed. Anal. 2007, 43, 869–1875. [Google Scholar] [CrossRef] [PubMed]
- Hossain, M.; Khan, A.Y.; Kumar, G.S. Interaction of the anticancer plant alkaloid sanguinarine with bovine serum albumin. PLoS ONE 2011, 6, e18333. [Google Scholar] [CrossRef] [PubMed]
- Liu, E.H.; Qi, L.W.; Li, P. Structural relationship and binding mechanisms of five flavonoids with bovine serum albumin. Molecules 2010, 15, 9092–9103. [Google Scholar] [CrossRef] [PubMed]
- Kandagal, P.B.; Ashoka, S.; Seetharamappa, J.; Shaikh, S.M.T.; Jadegoud, Y.; Ijare, O.B. Study of the interaction of an anticancer drug with human and bovine serum albumin: Spectroscopic approach. J. Pharmaceut. Biomed. Anal. 2006, 41, 393–399. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, B.; Basu, S. Interaction of BSA with proflavin: A spectroscopic approach. J. Lumin. 2009, 129, 34–39. [Google Scholar] [CrossRef]
- Vivian, J.T.; Callis, P.R. Mechanisms of tryptophan fluorescence shifts in proteins. Biophys. J. 2001, 80, 2093–2109. [Google Scholar] [CrossRef]
- Möller, M.; Denicola, A. Protein tryptophan accessibility studied by fluorescence quenching. Biochem. Mol. Biol. Educ. 2002, 30, 175–178. [Google Scholar] [CrossRef]
- Kraft, C.A.; Garrido, J.L.; Leiva-Vega, L.; Romero, G. Quantitative analysis of protein-lipid interactions using tryptophan fluorescence. Sci. Signal. 2009, 2. [Google Scholar] [CrossRef]
- Sonveaux, N.; Vigano, C.; Shapiro, A.B.; Ling, V.; Ruysschaert, J.M. Ligand-mediated tertiary structure changes of reconstituted P-glycoprotein. A tryptophan fluorescence quenching analysis. J. Biol. Chem. 1999, 274, 17649–17654. [Google Scholar] [CrossRef] [PubMed]
- Matveeva, E.G.; Morisseau, C.; Goodrow, M.H.; Mullin, C.; Hammock, B.D. Tryptophan fluorescence quenching by enzyme inhibitors as a tool for enzyme active site structure investigation: epoxide hydrolase. Curr. Pharm. Biotechnol. 2009, 10, 589–599. [Google Scholar] [CrossRef] [PubMed]
- Kagaya, N.; Kamiyoshi, A.; Tagawa, Y.; Nagashima, H.; Kawase, M.; Yagi, K. Interaction of rubratoxin B with serum albumin. Micotxins 2005, 55, 23–26. [Google Scholar] [CrossRef]
- Klajnert, B.; Bryszewska, M. Fluorescence studies on PAMAM dendrimers interactions with bovine serum albumin. Bioelectrochemistry 2002, 55, 33–53. [Google Scholar] [CrossRef]
- Moriyama, Y.; Ohta, D.; Hachiya, K.; Mitsui, Y.; Takeda, K. Fluorescence behavior of tryptophan residues of bovine and human serum albumins in ionic surfactant solutions: a comparative study of the two and one tryptophan(s) of bovine and human albumins. J. Protein Chem. 1996, 15, 265–272. [Google Scholar] [CrossRef] [PubMed]
- Papadopoulou, A.; Green, R.J.; Frazier, R.A. Interaction of flavonoids with bovine serum albumin: A fluorescence quenching study. J. Agric. Food Chem. 2005, 53, 158–163. [Google Scholar] [CrossRef] [PubMed]
- Shi, S.; Zhang, Y.; Xiong, X.; Huang, K.; Chen, X.; Peng, M. The influence of flavonoids on the binding of pantoprazole to bovine serum albumin by spectroscopic methods: with the viewpoint of food/drug interference. Food Chem. 2012, 135, 1083–1090. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Li, X.; Gui, M.; Zhou, H.; Yang, R.; Zhang, H.; Jin, Y. Studies on interaction between flavonoids and bovine serum albumin by spectral methods. J. Lumin. 2010, 130, 637–664. [Google Scholar] [CrossRef]
- Peters, T., Jr. Serum albumin. Adv. Protein Chem. 1985, 37, 161–245. [Google Scholar] [PubMed]
- Petitpas, I.; Bhattacharya, A.A.; Twine, S.; East, M.; Curry, S.J. Crystal structure analysis of warfarin binding to human serum albumin: anatomy of drug site I. Biol. Chem. 2011, 276, 22804–22809. [Google Scholar] [CrossRef] [PubMed]
- Sowell, J.; Mason, J.C.; Strekowski, L.; Patonay, G. Binding constant determination of drugs toward subdomain IIIA of human serum albumin by near-infrared dye-displacement capillary electrophoresis. Electrophoresis 2001, 22, 2512–2517. [Google Scholar] [CrossRef]
- Kragh-Hansen, U. Molecular aspects of ligand binding to serum albumin. Pharmacol. Rev. 1981, 33, 17–53. [Google Scholar] [PubMed]
- Kawase, M.; Fujiwara, S.; Nakanishi, S.; Itoh, H. Host-dependent optical dephasing of dye molecules doped in cross-linked polyvinyl alcohols. Chem. Phys. Lett. 1992, 194, 268–274. [Google Scholar] [CrossRef]
- Nakanishi, S.; Miyawaki, Y.; Nishikawa, M.; Amano, M.; Fujiwara, S.; Jitou, S.; Itoh, H.; Kawase, M. Optical dephasing of organic dye molecules doped in cross-linked polyvinyl alcohol derivatives: Incoherent photon echo and hole-burning studies. J. Chem. Phys. 1994, 100, 3442–3454. [Google Scholar] [CrossRef]
- Mori, S.; Miyake, S.; Kobe, T.; Nakaya, T.; Fuller, S.D.; Kato, N.; Kaihatsu, K. Enhanced anti-influenza A virus activity of (−)-epigallocatechin-3-O-gallate fatty acid monoester derivatives: effect of alkyl chain length. Bioorg. Med. Chem. Lett. 2008, 18, 4249–4252. [Google Scholar] [CrossRef] [PubMed]
- Matsubara, K.; Saito, A.; Tanaka, A.; Nakajima, N.; Akagi, R.; Mori, M.; Mizushina, Y. Catechin conjugated with fatty acid inhibits DNA polymerase and angiogenesis. DNA Cell Biol. 2006, 25, 95–103. [Google Scholar] [CrossRef] [PubMed]
- Molecular Operating Environment (MOE), 2016.08; Chemical Computing Group Inc.: Montreal, QC, Canada, 2016.
- Galli, C.L.; Sensi, C.; Fumagalli, A.; Parravicini, C.; Marinovich, M.; Eberini, I. A computational approach to evaluate the androgenic affinity of iprodione, procymidone, vinclozolin and their metabolites. PLoS ONE 2014, 9, e104822. [Google Scholar] [CrossRef] [PubMed]
- Qin, H.-L.; Shang, Z.-P.; Jantan, I.; Tan, O.U.; Hussain, M.A.; Sher, M.; Bukhari, S.N.A.B. Molecular docking studies and biological evaluation of chalcone based pyrazolines as tyrosinase inhibitors and potential anticancer agents. RSC Adv. 2015, 5, 46330–46338. [Google Scholar] [CrossRef]
- Liu, M.; Zheng, N.; Li, D.; Zheng, H.; Zhang, L.; Ge, H.; Liu, W. cyp51A-based mechanism of azole resistance in Aspergillus fumigatus: Illustration by a new 3D Structural Model of Aspergillus fumigatus CYP51A protein. Med. Mycol. 2016, 54, 400–408. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Samples of the compounds are not available.
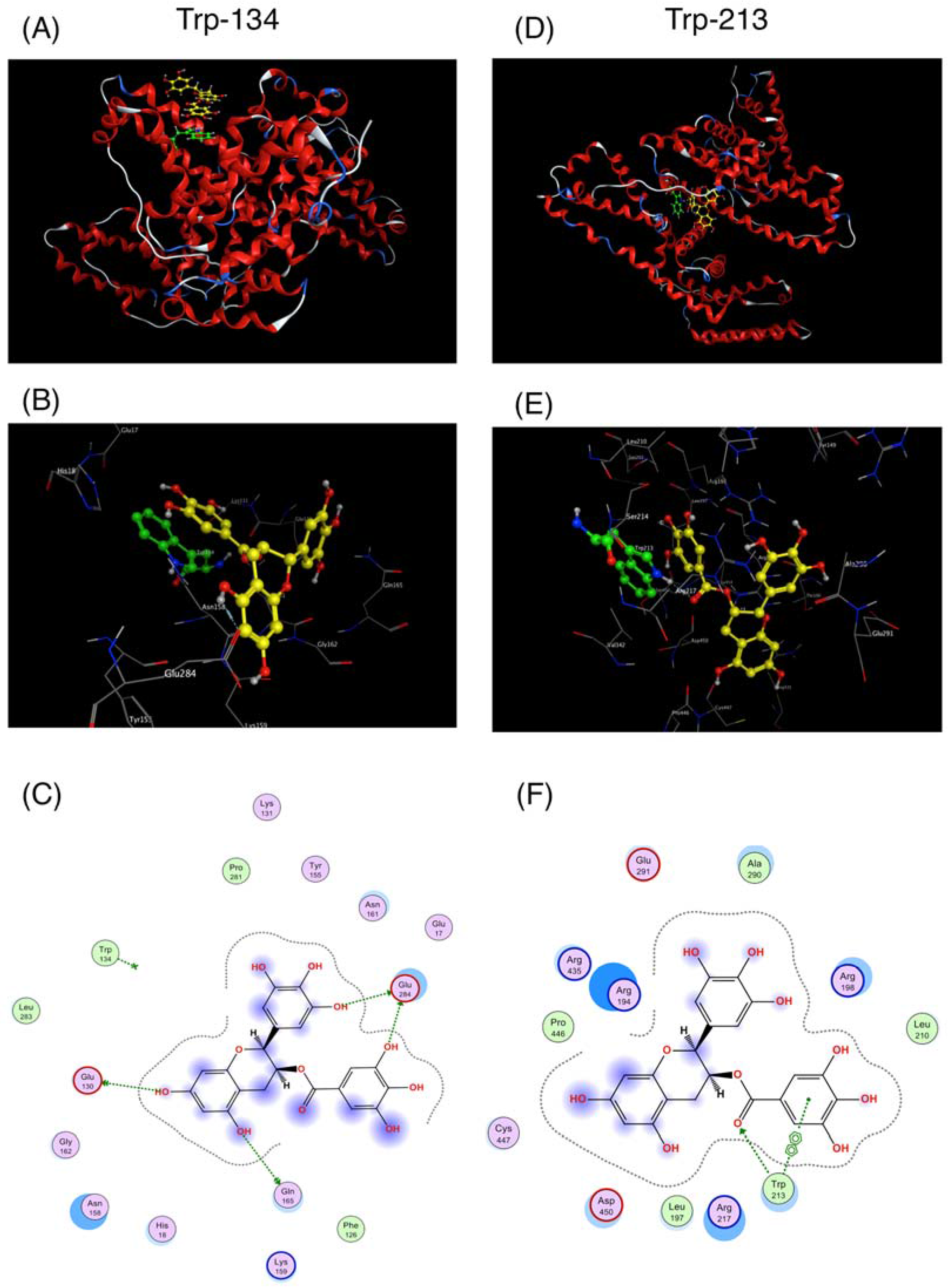
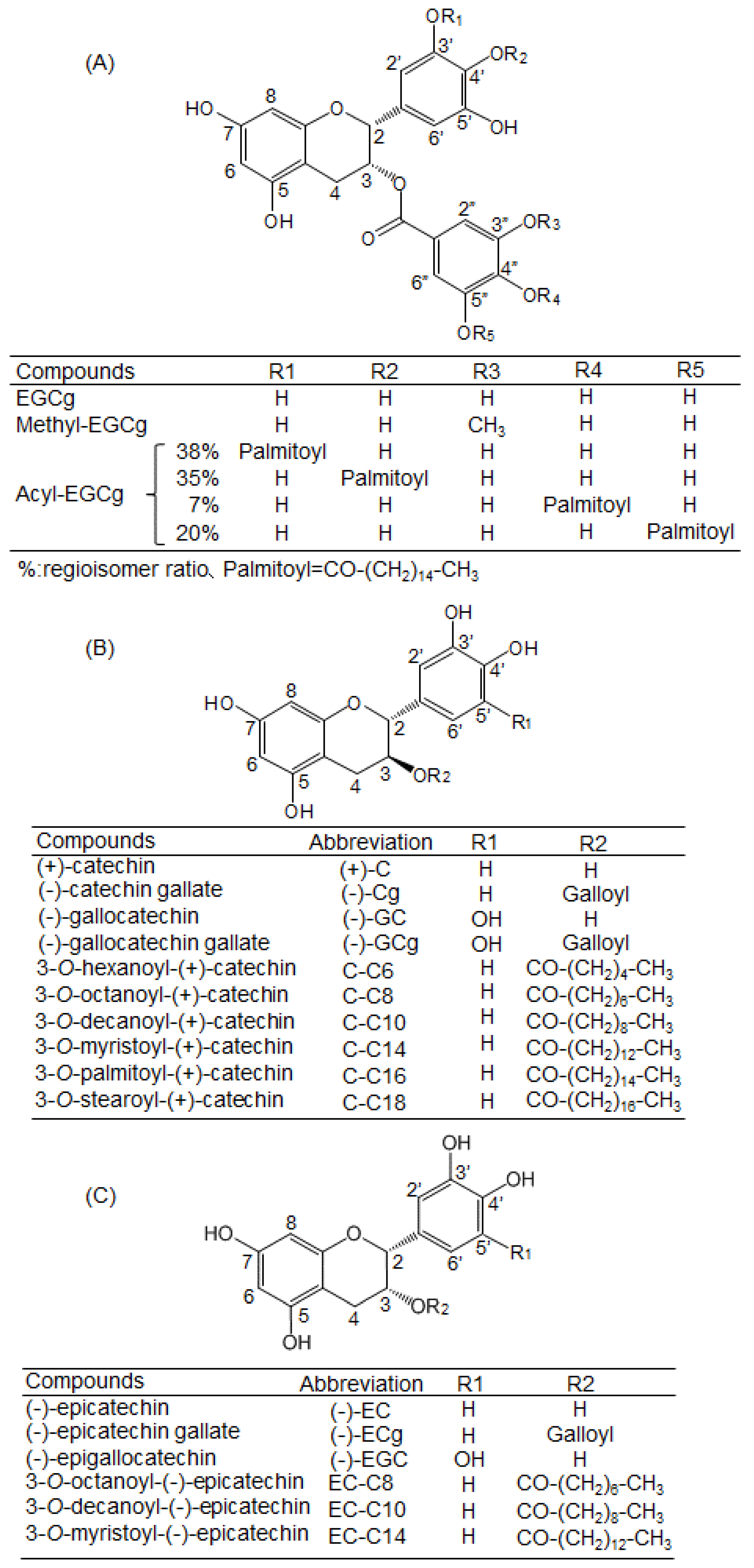
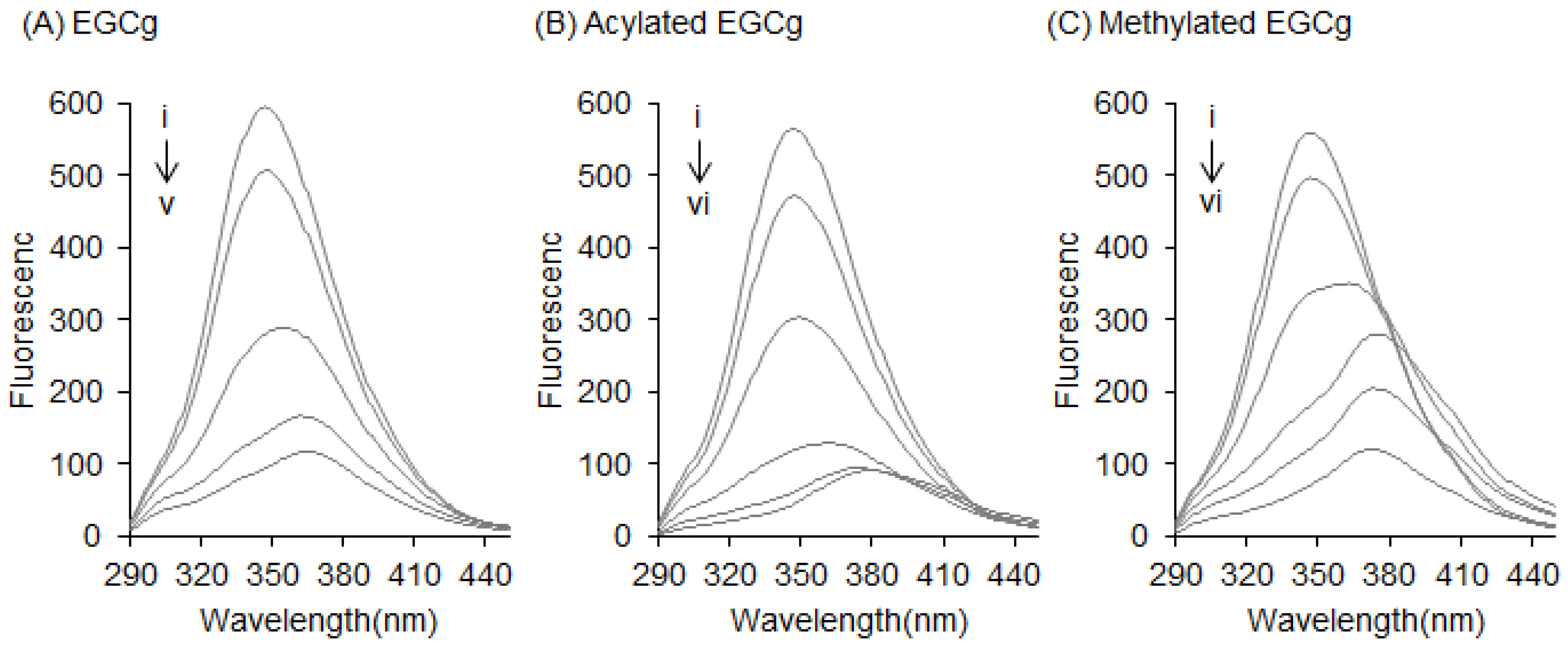
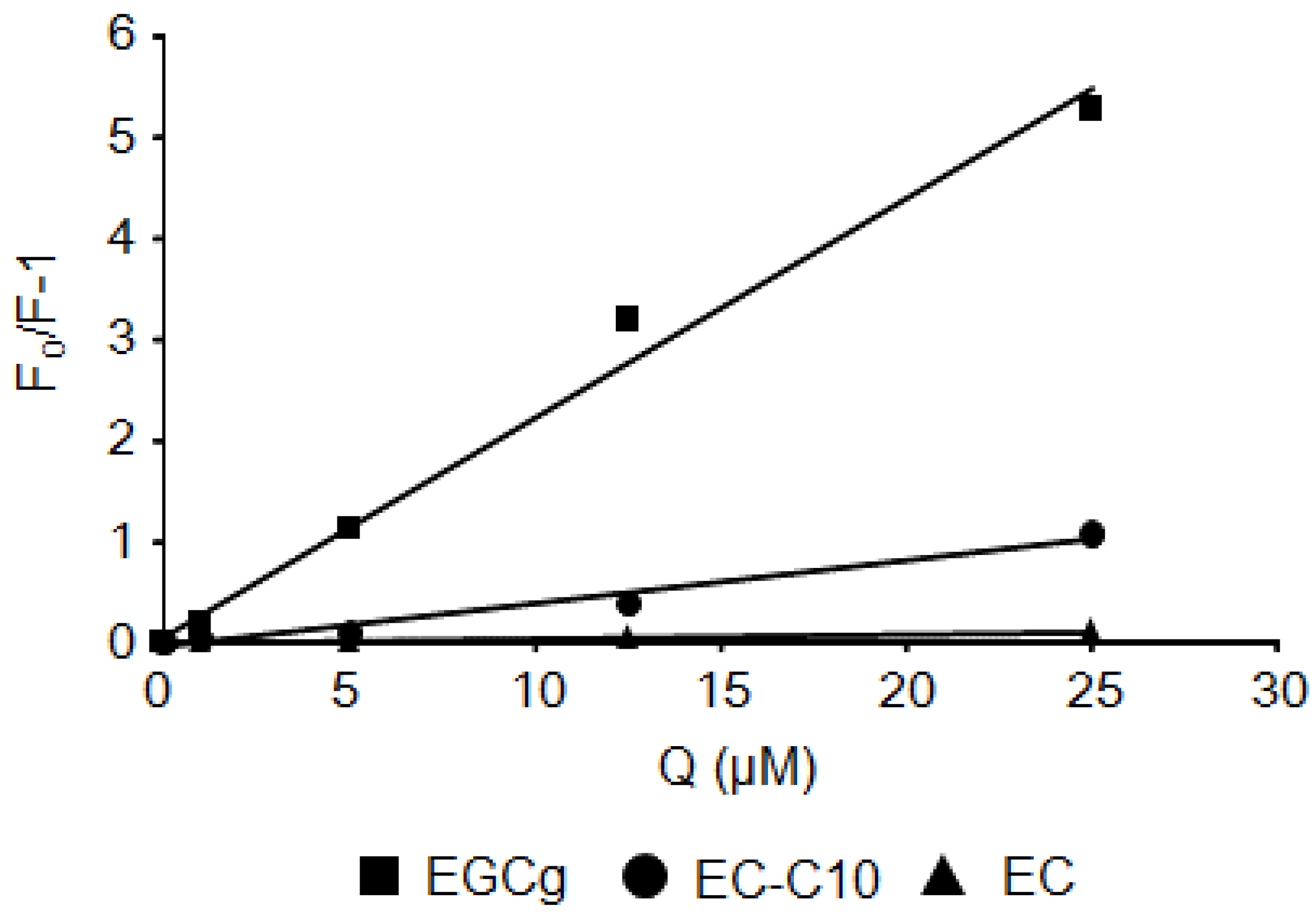
| Coumpunds | BSA | HSA |
|---|---|---|
| (+)-C | 0.005 ± 0.0003 | 0.002 ± 0.0001 |
| Cg | 0.034 ± 0.0092 | 0.011 ± 0.0011 |
| GC | 0.001 ± 0.0001 | 0.001 ± 0.0001 |
| GCg | 0.019 ± 0.0030 | 0.009 ± 0.0008 |
| (−)-EC | 0.005 ± 0.0011 | 0.006 ± 0.0013 |
| ECg | 0.060 ± 0.0118 | 0.007 ± 0.0011 |
| EGC | 0.001 ± 0.0003 | 0.001 ± 0.0002 |
| EGCg | 0.225 ± 0.0192 | 0.033 ± 0.0065 |
| Acyl-EGCg | 0.286 ± 0.0323 | 0.023 ± 0.0055 |
| Methyl-EGCg | 0.150 ± 0.0109 | 0.014 ± 0.0021 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ikeda, M.; Ueda-Wakagi, M.; Hayashibara, K.; Kitano, R.; Kawase, M.; Kaihatsu, K.; Kato, N.; Suhara, Y.; Osakabe, N.; Ashida, H. Substitution at the C-3 Position of Catechins Has an Influence on the Binding Affinities against Serum Albumin. Molecules 2017, 22, 314. https://doi.org/10.3390/molecules22020314
Ikeda M, Ueda-Wakagi M, Hayashibara K, Kitano R, Kawase M, Kaihatsu K, Kato N, Suhara Y, Osakabe N, Ashida H. Substitution at the C-3 Position of Catechins Has an Influence on the Binding Affinities against Serum Albumin. Molecules. 2017; 22(2):314. https://doi.org/10.3390/molecules22020314
Chicago/Turabian StyleIkeda, Masaki, Manabu Ueda-Wakagi, Kaori Hayashibara, Rei Kitano, Masaya Kawase, Kunihiro Kaihatsu, Nobuo Kato, Yoshitomo Suhara, Naomi Osakabe, and Hitoshi Ashida. 2017. "Substitution at the C-3 Position of Catechins Has an Influence on the Binding Affinities against Serum Albumin" Molecules 22, no. 2: 314. https://doi.org/10.3390/molecules22020314






