Ent-Abietanoids Isolated from Isodon serra
Abstract
:1. Introduction
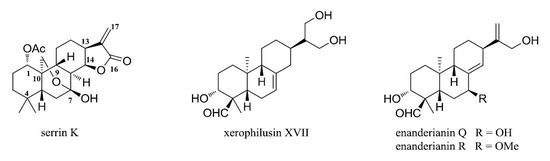
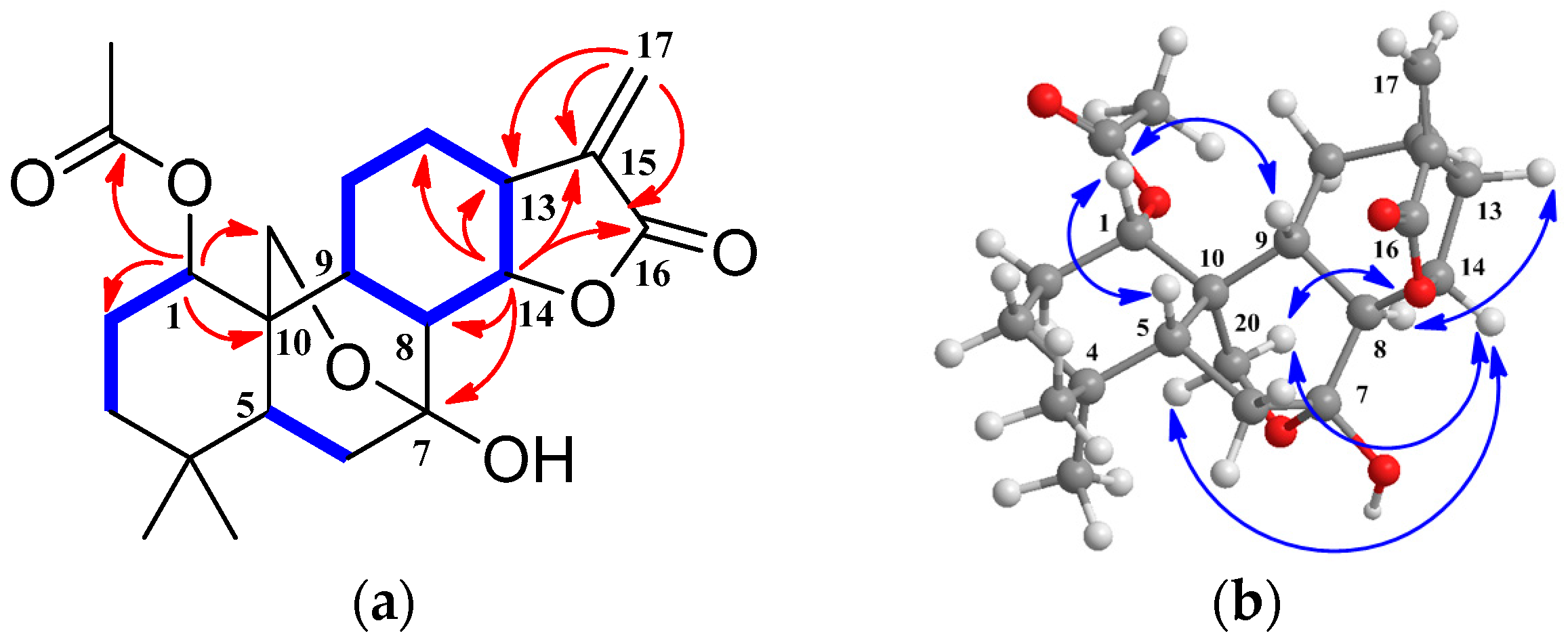
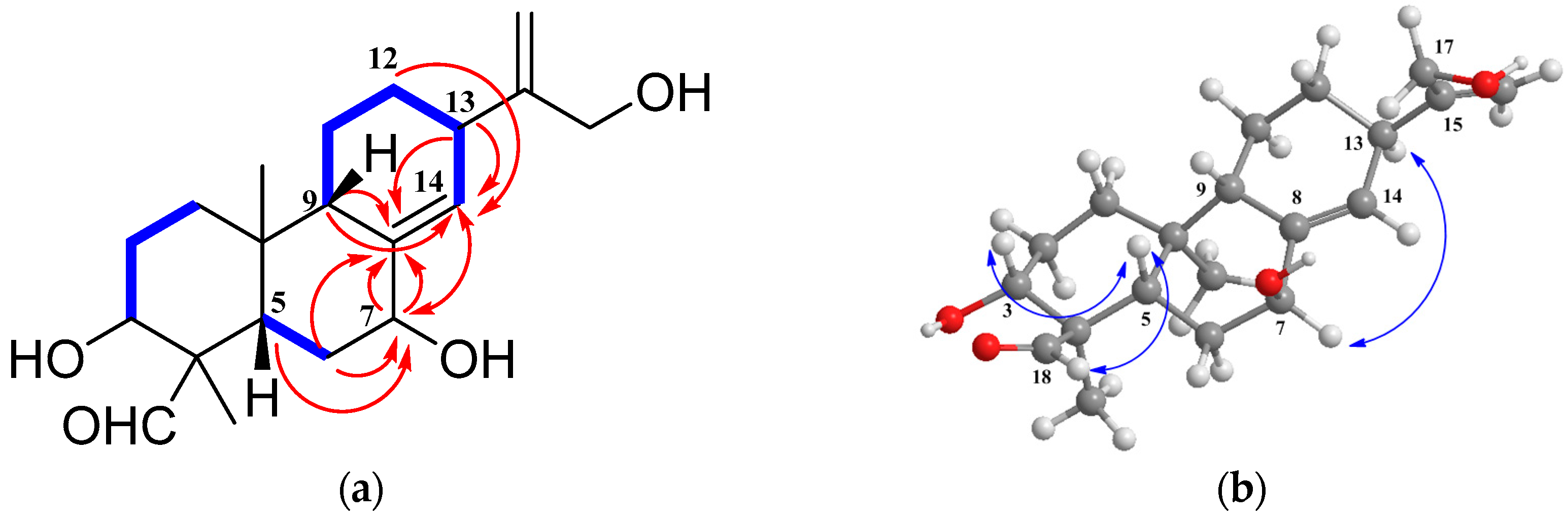
2. Results and Discussion
3. Experimental Section
3.1. General Information
3.2. Plant Material
3.3. Extraction and Isolation
3.4. Cytotoxicity Assays
3.5. Nitric Oxide Production in RAW264.7 Macrophages
3.6. Determination of Cytotoxic Effects
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Editorial Board of the Flora of China of Chinese Academy of Sciences. Flora of China; Science Press: Beijing, China, 2004; Volume 66, pp. 433–434. [Google Scholar]
- Wang, G.Q. The National Assembly of Chinese Herbal Medicine, 3rd ed.; People’s Medical Publishing House: Beijing, China, 2014; pp. 1094–1095. [Google Scholar]
- Sun, H.D.; Xu, Y.L.; Jiang, B. Diterpenoids from Isodon Species; Science Press: Beijing, China, 2001; p. 2. [Google Scholar]
- Jin, R.L.; Cheng, P.Y.; Xu, G.Y. The structure of rabdoserrin A, isolated from Rabdosia serra (Maxim) Hara. Yaoxue Xuebao 1985, 20, 366–371. [Google Scholar]
- Jin, R.L.; Cheng, P.Y.; Xu, G.Y. Structure of rabdoserrin B, isolated from Rabdosia serra. J. China Pharm. Univ. 1987, 18, 172–174. [Google Scholar]
- Lin, L.Z.; Gao, Q.; Cui, C.; Zhao, H.F.; Fu, L.W.; Chen, L.M.; Yang, B.; Luo, W.; Zhao, M.M. Isolation and identification of ent-kaurane-type diterpenoids from Rabdosia serra (Maxim.) Hara leaf and their inhibitory activities against HepG-2, MCF-7, and HL-60 cell lines. Food Chem. 2012, 131, 1009–1014. [Google Scholar] [CrossRef]
- Wan, J.; Liu, M.; Jiang, H.Y.; Yang, J.; Du, X.; Li, X.N.; Wang, W.G.; Li, Y.; Pu, J.X.; Sun, H.D. Bioactive ent-kaurane diterpenoids from Isodon serra. Phytochemistry (Elsevier) 2016, 130, 244–251. [Google Scholar] [CrossRef] [PubMed]
- Yan, F.L.; Zhang, L.B.; Zhang, J.X.; Sun, H.D. Two new diterpenoids and other constituents from Isodon serra. J. Chem. Res. 2007, 362–364. [Google Scholar] [CrossRef]
- Liu, P.W.; Du, Y.F.; Zhang, X.W.; Sheng, X.N.; Shi, X.W.; Zhao, C.C.; Zhu, H.; Wang, N.; Wang, Q.; Zhang, L.T. Rapid Analysis of 27 Components of Isodon serra by LC-ESI-MS-MS. Chromatographia 2010, 72, 265–273. [Google Scholar] [CrossRef]
- Wang, W.Q.; Xuan, L.J. ent-6,7-Seco-kaurane diterpenoids from Rabdosia serra and their cytotoxic activities. Phytochemistry (Elsevier) 2016, 122, 119–125. [Google Scholar] [CrossRef] [PubMed]
- Han, Q.B.; Li, R.T.; Zhang, J.X.; Sun, H.D. New ent-abietanoids from Isodon rubescens. Helv. Chim. Acta 2004, 87, 1007–1015. [Google Scholar] [CrossRef]
- Li, L.M.; Pu, J.X.; Xiao, W.L.; Sun, H.D. ent-Abietane diterpenoids from Isodon xerophilus. Arch. Pharm. Res. 2011, 34, 875–879. [Google Scholar] [CrossRef] [PubMed]
- Xiang, W.; Na, Z.; Li, S.H.; Li, M.L.; Li, R.T.; Tian, Q.E.; Sun, H.D. Cytotoxic diterpenoids from Isodon enanderianus. Planta Med. 2003, 69, 1031–1035. [Google Scholar] [PubMed]
- Chen, C.; Chen, Y.; Zhu, H.Y.; Xiao, Y.Y.; Zhang, X.Z.; Zhao, J.F.; Chen, Y.X. Effective compounds screening from Rabdosia serra (Maxim) Hara against HBV and tumor in vitro. Int. J. Clin. Exp. Med. 2014, 7, 384–392. [Google Scholar] [PubMed]
- Guo, L.Q.; Hai, G.F.; Yan, J.W.; Liu, W.; Yang, L.J.; Huang, M.G.; Wang, L. Chemical constituents of Isodon serra and their cytotoxicity. Xinxiang Yixueyuan Xuebao 2014, 31, 96–99. [Google Scholar]
- Hai, G.F.; Yan, Y.; Liu, J.Y.; Yang, J.N. Inhibitory Effects of enmein, serrin B, nodosin and lasiodonin on growth of HL60 cells and LOVO cells in vitro. Xinxiang Yixueyuan Xuebao 2008, 25, 564–566. [Google Scholar]
- Hu, A.P.; Du, J.M.; Li, J.Y.; Liu, J.W. Oridonin promotes CD4+/CD25+ Treg differentiation, modulates Th1/Th2 balance and induces HO-1 in rat splenic lymphocytes. Inflamm. Res. 2008, 57, 163–170. [Google Scholar] [CrossRef] [PubMed]
- Li, J.Y.; Du, J.M.; Sun, L.J.; Liu, J.W.; Quan, Z.W. Anti-inflammatory function of Nodosin via inhibition of IL-2. Am. J. Chin. Med. 2010, 38, 127–142. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.W.; Yang, F.; Zhang, Y.; Li, J.Y. Studies on the cell-immunosuppressive mechanism of Oridonin from Isodon serra. Int. Immunopharmacol. 2007, 7, 945–954. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Liu, J.W.; Jia, W.; Zhao, A.H.; Li, T. Distinct immunosuppressive effect by Isodon serra extracts. Int. Immunopharmacol. 2005, 5, 1957–1965. [Google Scholar] [CrossRef] [PubMed]
- Zhao, A.H.; Zhang, Y.; Xu, Z.H.; Liu, J.W.; Jia, W. Immunosuppressive ent-kaurene diterpenoids from Isodon serra. Helv. Chim. Acta 2004, 87, 3160–3166. [Google Scholar] [CrossRef]
- Zhou, L.; Sun, L.J.; Wu, H.K.; Zhang, L.Z.; Chen, M.C.; Liu, J.W.; Zhong, R.Q. Oridonin ameliorates lupus-like symptoms of MRLlpr/lpr mice by inhibition of B-cell activating factor (BAFF). Eur. J. Pharmacol. 2013, 715, 230–237. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.P.; Sun, H.D.; Lin, Z.W. Diterpenoids of Rabdosia coetsa. Zhiwu Xuebao 1990, 32, 292–296. [Google Scholar]
- Sun, H.D.; Huang, S.X.; Han, Q.B. Diterpenoids from Isodon species and their biological activities. Nat. Prod. Rep. 2006, 23, 673–698. [Google Scholar] [CrossRef] [PubMed]
- Monks, A.; Scudiero, D.; Skehan, P.; Shoemaker, R.; Paull, K.; Vistica, D.; Hose, C.; Langley, J.; Cronise, P. Feasibility of a high-flux anticancer drug screen using a diverse panel of cultured human tumor cell lines. J. Natl. Cancer Inst. 1991, 83, 757–766. [Google Scholar] [CrossRef] [PubMed]
- Reed, L.J.; Muench, H. A simple method of estimating fifty percent endpoints. Am. J. Epidemiol. 1938, 27, 493–497. [Google Scholar] [CrossRef]
- Fan, J.T.; Su, J.; Peng, Y.M.; Li, Y.; Li, J.; Zhou, Y.B.; Zeng, G.Z.; Yan, H.; Tan, N.H. Rubiyunnanins C-H, cytotoxic cyclic hexapeptides from Rubia yunnanensis inhibiting nitric oxide production and NF-κB activation. Bioorg. Med. Chem. 2010, 18, 8226–8234. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Samples of the compounds are available from the authors.

| Position | 1 a | 2 a | 3 b | 4 c |
|---|---|---|---|---|
| 1a | 4.81 (dd, 11.5, 5.1) | 1.80 (dt, 13.2, 3.2) | 1.67 (dt, 13.1, 3.3) | 1.60 (dt, 13.1, 3.3) |
| 1b | 1.19 (m) | 1.32 (dt, 13.1, 3.3) | 1.21 (dt, 13.1, 3.3) | |
| 2a | 1.80 (m) | 1.90 (2H, overlap) | 1.95 (overlap) | 1.92 (overlap) |
| 2b | 1.55 (overlap) | 1.88 (overlap) | 1.85 (m) | |
| 3a | 1.32 (dt, 13.5, 3.3) | 4.12 (dd, 11.0, 4.6) | 4.18 (br d, 11.1) | 4.12 (br d, 8.8) |
| 3b | 1.17 (overlap) | |||
| 5 | 1.41 (dd, 11.4, 5.8) | 1.70 (overlap) | 2.61 (overlap) | 2.27 (dd, 13.2, 2.5) |
| 6a | 2.03 (2H, overlap) | 2.01 (overlap) | 1.78 (dd, 13.6, 2.8) | 1.68 (overlap) |
| 6b | 1.48 (m) | 1.57 (dt, 13.6, 2.8) | 1.44 (dt, 13.2, 2.8) | |
| 7 | 5.26 (d, 4.1) | 4.40 (overlap) | 3.47 (t, 2.8) | |
| 8 | 2.16 (t, 11.0) | |||
| 9 | 1.92 (m) | 1.70 (overlap) | 2.64 (overlap) | 2.11 (br s) |
| 11a | 1.62 (m) | 1.70 (overlap) | 1.74 (overlap) | 1.65 (overlap) |
| 11b | 1.49 (m) | 1.05 (m) | 1.37 (overlap) | 1.30 (overlap) |
| 12a | 1.19 (overlap) | 1.25 (m) | 1.92 (overlap) | 1.92 (overlap) |
| 12b | 2.06 (overlap) | 2.03 (overlap) | 1.37 (overlap) | 1.30 (overlap) |
| 13 | 3.30 (m) | 1.85 (m) | 2.89 (br s) | 2.88 (br s) |
| 14a | 5.31 (t, 9.8) | 2.57 (br d, 13.9) | 5.86 (s) | 5.73 (s) |
| 14b | 2.04 (overlap) | |||
| 15 | 1.93 (overlap) | |||
| 16 | 4.19 (2H, overlap) | 4.42 (2H, overlap) | 4.47 (2H, s) | |
| 17a | 6.33 (d, 3.1) | 4.23 (2H, overlap) | 5.44 (br s) | 5.54 (br s) |
| 17b | 5.52 (d, 3.1) | 4.98 (br s) | 5.10 (br s) | |
| 18 18b | 0.74 (3H, s) | 9.62 (s) | 9.69 (s) 3.54 (2H, d, 10.6) | 9.61 (s) |
| 19 | 1.07 (3H, s) | 1.45 (3H, s) | 1.42 (3H, s) | 1.37 (3H, s) 3.54 (2H, d, 10.6) |
| 20a | 4.35 (d, 10.3) | 0.82 (3H, s) | 0.85 (3H, s) | 0.80 (3H, s) |
| 20b | 4.21 (d, 10.3) | |||
| OAc-1 | 2.07 (s) | |||
| OMe | 3.16 (3H, s) |
| Position | 1 a | 2 a | 3 c | 4 c |
|---|---|---|---|---|
| 1 | 76.5 (d) | 38.2 (t) | 37.1 (t) | 36.8 (t) |
| 2 | 25.8 (t) | 27.8 (t) | 27.5 (t) | 27.4 (t) |
| 3 | 38.6 (t) | 72.8 (d) | 71.8 (d) | 71.7 (d) |
| 4 | 34.1 (s) | 56.2 (s) | 56.2 (s) | 56.0 (s) |
| 5 | 49.8 (d) | 42.6 (d) | 39.1 (d) | 39.6 (d) |
| 6 | 36.1 (t) | 25.2 (t) | 32.4 (t) | 30.7 (t) |
| 7 | 96.8 (s) | 119.7 (d) | 71.9 (d) | 81.3 (d) |
| 8 | 45.4 (d) | 138.6 (s) | 141.3 (s) | 135.8 (s) |
| 9 | 47.0 (d) | 52.9 (d) | 46.5 (d) | 46.5 (d) |
| 10 | 37.7 (s) | 34.8 (s) | 37.7 (s) | 37.3 (s) |
| 11 | 21.9 (t) | 26.3 (t) | 22.5 (t) | 22.4 (t) |
| 12 | 27.7 (t) | 30.5 (t) | 29.5 (t) | 29.8 (t) |
| 13 | 39.5 (d) | 37.4 (d) | 39.4 (d) | 39.9 (d) |
| 14 | 77.7 (d) | 40.1 (t) | 129.3 (d) | 132.6 (d) |
| 15 | 140.9 (s) | 49.6(d) | 155.0 (s) | 155.2 (s) |
| 16 | 170.8 (s) | 62.4 (t) | 64.3 (t) | 64.3 (t) |
| 17 | 121.8 (t) | 62.3 (t) | 107.9 (t) | 108.3 (t) |
| 18 | 31.8 (q) | 207.3 (d) | 206.8 (d) | 206.7 (d) |
| 19 | 20.3 (q) | 10.3 (q) | 9.7 (q) | 9.6 (q) |
| 20 | 64.1 (t) | 16.0 (q) | 14.4 (q) | 14.5 (q) |
| OAc-1 | 170.6 (s) | |||
| 21.9 (q) | ||||
| OMe | 54.8 (q) |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wan, J.; Jiang, H.-Y.; Tang, J.-W.; Li, X.-R.; Du, X.; Li, Y.; Sun, H.-D.; Pu, J.-X. Ent-Abietanoids Isolated from Isodon serra. Molecules 2017, 22, 309. https://doi.org/10.3390/molecules22020309
Wan J, Jiang H-Y, Tang J-W, Li X-R, Du X, Li Y, Sun H-D, Pu J-X. Ent-Abietanoids Isolated from Isodon serra. Molecules. 2017; 22(2):309. https://doi.org/10.3390/molecules22020309
Chicago/Turabian StyleWan, Jun, Hua-Yi Jiang, Jian-Wei Tang, Xing-Ren Li, Xue Du, Yan Li, Han-Dong Sun, and Jian-Xin Pu. 2017. "Ent-Abietanoids Isolated from Isodon serra" Molecules 22, no. 2: 309. https://doi.org/10.3390/molecules22020309
APA StyleWan, J., Jiang, H.-Y., Tang, J.-W., Li, X.-R., Du, X., Li, Y., Sun, H.-D., & Pu, J.-X. (2017). Ent-Abietanoids Isolated from Isodon serra. Molecules, 22(2), 309. https://doi.org/10.3390/molecules22020309