Synthesis of 16 New Hybrids from Tetrahydropyrans Derivatives and Morita˗Baylis˗Hillman Adducts: In Vitro Screening against Leishmania donovani
Abstract
:1. Introduction
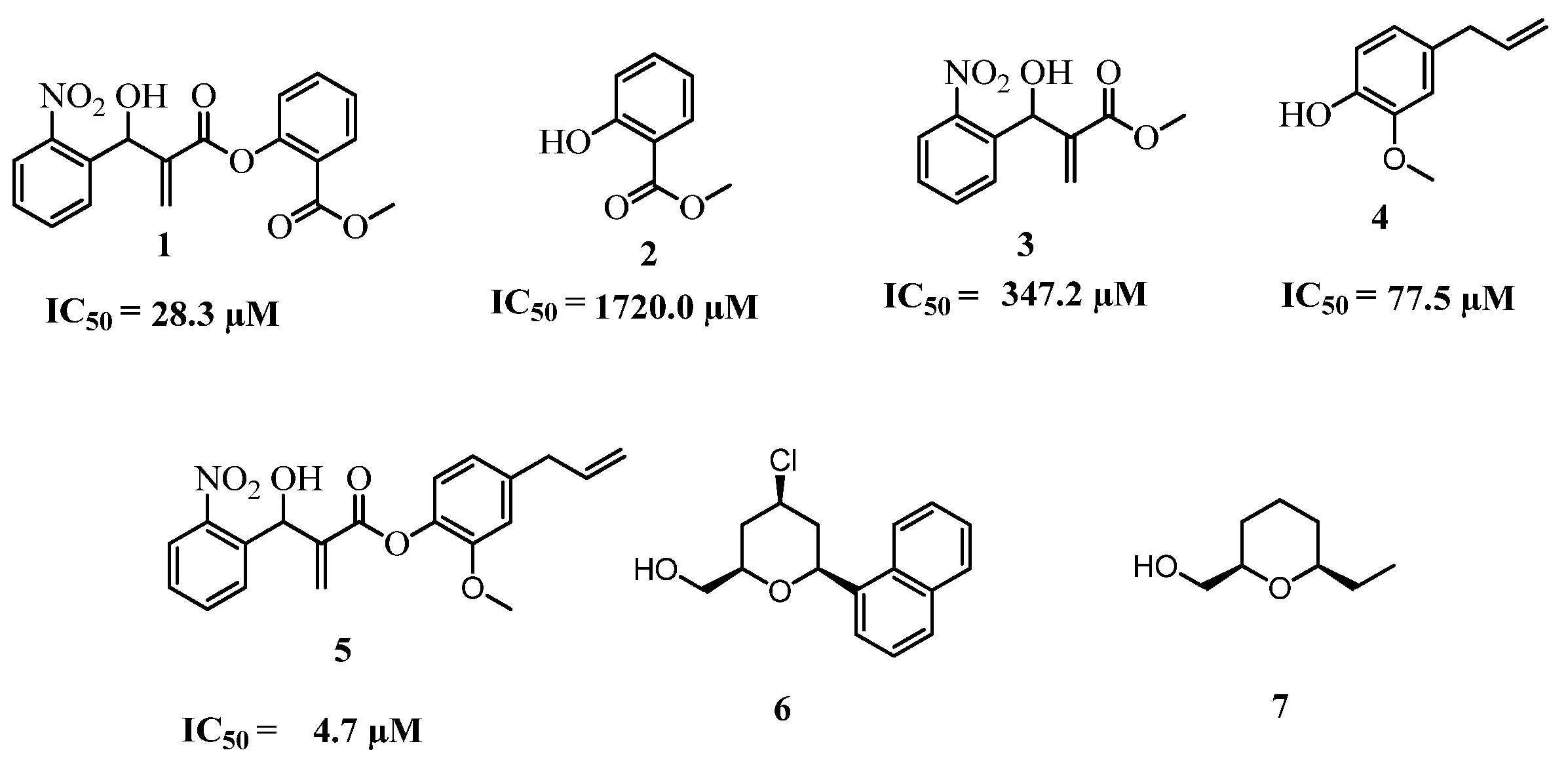
2. Results and Discussion
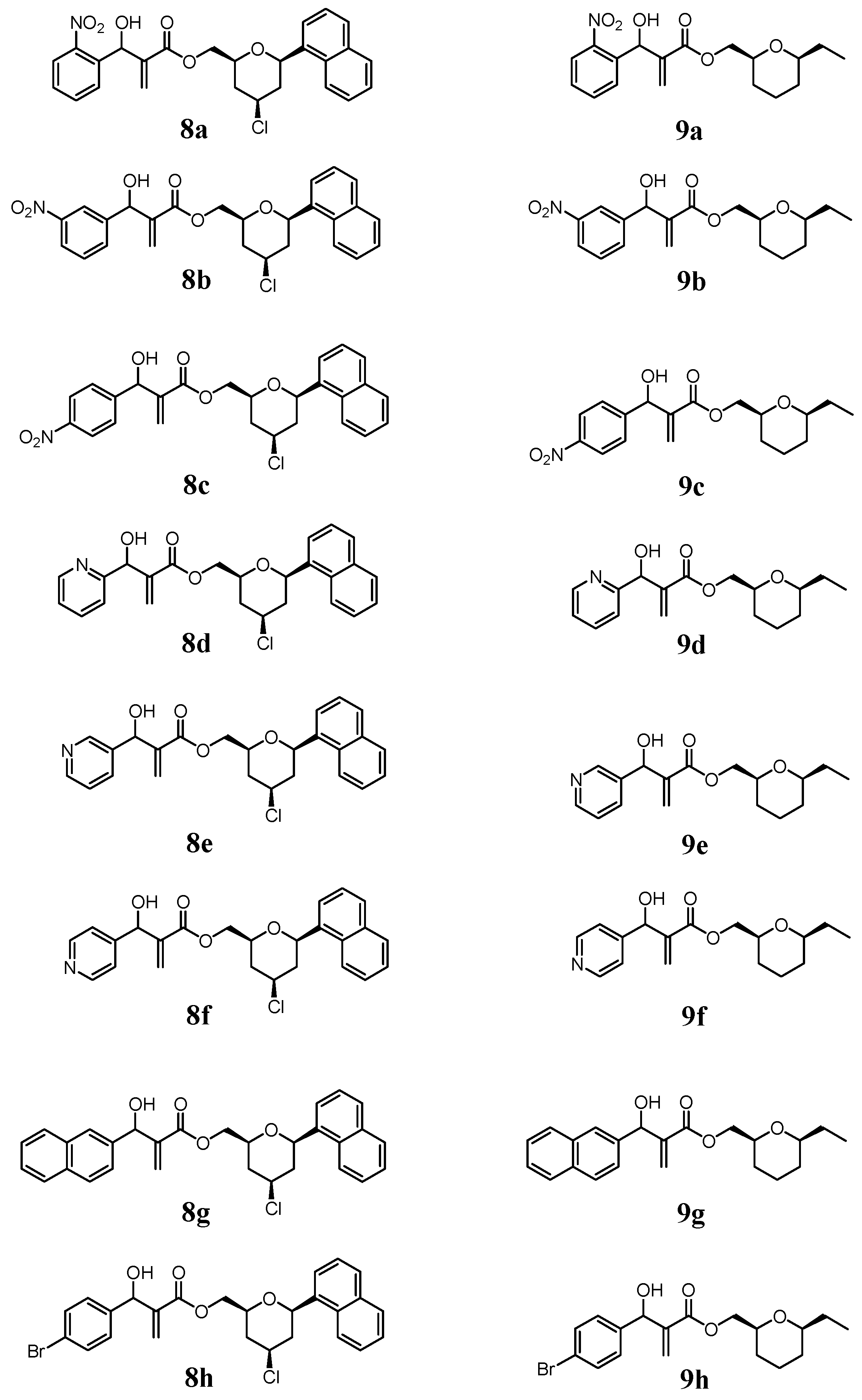
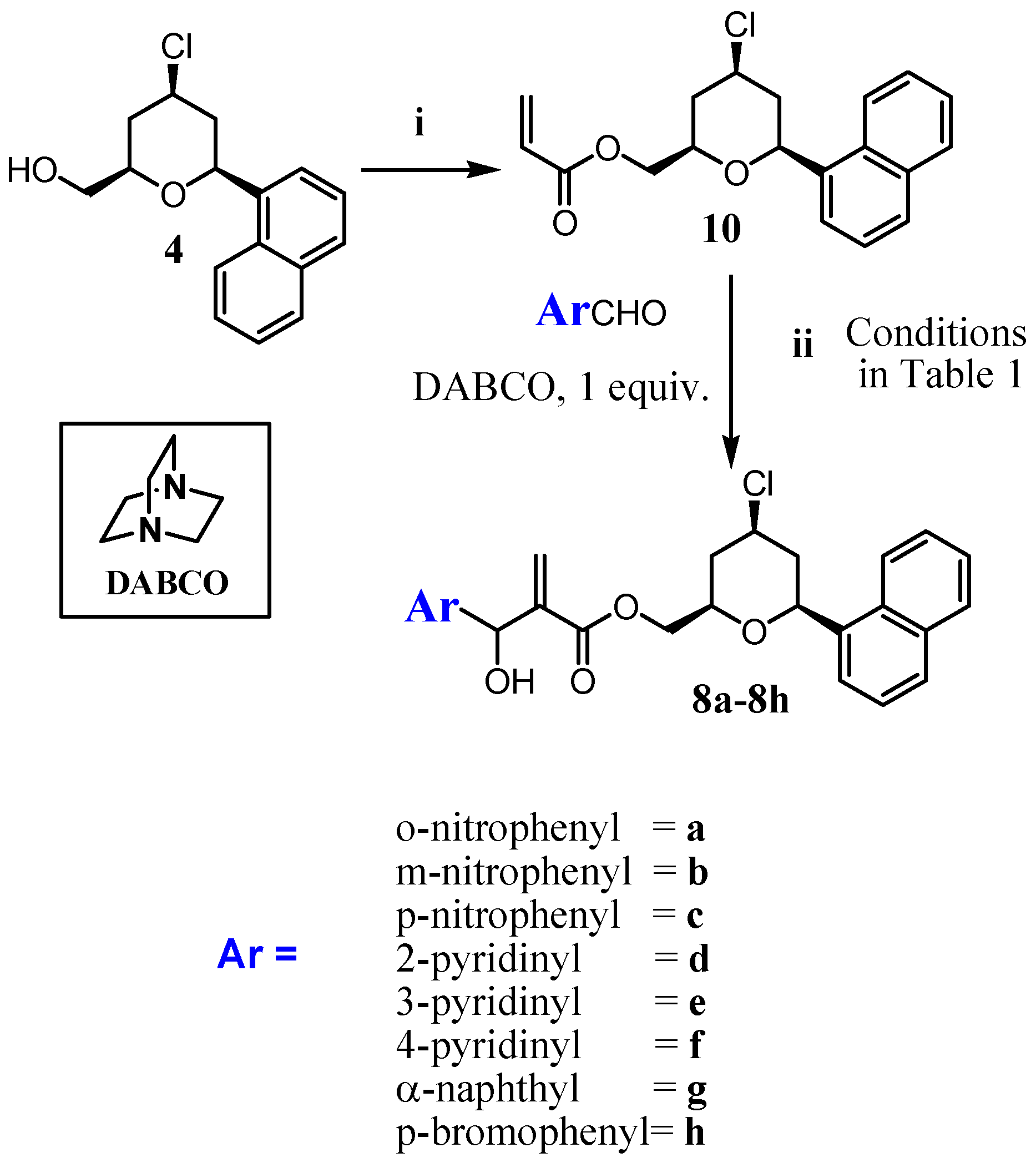
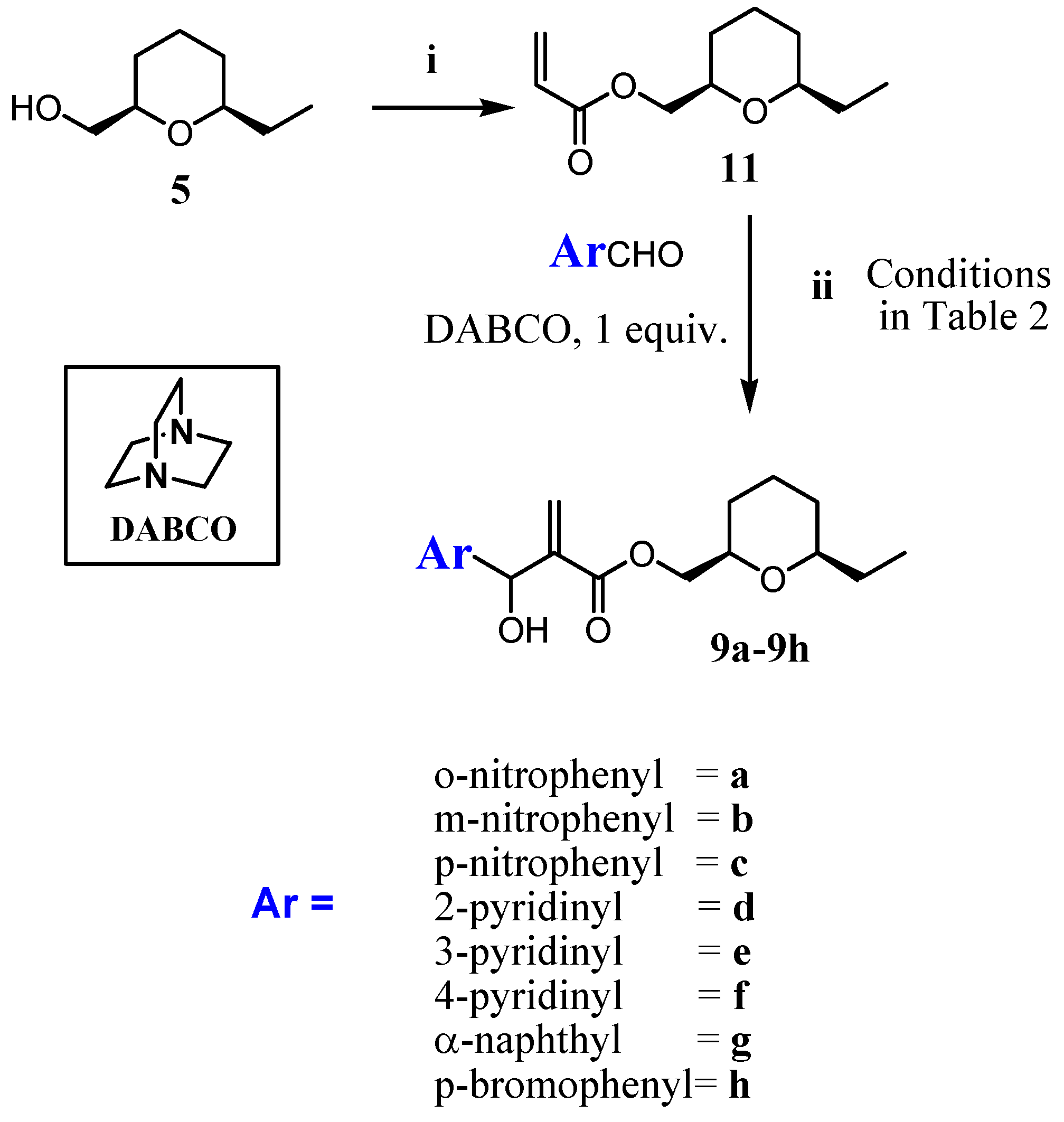
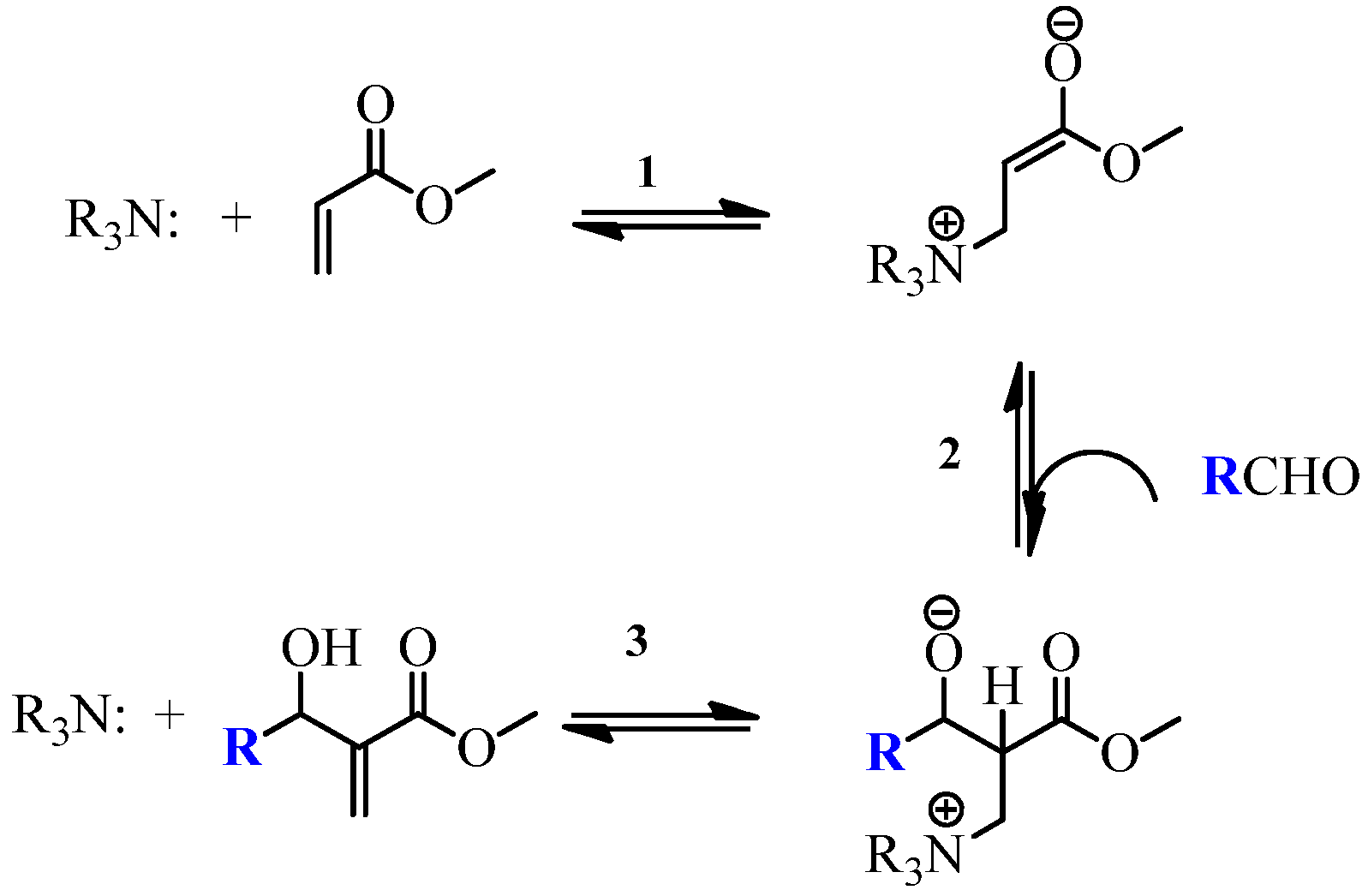
2.1. Chemistry
2.2. Biology
3. Materials and Methods
3.1. Chemistry
3.1.1. General
3.1.2. Procedure for the Synthesis of Acrylate 10 and 11
3.1.3. General Procedure for the Synthesis of MBHA (8a–8h) and (9a–9h)
3.5. Biology
3.5.1. Leishmania Culture
3.5.2. Effect of Morita-Baylis-Hillman Adduct in Promastigotes of Leishmania donovani
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References and Notes
- Feasey, N.; Wansbrough-Jones, M.; Mabey, D.C.W.; Solomon, A.W. Neglected tropical diseases. Br. Med. Bull. 2010, 93, 179–200. [Google Scholar] [CrossRef] [PubMed]
- Alvar, J.; Vélez, I.D.; Bern, C.; Herrero, M.; Desjeux, P.; Cano, J.; Jannin, J.; den-Boer, M. Leishmaniasis Worldwide and Global Estimates of Its Incidence. PLoS ONE 2012, 7, e35671. [Google Scholar] [CrossRef] [PubMed]
- Romero, G.A.S.; Boelaert, M. Control of Visceral Leishmaniasis in Latin America—A Systematic Review. PLoS NTD 2010, 1, e584. [Google Scholar] [CrossRef] [PubMed]
- Forestier, C.L. Imaging host-Leishmania interactions: significance in visceral leishmaniasis. Parasite Immunol. 2013, 35, 256–266. [Google Scholar] [CrossRef] [PubMed]
- Lima–Junior, C.G.; Vasconcellos, M.L.A.A. Morita–Baylis–Hillman adducts: Biological activities and potentialities to the discovery of new cheaper drugs. Bioorg. Med. Chem. 2012, 20, 3954–3971. [Google Scholar] [CrossRef] [PubMed]
- Basavaiah, D.; Reddy, B.S.; Badsara, S.S. Recent Contributions from the Baylis−Hillman Reaction to Organic Chemistry. Chem. Rev. 2010, 110, 5447–5674. [Google Scholar] [CrossRef] [PubMed]
- Santos, M.S.; Coelho, F.; Lima-Junior, C.G.; Vasconcellos, M.L.A.A. The Morita-Baylis-Hillman Reaction: Advances and Contributions from Brazilian Chemistry. Curr. Org. Synt. 2015, 12, 830–852. [Google Scholar] [CrossRef]
- Viegas-Junior, C.; Danuello, A.; Bolzani, V.S.; Barreiro, E.J.; Fraga, C.A.M. Molecular Hybridization: A Useful Tool in the Design of New Drug Prototypes. Curr. Med. Chem. 2007, 14, 1829–1852. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, T.P.; Sousa, S.C.O.; Amorim, F.M.; Rodrigues, Y.K.S.; de Assis, P.A.C.; Caldas, J.P.A.; Oliveira, M.R.; Vasconcellos, M.L.A.A. Design, synthesis and antileishmanial in vitro activity of new series of chalcones-like compounds: A molecular hybridization approach. Bioorg. Med. Chem. 2011, 19, 4250–4256. [Google Scholar] [CrossRef] [PubMed]
- Xavier, F.J.S.; Rodrigues, K.A.F.; Oliveira, R.G.; Lima-Junior, C.G.; Rocha, J.C.; Keesen, T.S.L.; Oliveira, M.R.; Silva, F.P.L.; Vasconcellos, M.L.A.A. Synthesis and In Vitro Anti Leishmania amazonenses Biological Screening of Morita-Baylis-Hillman Adducts Prepared from Eugenol, Thymol and Carvacrol. Molecules 2016, 21, 1483. [Google Scholar] [CrossRef] [PubMed]
- Capim, S.L.; Carneiro, P.H.P.; Castro, P.C.; Barros, M.R.M.; Marinho, B.G.; Vasconcellos, M.L.A.A. Design, Prins-cyclization reaction promoting diastereoselective synthesis of 10 new tetrahydropyran derivatives and in vivo antinociceptive evaluations. Eur. J. Med. Chem. 2012, 58, 1–11. [Google Scholar] [CrossRef] [PubMed]
- The 2D-NOESY spectrum of compound 6 described on reference 11, presented significant signals of correlation between Ha/ Hb, Ha/Hc and Hb/Hc that determine unequivocally the 2,4,6-cis geometry of tetrahydropyran ring. Thus, the relative configuration of the C2 -C4-C6 in the compounds 8a-8h in the tetrahydropyran ring are the same as those determined for 6. However, the relative configuration in the carbinolic carbon in 8a-8h has not been determined in this article.
- Miranda, L.S.M.; Marinho, B.G.; Leitão, S.G.; Matheus, M.E.; Fernandes, P.D.; Vasconcellos, M.L.A.A. (±)-cis-(6-Ethyl-tetrahydropyran-2-yl)-formic acid: A novel substance with antinociceptive properties. Bioorg. Med. Chem. Lett. 2004, 14, 1573–1575. [Google Scholar] [CrossRef] [PubMed]
- Eliel, E.L.; Manoharan, M.; Pietrusiewicz, K.M.; Hargrave, K.D. Carbon 13 NMR Spectra of Saturated Heterocycles. XI:-Tetrahydropyrans (Oxanes). Org. Magn. Res. 1983, 21, 94–107. [Google Scholar] [CrossRef]
- Gonçalves, G.M.; Capim, S.L.; Vasconcellos, M.L.A.A.; Marinho, B.G. Antihyperalgesic effect of [(±)-(2,4,6-cis)-4-chloro-6-(naphthalen-1-yl)-tetrahydro-2H-pyran-2-yl]methanol:participation of the NO/cGMP/KATP pathway and κ-opioid receptor. Behav. Pharmacol. 2016, 27, 506–515. [Google Scholar] [CrossRef] [PubMed]
- Calculated ED50 values from the acetic acid-induced abdominal writhes in mice to 6 = 27.97 μmol·kg–1 Capim, S.L.; Gonçalves, G.M.; dos Santos, G.C.M.; Marinho, B.G.; Vasconcellos, M.L.A.A. High analgesic and anti-inflammatory in vivo activities of six new hybrids NSAIAs tetrahydropyran derivatives. Bioorg. Med. Chem. 2013, 21, 6003–6010. [Google Scholar] [CrossRef] [PubMed] Calculated ED50 values from the acetic acid-induced abdominal writhes in mice to 7 = 41.67 μmol·kg–1 (unpublished results).
- The relative cis configuration of the groups present in the C2 and C6 positions of the corresponding carboxylic acid prepared from the alcohol 7, were determinated by 13C NMR spectroscopy through the characteristic down field shift of the C2, C6 carbons (see references 28 and 29). Thus, the relative configuration of the C2-C6 in the compounds 9a–9h in the tetrahydropyran ring are the same as those determined for 7. However, the relative configuration in the carbinolic carbon in 9a-9h has not been determined in this article.
- De Souza, R.O.M.A.; Pereira, V.L.P.; Muzitano, M.F.; Rossi-Bergmann, B.; Filho, E.B.A.; Vasconcellos, M.L.A.A. High selective leishmanicidal activity of 3-hydroxy-2-methylene-3-(4-bromophenyl) propanenitrile and analogous compounds. Eur. J. Med. Chem. 2007, 42, 99–102. [Google Scholar] [CrossRef] [PubMed]
- Junior, C.G.L.; de Assis, P.A.C.; Silva, F.P.L.; Sousa, S.C.O.; de Andrade, N.G.; Barbosa, T.P.; Nerís, P.L.N.; Segundo, L.V.G.; Anjos, I.C.; Carvalho, G.A.U.; et al. Efficient synthesis of 16 aromatic Morita-Baylis-Hillman adducts: biological evaluation on Leishmania amazonensis and Leishmania chagasi. Bioorg. Chem. 2010, 38, 279–284. [Google Scholar] [CrossRef] [PubMed]
- Silva, F.P.L.; de Assis, P.A.C.; Lima-Junior, C.G.; de Andrade, N.G.; da Cunha, S.M.D.; Oliveira, M.R.; Vasconcellos, M.L.A.A. Synthesis, evaluation against Leishmania amazonensis and cytotoxicity assays in macrophages of sixteen new congeners Morita-Baylis-Hillman adducts. Eur. J. Med. Chem. 2011, 46, 4295–4301. [Google Scholar] [CrossRef] [PubMed]
- Junior, C.G.L.; Silva, F.P.L.; Oliveira, R.G.; Subrinho, F.L.; Andrade, N.G.; Vasconcellos, M.L.A.A. Microwave Irradiation or Low Temperature Improved Synthesis of Antiparasitic Morita-Baylis-Hillman Adducts. J. Braz. Chem. Soc. 2011, 22, 2220–2224. [Google Scholar] [CrossRef]
- Muylder, G.D.; Ang, K.K.H.; Chen, S.; Arkin, M.R.; Engel, J.C.; McKerrow, J.H. A Screen against Leishmania Intracellular Amastigotes: Comparison to a Promastigote Screen and Identificationof a Host Cell-Specific Hit. PLoS NTD 2011, 5, e1253. [Google Scholar] [CrossRef] [PubMed]
- Rafel, S.; Leahy, J.W. An unexpected rate acceleration practical improvements in the Baylis-Hillman reaction. J. Org. Chem. 1997, 62, 1521–1522. [Google Scholar] [CrossRef]
- Vasconcellos, M.L.A.A.; de Souza, R.O.M.A. Intrinsic catalytic activity of tertiary amines: a mechanistic proposal to the unexpected temperature effect in Baylis-Hillman reaction. Catal. Commun. 2004, 5, 21–24. [Google Scholar]
- Price, K.E.; Broadwater, S.J.; Walker, B.J.; McQuade, D.T. A new interpretation of the Baylis-Hillman mechanism. J. Org. Chem. 2005, 70, 3980–3987. [Google Scholar] [CrossRef] [PubMed]
- Robiette, R.; Aggarwal, V.K.; Harvey, J.N. Mechanism of the Morita-Baylis-Hillman reaction: a computational investigation. J. Am. Chem. Soc. 2007, 129, 15513–15525. [Google Scholar] [CrossRef] [PubMed]
- Plata, R.E.; Singleton, D.A. A Case Study of the Mechanism of Alcohol-Mediated Morita Baylis–Hillman Reactions. The Importance of Experimental Observations. J. Am. Chem. Soc. 2015, 137, 3811–3826. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, W.A.V.; Rodrigues, D.C.; Oliveira, R.G.; Mendes, R.K.S.; Olegário, T.R.; Rocha, J.C.; Keesen, T.S.L.; Lima-Junior, C.G.; Vasconcellos, M.L.A.A. Synthesis and activity of novel homodimers of Morita–Baylis–Hillman adducts against Leishmania donovani: A twin drug approach. Bioor. Med. Chem. Lett. 2016, 26, 4523–4526. [Google Scholar] [CrossRef] [PubMed]
- Morais-Teixeira, E.; Damasceno, Q.S.; Galuppo, M.K.; Romanha, A.J.; Rubella, A. The in vitro leishmanicidal activity of hexadecylphosphocholine (miltefosine) against four medically relevant Leishmania species of Brazil. Mem. Inst. Oswaldo Cruz 2011, 106, 475–478. [Google Scholar] [CrossRef] [PubMed]
- Valadares, D.G.; Duarte, M.C.; Oliveira, J.S.; Chávez-Fumagalli, M.A.; Martins, V.T.; Costa, L.E.; Leite, J.P.V.; Santoro, M.M.; Régis, W.C.B.; Tavares, C.A.P.; et al. Leishmanicidal activity of the Agaricus blazei Murill in different Leishmania species. Parasitol. Int. 2011, 60, 357–363. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Samples of the compounds not available from the authors.
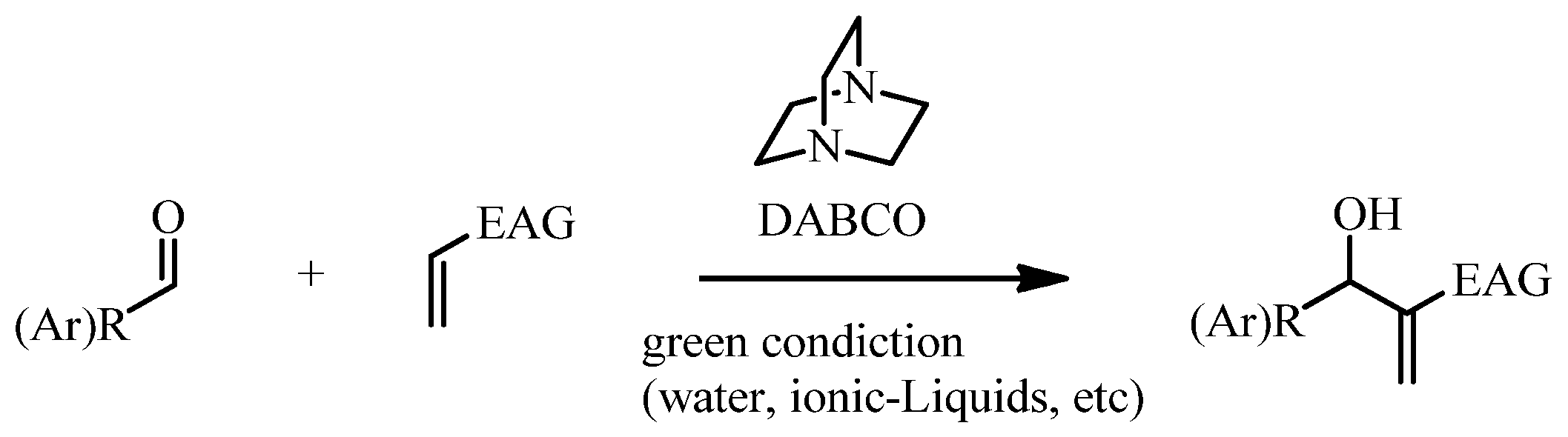

| Entry | Ar | Hybrid | Solvent a | Temp. (°C) b | Time (h) | Yield (%) c |
|---|---|---|---|---|---|---|
| 1 | o-nitrophenyl | 8a | t-buOH/H2O | 80 Mw | 1 | 85 |
| 2 | m-nitrophenyl | 8b | t-buOH/H2O | 80 Mw | 0.7 | 34d |
| 3 | m-nitrophenyl | 8b | DMF:H2O | 0 | 48 | 81 |
| 4 | p-nitrophenyl | 8c | t-buOH/H2O | 80 Mw | 0.7 | 86 |
| 5 | 2-pyridinyl | 8d | t-buOH/H2O | 80 Mw | 1.3 | 81 |
| 6 | 3-pyridinyl | 8e | t-buOH/H2O | 80 Mw | 2 | 80 |
| 7 | 4-pyridinyl | 8f | t-buOH/H2O | 80 Mw | 1 | 87 |
| 8 | α-naphthyl | 8g | t-buOH/H2O | 80 Mw | 2 | n.r. |
| 9 | α-naphthyl | 8g | DMF:H2O | 0 | 48 | 45 |
| 10 | p-bromophenyl | 8h | t-buOH/H2O | 80 Mw | 2 | n.r. |
| 11 | p-bromophenyl | 8h | DMF:H2O | 0 | 240 | 60 |
| Entry | Ar | Hybrid | Solvent a | Temp. (°C) b | Time (h) | Yield (%) c,d |
|---|---|---|---|---|---|---|
| 1 | o-nitrophenyl | 9a | t-buOH/H2O | 80 Mw | 0.7 | 74 |
| 2 | m-nitrophenyl | 9b | t-buOH/H2O | 80 Mw | 0.7 | 78 |
| 3 | p-nitrophenyl | 9c | t-buOH/H2O | 80 Mw | 0.3 | 95 |
| 4 | 2-pyridinyl | 9d | t-buOH/H2O | 80 Mw | 0.8 | 96 |
| 5 | 3-pyridinyl | 9e | t-buOH/H2O | 80 Mw | 1.3 | 80 |
| 6 | 4-pyridinyl | 9f | t-buOH/H2O | 80 Mw | 0.7 | 72 |
| 7 | 4-pyridinyl | 9f | t-buOH/H2O, PhOH | 80 Mw | 0.3 | 96 |
| 8 | α-naphthyl | 9g | t-buOH/H2O | 80 Mw | 2 | n.r. |
| 9 | α-naphthyl | 9g | DMF/H2O, PhOH | 0 | 96 | 65 |
| 10 | p-bromophenyl | 9h | t-buOH/H2O | 80 Mw | 2 | n.r. |
| 11 | p-bromophenyl | 9h | DMF/H2O, PhOH | 0 | 240 | 50 |
| Compounds | IC50 a (μg·mL−1) | IC50 a (μM) | HC50 b (μg·mL−1) | HC50 b (μM) | SIrb c |
|---|---|---|---|---|---|
| 6 | 47.53 ± 39 | 172.19 ± 141.25 | - | - | - |
| 7 | >400 | >2775.46 | - | - | - |
| 8a | 7.09 ± 3.0 | 14.74 ± 6.23 | >400 | >831.38 | >56.40 |
| 8b | 8.76 ± 4.0 | 18.21 ±8.31 | - | - | - |
| 8c | 9.05 ± 3.3 | 18.81 ± 6.85 | - | - | - |
| 8d | 61.93 ± 18 | 141.67 ± 41.17 | - | - | - |
| 8e | 8.76 ± 3.8 | 20.04 ± 8.69 | - | - | - |
| 8f | 8.96 ± 4.5 | 20.50 ± 10.29 | - | - | - |
| 8g | 24.51± 7.5 | 50.42 ± 15.42 | - | - | - |
| 8h | 17.17 ± 5.6 | 33.40 ± 10.90 | - | - | - |
| 9a | 5.64 ± 3.0 | 16.15 ± 8.60 | >400 | >1145.64 | >70.94 |
| 9b | 13.75 ± 9.8 | 39.38 ± 28.06 | - | - | - |
| 9c | 7.03 ± 4.3 | 20.13 ± 12.31 | >400 | >1145.64 | >56.91 |
| 9d | 72.32 ± 26.0 | 240.27 ± 85.20 | - | - | - |
| 9e | 17.68 ± 7.7 | 57.94 ± 25.23 | - | - | - |
| 9f | 57.76 ± 20.0 | 189.28 ± 65.53 | - | - | - |
| 9g | 9.73 ± 4.8 | 27.47 ± 13.55 | - | - | - |
| 9h | 2.22 ± 7.6 | 5.81 ± 19.90 | >400 | >1046.90 | >180.19 |
| Amphotericin B | 0.35 ± 0.27 | 0.38 ± 0.30 | 11.61 | 12.56 | 33.05 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sousa, S.C.d.O.; Rocha, J.D.C.; Keesen, T.D.S.L.; Silva, E.D.P.; De Assis, P.A.C.; De Oliveira, J.P.G.; Capim, S.L.; Xavier, F.J.S.; Marinho, B.G.; Silva, F.P.L.; et al. Synthesis of 16 New Hybrids from Tetrahydropyrans Derivatives and Morita˗Baylis˗Hillman Adducts: In Vitro Screening against Leishmania donovani. Molecules 2017, 22, 207. https://doi.org/10.3390/molecules22020207
Sousa SCdO, Rocha JDC, Keesen TDSL, Silva EDP, De Assis PAC, De Oliveira JPG, Capim SL, Xavier FJS, Marinho BG, Silva FPL, et al. Synthesis of 16 New Hybrids from Tetrahydropyrans Derivatives and Morita˗Baylis˗Hillman Adducts: In Vitro Screening against Leishmania donovani. Molecules. 2017; 22(2):207. https://doi.org/10.3390/molecules22020207
Chicago/Turabian StyleSousa, Suervy Canuto de Oliveira, Juliana Da Câmara Rocha, Tatjana De Souza Lima Keesen, Everton Da Paz Silva, Priscilla Anne Castro De Assis, João Paulo Gomes De Oliveira, Saulo Luís Capim, Francisco José Seixas Xavier, Bruno Guimarães Marinho, Fábio Pedrosa Lins Silva, and et al. 2017. "Synthesis of 16 New Hybrids from Tetrahydropyrans Derivatives and Morita˗Baylis˗Hillman Adducts: In Vitro Screening against Leishmania donovani" Molecules 22, no. 2: 207. https://doi.org/10.3390/molecules22020207







