Effects of Bacterial Fermentation on the Biochemical Constituents and Antioxidant Potential of Fermented and Unfermented Soybeans Using Probiotic Bacillus subtilis (KCTC 13241)
Abstract
:1. Introduction
2. Results and Discussion
2.1. Viable Cell Number and pH of Unfermented Soybeans and Cheonggukjang
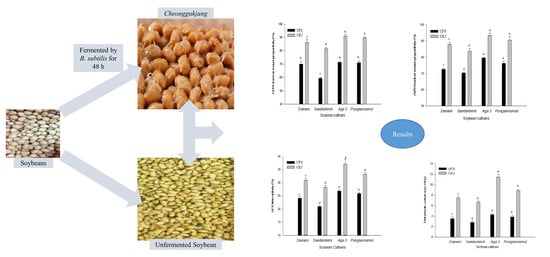
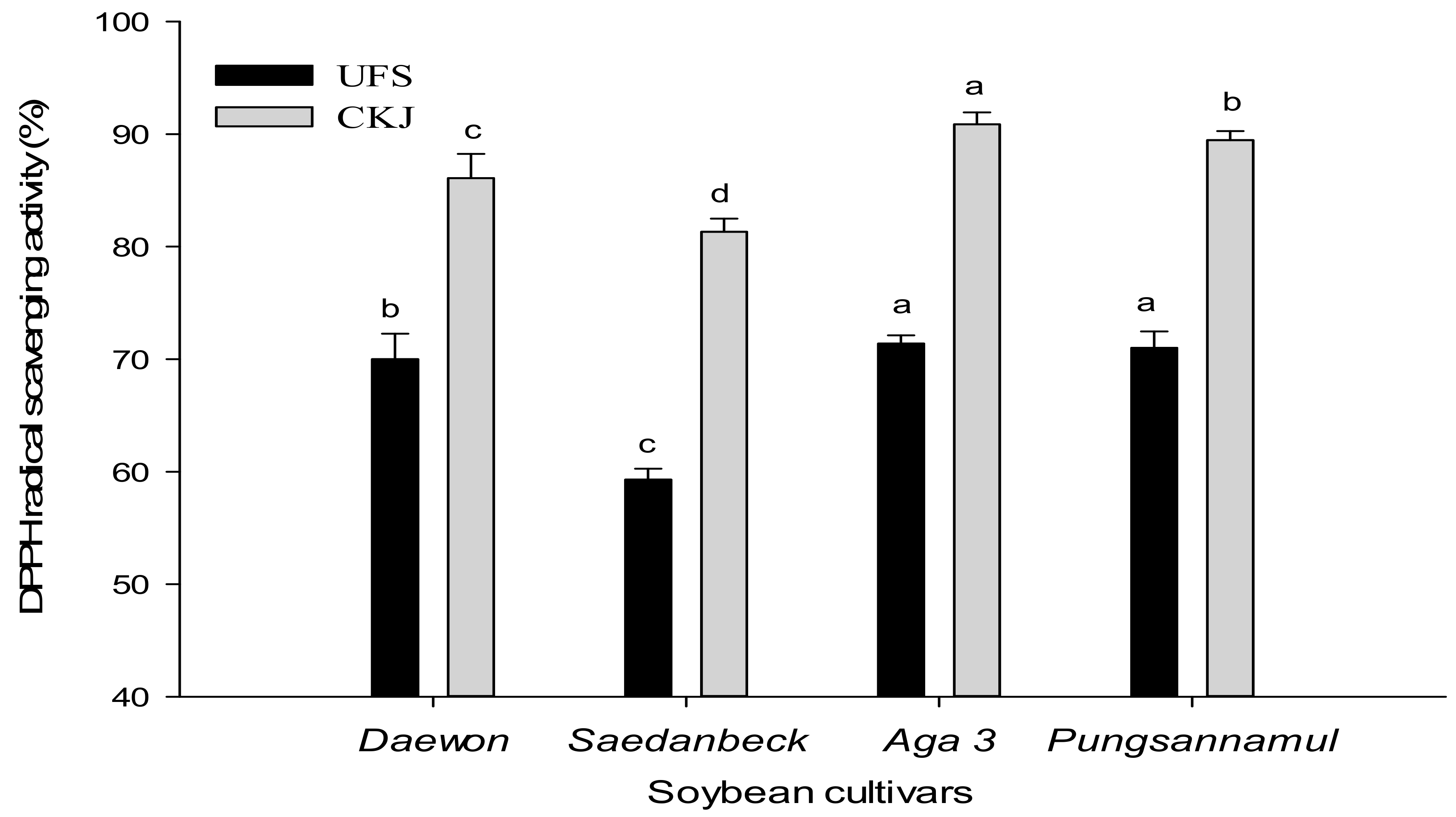
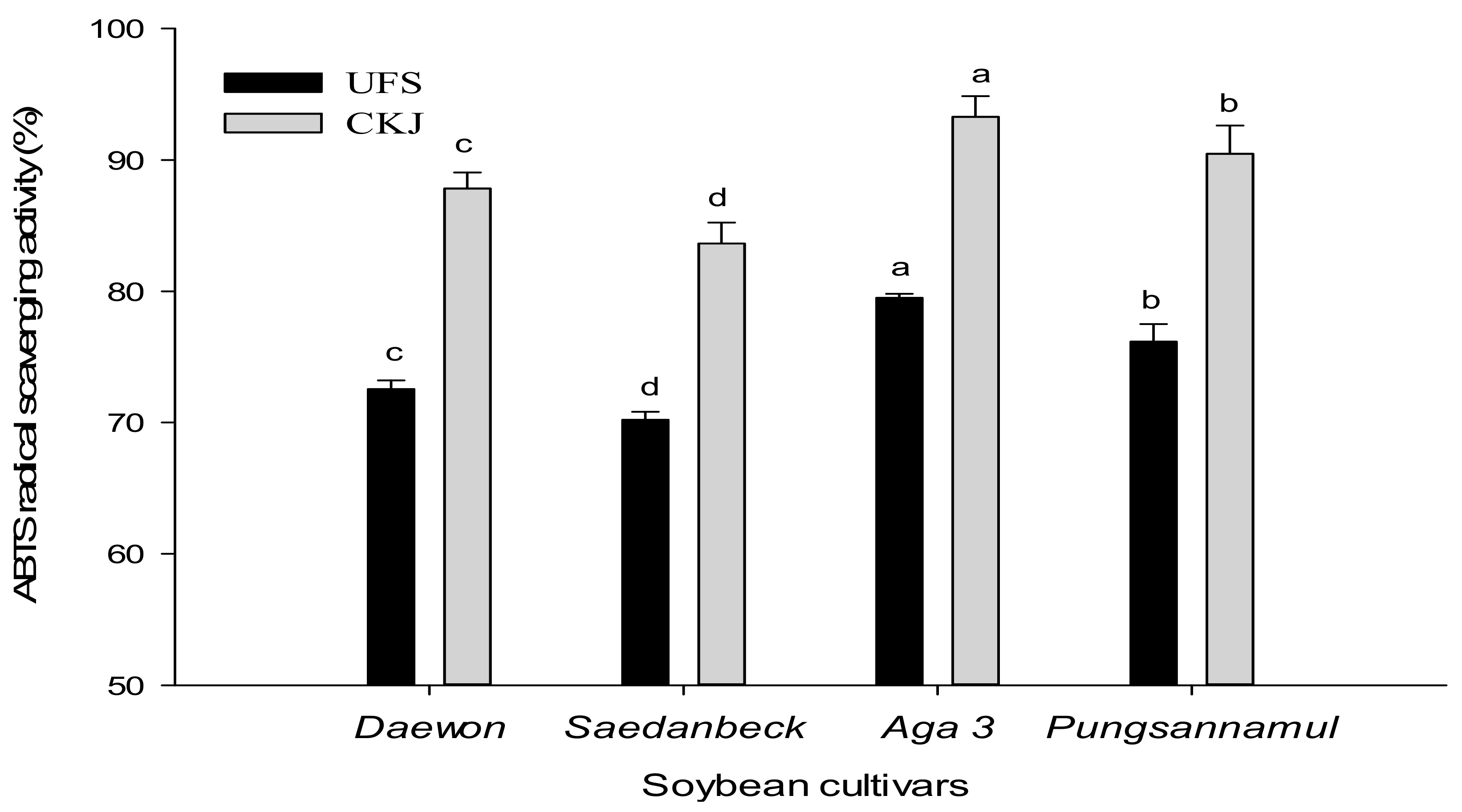
2.2. Antioxidant Activity of Unfermented Soybeans and Cheonggukjang
2.3. Total Phenolic Contents of Unfermented Soybeans and Cheonggukjang
2.4. Isoflavone Composition of Unfermented Soybeans and Cheonggukjang
2.5. Free Amino Acid Composition
3. Materials and Methods
3.1. Chemical
3.2. Soybean Cultivars and Microorganisms
3.3. Preparation of Cheonggukjang
3.4. Viable Cell Number and pH
3.5. Sample Extraction
3.6. DPPH Radical-Scavenging Activity
3.7. ABTS Radical-Scavenging Activity
3.8. Superoxide Dismutase (SOD)-Like Activity
3.9. Isoflavone Analysis
3.10. Total Phenolic Contents (TPC)
3.11. Free Amino Acid Composition
3.12. Statistical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- García, M.C.; Puchalska, P.; Esteve, C.; Marina, M.L. Vegetable foods: A cheap source of proteins and peptides with antihypertensive, antioxidant, and other less occurrence bioactivities. Talanta 2013, 106, 328–349. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.H.; Kim, S.H.; Chung, J.I.; Chi, H.Y.; Kim, J.A.; Chung, I.M. Analysis of phenolic compounds and isoflavones in soybean seeds (Glycine max (L.) Merill) and sprouts grown under different conditions. Eur. Food Res. Technol. 2006, 222, 201–208. [Google Scholar] [CrossRef]
- Coward, L.; Barnes, N.C.; Setchell, K.D.; Barnes, S. Genistein, Daidzein, and Their & Glycoside Conjugates: Antitumor Isoflavones in Soybean Foods from American and Asian Diets. J. Agric. Food Chem. 1993, 41, 1961–1967. [Google Scholar]
- Robert, L.; Walter, J. Compositional changes in trypsin inhibitors, phytic acid, saponins and isoflavones related to soybean processing. J. Nutr. 1995, 125, S581. [Google Scholar]
- Kumar, M.; Nagpal, R.; Kumar, R.; Hemalatha, R.; Verma, V.; Kumar, A.; Chakraborty, C.; Singh, B.; Marotta, F.; Jain, S.; et al. Cholesterol-lowering probiotics as potential biotherapeutics for metabolic diseases. Exp. Diabetes Res. 2012, 2012. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Ge, C.; Yuan, W.; Zhu, R.; Zhang, W.; Du, L.; Xue, J. Characterization of fermented black soybean natto inoculated with Bacillus natto during fermentation. J. Sci. Food Agric. 2010, 1194–1202. [Google Scholar] [CrossRef] [PubMed]
- Sheih, I.C.; Wu, H.Y.; Lai, Y.J.; Lin, C.F. Preparation of high free radical scavenging tempeh by a newly isolated Rhizopus sp. R-69 from Indonesia. Food Sci. Agric. Chem. 2000, 2, 35–40. [Google Scholar]
- Yen, G.; Chang, Y.; Su, S. Antioxidant activity and active compounds of rice koji fermented with Aspergillus candidus. Food Chem. 2003, 83, 49–54. [Google Scholar] [CrossRef]
- Cao, X.; Wang, A.H.; Jiao, R.Z.; Wang, C.L.; Mao, D.Z.; Yan, L.; Zeng, B. Surfactin Induces Apoptosis and G 2/M Arrest in Human Breast Cancer MCF-7 Cells Through Cell Cycle Factor Regulation. Cell Biochem. Biophys. 2009, 163–171. [Google Scholar] [CrossRef] [PubMed]
- Yee, N.; Jeong, E.; Young, D.; Pyo, H. Antioxidant and antigenotoxic activities of Korean fermented soybean. Food Chem. Toxicol. 2008, 46, 1184–1189. [Google Scholar] [CrossRef]
- Byun, M.; Son, J.; Yook, H.; Jo, C.; Kim, D. Effect of gamma irradiation on the physiological activity of Korean soybean fermented foods, Chungkookjang and Doenjang. Radiat. Phys. Chem. 2002, 64, 245–248. [Google Scholar] [CrossRef]
- Omura, K.; Hitosugi, M.; Zhu, X.; Ikeda, M.; Maeda, H. A Newly Derived Protein from Bacillus subtilis natto with Both Antithrombotic and Fibrinolytic Effects. J. Pharmacol. Sci. 2005, 251, 247–251. [Google Scholar] [CrossRef]
- D’Adamo, C.R.; Sahin, A. Soy foods and supplementation: A review of commonly perceived health benefits and risks. Altern. Ther. Health Med. 2014, 20 (Suppl. 1), 39–51. [Google Scholar] [CrossRef] [PubMed]
- Cho, K.M.; Lee, J.H.; Yun, H.D.; Ahn, B.Y.; Kim, H.; Seo, W.T. Changes of phytochemical constituents (isoflavones, flavanols, and phenolic acids) during cheonggukjang soybeans fermentation using potential probiotics Bacillus subtilis CS90. J. Food Compos. Anal. 2011, 24, 402–410. [Google Scholar] [CrossRef]
- Cho, K.M.; Hong, S.Y.; Math, R.K.; Lee, J.H.; Kambiranda, D.M.; Kim, J.M.; Islam, S.M.A.; Yun, M.G.; Cho, J.J.; Lim, W.J.; et al. Biotransformation of phenolics (isoflavones, flavanols and phenolic acids) during the fermentation of cheonggukjang by Bacillus pumilus HY1. Food Chem. 2009, 114, 413–419. [Google Scholar] [CrossRef]
- Kim, M.; Han, S.; Ko, J.; Kim, Y. Degradation Characteristics of Proteins in Cheonggukjang (Fermented Unsalted Soybean Paste) Prepared with Various Soybean Cultivars. Food Sci. Biotechnol. 2012, 21, 9–18. [Google Scholar] [CrossRef]
- Shon, M.; Kim, T.; Sung, N. Antioxidants and free radical scavenging activity of Phellinus baumii (Phellinus of Hymenochaetaceae) extracts. Food Chem. 2003, 82, 593–597. [Google Scholar] [CrossRef]
- Haque, A.; Hwang, C.E.; Lee, H.Y.; Ahn, M.J.; Sin, E. Comparison of Isoflavone Contents and Antioxidant Effect in Cheonggukjang with Black Soybean Cultivars by Bacillus subtilis CSY191. Korean J. Environ. Agric. 2016, 35, 62–71. [Google Scholar] [CrossRef]
- Almeida, M.M.B.; de Sousa, P.H.M.; Arriaga, Â.M.C.; do Prado, G.M.; de Carvalho Magalhães, C.E.; Maia, G.A.; de Lemos, T.L.G. Bioactive compounds and antioxidant activity of fresh exotic fruits from northeastern Brazil. Food Res. Int. 2011, 44, 2155–2159. [Google Scholar] [CrossRef]
- Morales, G.; Paredes, A. Antioxidant activities of Lampaya medicinalis extracts and their main chemical constituents. BMC Complement. Altern. Med. 2014, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Cho, M.J.; Lee, J.Y.; Kim, J.H. Microbial and Physiochemical Properties of Cheonggukjang Fermented Using Bacillus Strains with Antibacterial or Antifungal Activities. Food Sci. Biotechnol. 2014, 23, 1525–1532. [Google Scholar] [CrossRef]
- Hwang, C.E.; Seo, W.T.; Cho, K.M. Enhanced Antioxidant Effect of Black Soybean by Cheonggukjang with Potential Probiotic Bacillus subtilis CSY191. Korean J. Environ. Agric. 2013, 49, 391–397. [Google Scholar] [CrossRef]
- Oh, H.J.; Kim, C.S. Antioxidant and Nitrite Scavenging Ability of Fermented Soybean Foods (Chungkukjang, Doenjang). J. Korean Soc. Food Sci. Nutr. 2007, 36, 1503–1510. [Google Scholar] [CrossRef]
- Choi, U.; Kim, M.; Lee, N.H.; Jeong, Y.; Kwon, O.; Kim, Y.; Hwang, Y. The Characteristics of Cheonggukjang, a Fermented Soybean Product, by the Degree of Germination of Raw Soybeans. Food Sci. Biotechnol. 2007, 16, 734–739. [Google Scholar]
- Wojdyło, A.; Oszmiański, J.; Czemerys, R. Antioxidant activity and phenolic compounds in 32 selected herbs. J. Food Chem. 2007, 105, 940–949. [Google Scholar] [CrossRef]
- Shin, E.C.; Lee, J.H.; Hwang, C.E.; Lee, B.W.; Kim, H.T.; Ko, J.M.; Baek, I.Y.; Shin, J.H.; Nam, S.H.; Seo, W.T.; et al. Enhancement of total phenolic and isoflavone-aglycone contents and antioxidant activities during Cheonggukjang fermentation of brown soybeans by the potential probiotic Bacillus subtilis CSY191. Food Sci. Biotechnol. 2014, 23, 531–538. [Google Scholar] [CrossRef]
- Vaya, J.; Tamir, S. The Relation Between the Chemical Structure of Flavonoids and Their Estrogen-Like Activities. Curr. Med. Chem. 2004, 11, 1333–1343. [Google Scholar] [CrossRef] [PubMed]
- Prabhakaran, M.P.; Perera, C.O.; Valiyaveettil, S. Effect of different coagulants on the isoflavone levels and physical properties of prepared firm tofu. J. Food Chem. 2006, 99, 492–499. [Google Scholar] [CrossRef]
- Yang, S.O.; Chang, P.S.; Lee, J.H. Isoflavone Distribution and β-Glucosidase Activity in Cheonggukjang, a Traditional Korean Whole Soybean-Fermented Food. Food Sci. Biotechnol. 2006, 15, 96–101. [Google Scholar]
- Velioglu, Y.S.; Mazza, G.; Gao, L.; Oomah, B.D. Antioxidant Activity and Total Phenolics in Selected Fruits, Vegetables, and Grain Products. J. Agric. Food Chem. 1998, 46, 4113–4117. [Google Scholar] [CrossRef]
- Mayer, R.R.; Cherry, J.H.; Rhodes, D. Effects of Heat Shock on Amino Acid Metabolism of Cowpea Cells. Plant Physiol. 1990, 94, 796–810. [Google Scholar] [CrossRef] [PubMed]
- Gerich, J.E.; Meyer, C.; Woerle, H.J.; Stumvoll, M. Renal Gluconeogenesis Its importance in human glucose homeostasis. Diabetes Care 2001, 24, 382–391. [Google Scholar] [CrossRef] [PubMed]
- Ruth, M.R.; Field, C.J. The immune modifying effects of amino acids on gut-associated lymphoid tissue. J. Anim. Sci. Biotechnol. 2013. [Google Scholar] [CrossRef] [PubMed]
- Genene, A.; Winans, S. Distribution of methionine and leucine enkephalin neurons within the social behavior circuitry of the male Syrian hamster brain. Brain Res. 2004, 1030, 28–48. [Google Scholar]
- Chen, P.E.; Geballe, M.T.; Stansfeld, P.J.; Johnston, A.R.; Yuan, H.; Jacob, A.L.; Snyder, J.P.; Traynelis, S.F.; Wyllie, D.J.A. Structural Features of the Glutamate Binding Site in Recombinant NR1/NR2A N-Methyl-d-aspartate Receptors Determined by Site-Directed Mutagenesis and Molecular Modeling. Mol. Pharmacol. 2005, 67, 1470–1484. [Google Scholar] [CrossRef] [PubMed]
- Vaquero, J.; Butterworth, R.F. The brain glutamate system in liver failure. J. Neurochem. 2006, 661–669. [Google Scholar] [CrossRef] [PubMed]
- Park, M.K.; Cho, I.H.; Lee, S.; Choi, H.K.; Kwon, D.Y.; Kim, Y.S. Metabolite profiling of Cheonggukjang, a fermented soybean paste, during fermentation by gas chromatography-mass spectrometry and principal component analysis. Food Chem. 2010, 122, 1313–1319. [Google Scholar] [CrossRef]
- Lee, N.K.; Park, J.W.; Cho, I.J.; Kim, B.; Kwon, K.; Hahm, Y.T. Isolation of Bacillus spp. from Cheonggukjang and Its Antagonistic Effect against Bacillus cereus. Korean J. Food Sci. Technol. 2008, 40, 669–673. [Google Scholar]
- Kim, M.-H.; Kim, S.-Y.; Ko, J.-M.; Jeong, D.-Y.; Kim, Y.-S. Biological activities of cheonggukjang prepared with several soybean cultivars. Food Sci. Biotechnol. 2012, 21, 475–483. [Google Scholar] [CrossRef]
- Xu, B.J.; Chang, S.K.C. A Comparative Study on Phenolic Profiles and Antioxidant Activities of Legumes as Affected by Extraction Solvents. J. Food Sci. 2005. [Google Scholar] [CrossRef] [PubMed]
- Blois, M.S. Antioxidant Determinations by the Use of a Stable Free Radical. Nature 1958, 181, 1199–1200. [Google Scholar] [CrossRef]
- Bilal, S.; Khan, A.L.; Waqas, M.; Shahzad, R.; Kim, I.D.; Lee, I.J.; Shin, D.H. Biochemical constituents and in vitro antioxidant and anticholinesterase potential of seeds from Native Korean Persimmon Genotypes. Molecules 2016, 21, 893. [Google Scholar] [CrossRef] [PubMed]
- Cho, K.M.; Seo, W.T. Bacterial Diversity in a Korean Traditional Soybean Fermented Foods (Doenjang and Ganjang) by 16S rRNA Gene Sequence Analysis. Food Sci. Biotechnol. 2007, 16, 320. [Google Scholar]
- Kuan, S. A Simplified HPLC Method for the Determination of Phytoestrogens in Soybean and Its Processed Products. J. Agric. Food Chem. 1990, 35, 185–190. [Google Scholar]
- Dewanto, V.; Wu, X.; Adom, K.K.; Liu, R.H. Thermal Processing Enhances the Nutritional Value of Tomatoes by Increasing Total Antioxidant Activity. J. Agric. Food Chem. 2002, 50, 3010–3014. [Google Scholar] [CrossRef] [PubMed]
- Waqas, M.; Shahzad, R.; Latif, A.; Asaf, S.; Kim, Y.; Kang, S.; Bilal, S.; Hamayun, M.; Lee, I. Plant Physiology and Biochemistry Salvaging effect of triacontanol on plant growth, thermotolerance, macro-nutrient content, amino acid concentration and modulation of defense hormonal levels under heat stress. Plant Physiol. Biochem. 2016, 99, 118–125. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are available from the authors. |


| Cultivars | Samples 1 | Viable Cell Numbers (log CFU/g) | pH |
|---|---|---|---|
| Daewon | UFS 2 | 4.85 ± 0.72 a | 5.87 ± 0.18 b |
| CKJ 3 | 10.21 ± 0.84 a | 7.79 ± 0.55 b | |
| Saedanbeck | UFS | 4.81 ± 0.41 a | 5.19 ± 0.35 c |
| CKJ | 10.11 ± 1.03 a | 7.02 ± 0.22 c | |
| Aga 3 | UFS | 5.18 ± 0.80 a | 6.26 ± 0.20 a |
| CKJ | 10.46 ± 1.21 a | 8.58 ± 0.28 a | |
| Pungsannamul | UFS | 5.08 ± 0.37 a | 6.19 ± 0.14 a |
| CKJ | 10.32 ± 0.36 a | 8.33 ± 0.12 a |
| Isoflavone µg/g | Sample 1 | Soybean Cultivars | |||
|---|---|---|---|---|---|
| Daewon | Saedanbeck | Aga 3 | Pungsannamul | ||
| Daidzin | UFS 2 | 145.60 ± 6.20 c | 126.54 ± 4.11 d | 347.04 ± 2.41 a | 236.39 ± 2.50 b |
| CKJ 3 | 47.70 ± 5.36 c | 26.84 ± 1.91 d | 162.05 ± 1.12 a | 130.68 ± 4.37 b | |
| Genistin | UFS | 163.01 ± 3.19 c | 145.01 ± 2.76 d | 424.25 ± 4.35 a | 262.71 ± 4.12 b |
| CKJ | 55.12 ± 4.11 c | 43.34 ± 4.03 d | 244.73 ± 2.59 a | 155.66 ± 3.46 b | |
| Glycitin | UFS | 17.39 ± 2.24 c | 10.91 ± 1.20 d | 93.85 ± 2.00 a | 55.79 ± 2.22 b |
| CKJ | 3.18 ± 0.90 c | 1.96 ± 0.39 d | 30.42 ± 2.50 a | 15.87 ± 1.97 b | |
| Daidzein | UFS | 66.48 ± 2.59 c | 34.82 ± 3.43 d | 119.45 ± 3.75 a | 88.60 ± 0.70 b |
| CKJ | 85.50 ± 1.42 c | 65.81 ± 2.58 d | 213.91 ± 4.17 a | 101.87 ± 2.36 b | |
| Glycitein | UFS | 40.35 ± 1.14 c | 30.34 ± 2.97 d | 91.48 ± 1.94 a | 74.68 ± 2.39 b |
| CKJ | 75.26 ± 3.74 c | 46.04 ± 2.49 d | 110.38 ± 2.45 a | 88.04 ± 3.87 b | |
| Genistein | UFS | 3.18 ± 0.31 a | 1.32 ± 0.48 b | 2.43 ± 0.45 a,b | 3.17 ± 0.89 a |
| CKJ | 7.64 ± 0.05 b | 4.16 ± 0.69 c | 24.50 ± 1.53 a | 8.63 ± 0.70 b | |
| Total isoflavones | UFS | 436.00 ± 0.54 c | 348.94 ± 0.36 d | 1078.50 ± 1.12 a | 721.34 ± 0.65 b |
| CKJ | 274.40 ± 0.23 c | 188.15 ± 0.43 d | 785.54 ± 0.87 a | 500.76 ± 0.87 b | |
| Amino Acids µg/g | Sample 1 | Daewon | Saedanbeck | Aga 3 | Pungsannamul |
|---|---|---|---|---|---|
| aspartic acid | UFS 2 | 99.38 ± 2.20 b | 43.39 ± 1.14 d | 138.33 ± 1.96 a | 56.52 ± 0.22 c |
| CKJ 3 | 443.95 ± 2.15 a | 368.56 ± 1.42 b | 288.69 ± 1.50 d | 320.98 ± 1.65 c | |
| threonine | UFS | 47.95 ± 2.68 b | 5.20 ± 0.36 d | 79.22 ± 1.33 a | 39.38 ± 0.57 c |
| CKJ | 171.72 ± 2.09 a | 139.46 ± 1.65 c | 143.79 ± 2.12 b | 111.39 ± 1.55 d | |
| serine | UFS | 26.69 ± 1.30 b | 1.13 ± 0.83 d | 52.40 ± 1.28 a | 15.98 ± 3.42 c |
| CKJ | 44.99 ± 1.29 a | 13.90 ± 1.47 b | 13.32 ± 0.53 b | 6.73 ± 0.60 c | |
| glutamic acid | UFS | 531.55 ± 3.64 b | 381.82 ± 2.97 c | 603.74 ± 1.27 a | 348.73 ± 1.17 d |
| CKJ | 1028.83 ± 2.19 a | 975.96 ± 0.78 b | 619.80 ± 2.31 c | 430.90 ± 0.84 d | |
| α-amino adipic acid | UFS | 96.99 ± 4.46 b | 267.05 ± 0.53 a | 54.41 ± 0.97 c | 55.32 ± 1.58 c |
| CKJ | 106.79 ± 0.76 b | 115.53 ± 1.56 a | 39.16 ± 2.41 d | 92.93 ± 3.26 c | |
| glycine | UFS | 176.04 ± 1.03 b | 41.48 ± 1.00 d | 193.33 ± 1.69 a | 116.88 ± 3.03 c |
| CKJ | 291.14 ± 2.20 a | 218.58 ± 1.04 b | 214.41 ± 0.52 c | 204.40 ± 2.71 d | |
| alanine | UFS | 580.17 ± 1.45 a | 73.53 ± 0.50 d | 420.89 ± 2.99 b | 234.57 ± 1.00 c |
| CKJ | 1017.40 ± 2.25 a | 544.99 ± 1.23 b | 542.55 ± 1.02 c | 392.15 ± 1.40 d | |
| citrulline | UFS | 221.09 ± 3.43 c | 760.79 ± 1.53 a | 365.01 ± 0.64 b | 219.57 ± 2.79 d |
| CKJ | ND c | 291.41 ± 0.69 a | ND c | 158.76 ± 1.83 b | |
| α-amino-n-butyric acid | UFS | 20.87 ± 1.24 a | ND c | 18.84 ± 1.27 b | ND c |
| CKJ | 12.50 ± 0.55 a | 10.98 ± 2.20 a,b | 9.92 ± 0.01 b | 10.55 ± 1.06 a,b | |
| valine | UFS | 39.09 ± 3.51 c | 39.58 ± 1.01 c | 85.02 ± 1.34 a | 46.17 ± 0.47 b |
| CKJ | 133.26 ± 1.18 b | 103.16 ± 0.82 c | 148.05 ± 1.19 a | 104.93 ± 1.17 c | |
| methionine | UFS | 28.18 ± 3.54 a | ND c | 28.95 ± 2.58 a | 24.45 ± 0.51 b |
| CKJ | 102.36 ± 2.52 a | 76.07 ± 1.87 b | 77.29 ± 0.83 b | 68.69 ± 1.26 c | |
| cystathionine | UFS | 15.95 ± 1.56 a | ND c | 11.65 ± 1.99 b | 14.71 ± 2.26 a,b |
| CKJ | 16.83 ± 1.86 a | 14.16 ± 2.55 a,b | 12.14 ± 0.82 b | 15.02 ± 1.46 a,b | |
| isoleucine | UFS | 17.37 ± 1.93 d | 30.35 ± 0.72 b | 42.78 ± 0.59 a | 26.82 ± 2.51 c |
| CKJ | 99.57 ± 1.59 b | 75.96 ± 1.67 c | 104.57 ± 2.91 a | 73.67 ± 0.37 d | |
| leucine | UFS | 23.71 ± 3.67 c | 4.33 ± 0.80 d | 82.42 ± 2.57 a | 39.60 ± 0.50 b |
| CKJ | 195.29 ± 2.46 b | 142.13 ± 1.87 d | 225.25 ± 0.75 a | 152.81 ± 1.59 c | |
| tyrosine | UFS | 88.21 ± 2.47 b | 56.87 ± 0.66 d | 136.28 ± 1.45 a | 76.58 ± 1.22 c |
| CKJ | 170.89 ± 3.89 c | 172.50 ± 1.87 b | 204.54 ± 1.00 a | 204.18 ± 0.33 a | |
| phenylalanine | UFS | 43.11 ± 1.38 d | 47.47 ± 1.14 c | 119.95 ± 2.27 a | 80.77 ± 0.97 b |
| CKJ | 184.06 ± 1.73 d | 189.72 ± 0.86 c | 224.30 ± 1.12 b | 235.76 ± 1.58 a | |
| β-alanine | UFS | 126.44 ± 2.22 a | 35.32 ± 0.57 c | 43.78 ± 1.05 b | 43.44 ± 1.85 b |
| CKJ | 161.16 ± 1.00 a | 105.92 ± 2.47 b | 60.75 ± 1.81 d | 101.36 ± 2.13 c | |
| β-amino isobutyric acid | UFS | 30.80 ± 2.03 c | 46.71 ± 1.89 a | 21.88 ± 1.73 d | 39.06 ± 0.73 b |
| CKJ | 56.61 ± 1.33 c | 71.27 ± 2.02 b | 41.55 ± 0.60 d | 78.31 ± 0.72 a | |
| γ-amino-n-butyric acid | UFS | 663.83 ± 3.58 a | 107.43 ± 1.07 d | 515.72 ± 1.51 b | 316.93 ± 1.37 c |
| CKJ | 853.14 ± 2.90 a | 724.61 ± 2.61 b | 611.31 ± 3.16 d | 617.14 ± 0.45 c | |
| ethanol amine | UFS | 68.16 ± 0.87 a | 36.23 ± 1.07 c | 62.03 ± 1.41 b | 29.72 ± 1.34 d |
| CKJ | 70.16 ± 1.29 a,b | 68.33 ± 1.24 b | 71.37 ± 0.99 a | 52.94 ± 2.29 c | |
| Total free amino acids | UFS | 2595.34 ± 0.82 c | 2243.26 ± 1.24 d | 3371.00 ± 2.06 a | 3082.94 ± 2.43 b |
| CKJ | 5879.04 ± 1.27 c | 5177.41 ± 1.23 d | 6989.48 ± 1.98 a | 6452.05± 2.81 b |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ali, M.W.; Kim, I.-D.; Bilal, S.; Shahzad, R.; Saeed, M.T.; Adhikari, B.; Nabi, R.B.S.; Kyo, J.R.; Shin, D.-H. Effects of Bacterial Fermentation on the Biochemical Constituents and Antioxidant Potential of Fermented and Unfermented Soybeans Using Probiotic Bacillus subtilis (KCTC 13241). Molecules 2017, 22, 2200. https://doi.org/10.3390/molecules22122200
Ali MW, Kim I-D, Bilal S, Shahzad R, Saeed MT, Adhikari B, Nabi RBS, Kyo JR, Shin D-H. Effects of Bacterial Fermentation on the Biochemical Constituents and Antioxidant Potential of Fermented and Unfermented Soybeans Using Probiotic Bacillus subtilis (KCTC 13241). Molecules. 2017; 22(12):2200. https://doi.org/10.3390/molecules22122200
Chicago/Turabian StyleAli, Muhammad Waqas, Il-Doo Kim, Saqib Bilal, Raheem Shahzad, Muhammad Tariq Saeed, Bishnu Adhikari, Rizwana Begum Syed Nabi, Jeong Rae Kyo, and Dong-Hyun Shin. 2017. "Effects of Bacterial Fermentation on the Biochemical Constituents and Antioxidant Potential of Fermented and Unfermented Soybeans Using Probiotic Bacillus subtilis (KCTC 13241)" Molecules 22, no. 12: 2200. https://doi.org/10.3390/molecules22122200