New Type of Halogen Bond: Multivalent Halogen Interacting with π- and σ-Electrons
Abstract
:1. Introduction
2. Results and Discussion
2.1. Energetic and Geometric Parameters
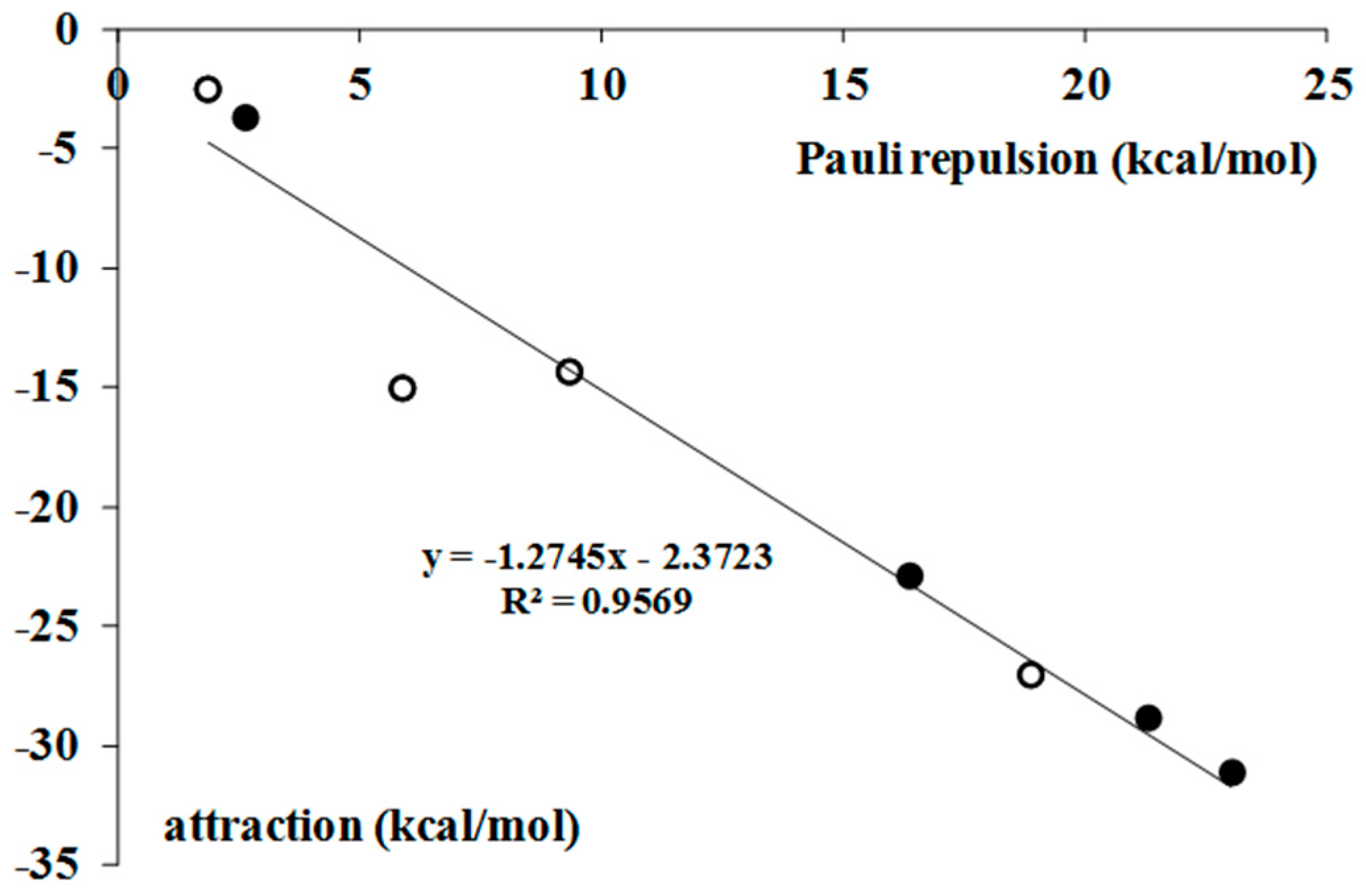
2.2. Nature of Interactions–Decomposition of Interaction Energy
2.3. QTAIM Parameters
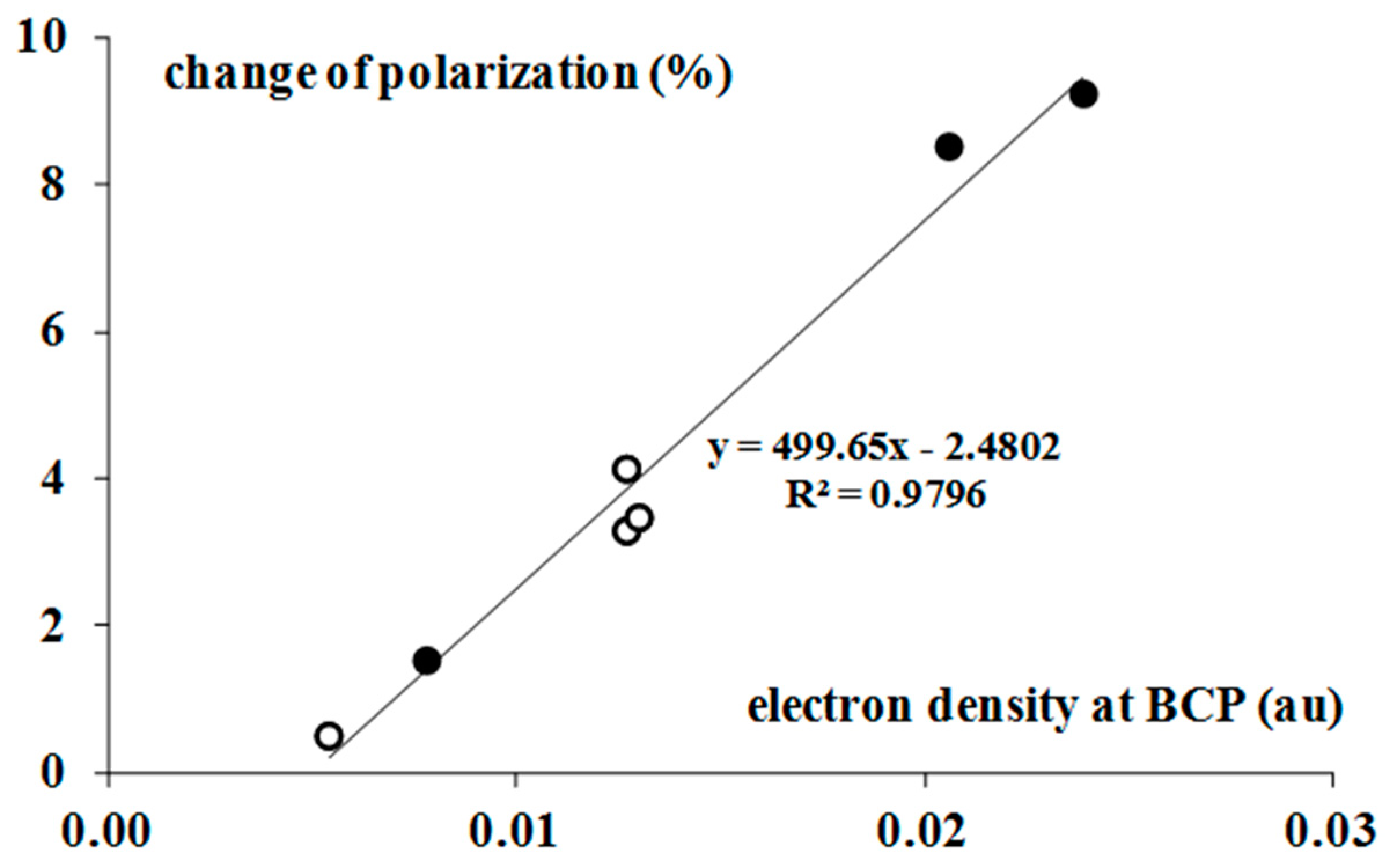
2.4. Electron Charge Density Shifts
3. Computational Details
4. Conclusions
Supplementary Materials
Acknowledgments
Conflicts of Interest
References
- Metrangolo, P.; Resnati, G. Halogen Bonding: A Paradigm in Supramolecular Chemistry. Chem. Eur. J. 2001, 7, 2511–2519. [Google Scholar] [CrossRef]
- Cavallo, G.; Metrangolo, P.; Pilati, T.; Resnati, G.; Sansotera, M.; Terraneo, G. Halogen bonding: A general route in anion recognition and coordination. Chem. Soc. Rev. 2010, 39, 3772–3783. [Google Scholar] [CrossRef] [PubMed]
- Clark, T.; Hennemann, M.; Murray, J.S.; Politzer, P. Halogen bonding: The σ-hole. J. Mol. Mod. 2007, 13, 291–296. [Google Scholar] [CrossRef] [PubMed]
- Politzer, P.; Lane, P.; Concha, M.C.; Ma, Y.; Murray, J.S. An overview of halogen bonding. J. Mol. Mod. 2007, 13, 305–311. [Google Scholar] [CrossRef] [PubMed]
- Metrangolo, P.; Neukirch, H.; Pilati, T.; Resnati, G. Halogen Bonding Based recognition Process: A World Parallel to Hydrogen Bonding. Acc. Chem. Res. 2005, 38, 386–395. [Google Scholar] [CrossRef] [PubMed]
- Politzer, P.; Murray, J.S.; Clark, T. Halogen bonding and other σ-hole interactions: A perspective. Phys. Chem. Chem. Phys. 2013, 15, 11178–11189. [Google Scholar] [CrossRef] [PubMed]
- Grabowski, S.J. Hydrogen and halogen bonds are ruled by the same mechanisms. Phys. Chem. Chem. Phys. 2013, 15, 7249–7259. [Google Scholar] [CrossRef] [PubMed]
- Zordan, F.; Brammer, L.; Sherwood, P. Supramolecular Chemistry of Halogens: Complementary Features of Inorganic (M-X) and Organic (C-X´) Halogens Applied to M-X…X´-C halogen Bond Formation. J. Am. Chem. Soc. 2005, 127, 5979–5989. [Google Scholar] [CrossRef] [PubMed]
- Grabowski, S.J. Hydrogen bonds, and σ-hole and π-hole bonds—Mechanisms protecting doublet and octet electron structures. Phys. Chem. Chem. Phys. 2017, 19, 29742–29759. [Google Scholar] [CrossRef] [PubMed]
- Politzer, P.; Riley, K.E.; Bulat, F.A.; Murray, J.S. Perspectives on halogen bonding and other σ-hole interactions: Lex parsimoniae (Occam’s Razor). Comput. Theor. Chem. 2012, 998, 2–8. [Google Scholar] [CrossRef]
- Bauzá, A.; Frontera, A. Aerogen Bonding Interaction: A New Supramolecular Force? Angew. Chem. Int. Ed. 2015, 54, 7340–7343. [Google Scholar] [CrossRef] [PubMed]
- Nishio, M.; Hirota, M.; Umezawa, Y. The CH/π Interaction, Evidence, Nature, and Consequences; Wiley-VCH: New York, NY, USA, 1998. [Google Scholar]
- Klooster, W.T.; Koetzle, T.F.; Siegbahn, P.E.M.; Richardson, T.B.; Crabtree, R.H. Study of the N-H…H-B Dihydrogen Bond Including the Crystal Structure of BH3NH3 by Neutron Diffraction. J. Am. Chem. Soc. 1999, 121, 6337–6343. [Google Scholar] [CrossRef]
- Grabowski, S.J.; Sokalski, W.A.; Leszczynski, J. Can H…σ, π…H+…σ and σ…H+…σ interactions be classified as H-bonded? Chem. Phys. Lett. 2006, 432, 33–39. [Google Scholar] [CrossRef]
- Grabowski, S.J. Dihydrogen bond and X-H…σ interaction as sub-classes of hydrogen bond. J. Phys. Org. Chem. 2013, 26, 452–459. [Google Scholar] [CrossRef]
- Fau, S.; Frenking, G. Theoretical investigation of the weakly bonded donor-acceptor complexes X3B-H2, X3B-C2H4, and X3B-C2H2 (X = H, F, Cl). Mol. Phys. 1999, 96, 519–527. [Google Scholar]
- Grabowski, S.J. Triel Bonds, π-Hole-π-Electrons Interactions in Complexes of Boron and Aluminium Trihalides and Trihydrides with Acetylene and Ethylene. Molecules 2015, 20, 11297–11316. [Google Scholar] [CrossRef] [PubMed]
- Alkorta, I.; Rozas, I.; Elguero, J. Charge-Transfer Complexes between Dihalogen Compounds and Electron Donors. J. Phys. Chem. A 1998, 102, 9278–9285. [Google Scholar] [CrossRef]
- Raghavendra, B.; Arunan, E. Unpaired and σ Bond Electrons as H, Cl, and Li Bond Acceptors: An Anomalous One-Electron Blue-Shifting Chlorine Bond. J. Phys. Chem. A 2007, 111, 9699–9706. [Google Scholar] [CrossRef] [PubMed]
- Vasilyev, A.V.; Lindeman, S.V.; Kochi, J.K. Noncovalent binding of the halogens to aromatic donors. Discrete structures of labile Br2 complexes with benzene and toluene. Chem. Commun. 2001, 909–910. [Google Scholar] [CrossRef]
- Duarte, D.J.R.; de las Vallejos, M.M.; Peruchena, N.M. Topological analysis of aromatic halogen/hydrogen bonds by electron charge density and electrostatic potentials. J. Mol. Model. 2010, 16, 737–748. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-S.; Yin, C.-C.; Chao, S.D. Intermolecular interactions of trifluorohalomethanes with Lewis bases in the gas phase: An ab initio study. J. Chem. Phys. 2014, 141, 134308. [Google Scholar] [CrossRef] [PubMed]
- Zhuo, H.; Li, Q.; Li, W.; Cheng, J. Is π halogen bonding or lone pair…π interaction formed between borazine and some halogenated compounds? Phys. Chem. Chem. Phys. 2014, 16, 159–165. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Zeng, Y.; Li, X.; Meng, L.; Zhang, X. A comprehensive analysis of P…π pnicogen bonds: Substitution effects and comparison with Br…π halogen bonds. J. Mol. Model. 2015, 21, 143. [Google Scholar] [CrossRef] [PubMed]
- Grabowski, S.J.; Sokalski, W.A. Are Various σ-Hole Bonds Steered by the Same Mechanisms? ChemPhysChem 2017, 18, 1569–1577. [Google Scholar] [CrossRef] [PubMed]
- Landrum, G.A.; Goldberg, N.; Hoffmann, R.; Minyaev, R.M. Intermolecular interactions between hypervalent molecules: Ph2IX and XF3 (X = Cl, Br, I) dimers. New J. Chem. 1998, 883–890. [Google Scholar] [CrossRef]
- Ochiai, M. Intermolecular hypervalent I(III)…O interactions: A new driving force for complexation of crown ethers. Coord. Chem. Rev. 2006, 250, 2771–2781. [Google Scholar] [CrossRef]
- Wang, W. Halogen Bond Involving Hypervalent Halogen: CSD Search and Theoretical Study. J. Phys. Chem. A 2011, 115, 9294–9299. [Google Scholar] [CrossRef] [PubMed]
- Wong, R.; Allen, F.H.; Willett, P. The scientific impact of the Cambridge Structural Database: A citation-based study. J. Appl. Crystallogr. 2010, 43, 811–824. [Google Scholar] [CrossRef]
- O’Hair, R.A.J.; Williams, C.M.; Clark, T. Neighboring group stabilization by σ-holes. J. Mol. Mod. 2010, 16, 559–565. [Google Scholar] [CrossRef] [PubMed]
- Politzer, P.; Murray, J.S.; Clark, T. Halogen bonding: An electrostatically-driven highly directional noncovalent interaction. Phys. Chem. Chem. Phys. 2010, 12, 7748–7757. [Google Scholar] [CrossRef] [PubMed]
- Grabowski, S.J. Halogen bond with the multivalent halogen acting as the Lewis acid center. Chem. Phys. Lett. 2014, 605–606, 131–136. [Google Scholar] [CrossRef]
- Cheng, N.; Bi, F.; Liu, Y.; Zhang, C.; Liu, C. The structures and properties of halogen bonds involving polyvalent halogen in complexes of FXOn (X = Cl, Br; n = 0–3)-CH3CN. New J. Chem. 2014, 38, 1256–1263. [Google Scholar] [CrossRef]
- Kirshenboim, O.; Kozuch, S. How to Twist, Split and Warp a σ-Hole with Hypervalent Halogens. J. Phys. Chem. A 2016, 120, 9431–9445. [Google Scholar] [CrossRef] [PubMed]
- Buralli, G.J.; Duarte, D.J.R.; Sosa, G.L.; Peruchena, N.M. Lewis acid-base behavior of hypervalent halogen fluorides in gas phase. Struct. Chem. 2017, 28, 1823–1830. [Google Scholar] [CrossRef]
- Eskandari, K.; Zariny, H. Halogen bonding: A lump-hole interaction. Chem. Phys. Lett. 2010, 492, 9–13. [Google Scholar] [CrossRef]
- Cavallo, G.; Murray, J.S.; Politzer, P.; Pilati, T.; Ursini, M.; Resnati, G. Halogen bonding in hypervalent iodine and bromine derivatives: Halonium salts. IUCrJ 2017, 4, 411–419. [Google Scholar] [CrossRef] [PubMed]
- Bader, R.F.W. Atoms in Molecules: A Quantum Theory; Oxford University Press: Oxford, UK, 1990. [Google Scholar]
- Weinhold, F.; Landis, C. Valency and Bonding: A Natural Bond Orbital Donor—Acceptor Perspective; Cambridge University Press: Cambridge, UK, 2005. [Google Scholar]
- Morokuma, K. Molecular Orbital Studies of Hydrogen Bonds. III. C=O…H–O Hydrogen Bond in H2CO…H2O and H2CO…2H2O. J. Chem. Phys. 1971, 55, 1236–1244. [Google Scholar] [CrossRef]
- Ziegler, T.; Rauk, A. On the calculation of bonding energies by the Hartree–Fock Slater method. Theor. Chim. Acta 1977, 46, 1–10. [Google Scholar] [CrossRef]
- Murray, J.S.; Politzer, P. Molecular electrostatic potentials and noncovalent interactions. WIREs Comput. Mol. Sci. 2017, 7, e1326. [Google Scholar] [CrossRef]
- Scheiner, S. Hydrogen Bonding: A Theoretical Perspective; Oxford University Press: New York, NY, USA, 1997; p. 78. [Google Scholar]
- Grabowski, S.J.; Sokalski, W.A.; Dyguda, E.; Leszczynski, J. Quantitative Classification of Covalent and Noncovalent H-Bonds. J. Phys. Chem. B 2006, 110, 6444–6446. [Google Scholar] [CrossRef] [PubMed]
- Grabowski, S.J. What is the Covalency of Hydrogen Bonding? Chem. Rev. 2011, 11, 2597–2625. [Google Scholar] [CrossRef] [PubMed]
- Grabowski, S.J.; Ugalde, J.M. Bond Paths Show Preferable Interactions: Ab Initio and QTAIM Studies on the X-H· · ·π Hydrogen Bond. J. Phys. Chem. A 2010, 114, 7223–7229. [Google Scholar] [CrossRef] [PubMed]
- Grabowski, S.J. What Can Be Learnt from a Location of Bond Paths and from Electron Density Distribution. In Applications of Topological Methods in Molecular Chemistry; Chauvin, R., Lepetit, C., Silvi, B., Alikhani, E., Eds.; Springer: Switzerland, Cham, 2016; pp. 399–433. [Google Scholar]
- Espinosa, E.; Molins, E.; Lecomte, C. Hydrogen bond strengths revealed by topological analyses of experimentally observed electron densities. Chem. Phys. Lett. 1998, 285, 170–173. [Google Scholar] [CrossRef]
- Hirshfeld, F.L. Bonded-Atom Fragments for Describing Molecular Charge Densities. Theor. Chim. Acta 1977, 44, 129–138. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09, Revision A.1; Gaussian, Inc.: Wallingford, UK, 2009. [Google Scholar]
- Møller, C.; Plesset, M.S. Note on an Approximation Treatment for Many-Electron Systems. Phys. Rev. 1934, 46, 618–622. [Google Scholar] [CrossRef]
- Woon, D.E.; Dunning, T.H., Jr. Gaussian Basis Sets for Use in Correlated Molecular Calculations. III. The second row atoms, Al–Ar. J. Chem. Phys. 1993, 98, 1358–1371. [Google Scholar] [CrossRef]
- Piela, L. Ideas of Quantum Chemistry; Elsevier Science Publishers: Amsterdam, The Netherlands, 2007; pp. 684–691. [Google Scholar]
- Grabowski, S.J.; Sokalski, W.A. Different types of hydrogen bonds: Correlation analysis of interaction energy components. J. Phys. Org. Chem. 2005, 18, 779–784. [Google Scholar] [CrossRef]
- Boys, S.F.; Bernardi, F. The calculation of small molecular interactions by the differences of separate total energies. Some procedures with reduced errors. Mol. Phys. 1970, 19, 553–561. [Google Scholar] [CrossRef]
- Keith, T.A. AIMAll (Version 11.08.23); TK Gristmill Software: Overland Park, KS, USA, 2011. [Google Scholar]
- Becke, A.D. Density-functional exchange-energy approximation with correct asymptotic behavious. Phys. Rev. A 1988, 38, 3098–3100. [Google Scholar] [CrossRef]
- Perdew, J.P. Density-functional approximation for the correlation energy of the inhomogeneous electron gas. Phys. Rev. B 1986, 33, 8822–8824. [Google Scholar] [CrossRef]
- Velde, G.T.E.; Bickelhaupt, F.M.; Baerends, E.J.; Guerra, C.F.; van Gisbergen, S.J.A.; Snijders, J.G.; Ziegler, T. Chemistry with ADF. J. Comput. Chem. 2001, 22, 931–967. [Google Scholar] [CrossRef]



| Complex | Distance | Eint | Ebin | Edef | BSSE | ENBO |
|---|---|---|---|---|---|---|
| BrF3–H2 | 2.969 | −0.9 | −0.8 | 0.0 | 0.5 | 0.6 |
| BrF3–C2H2 | 2.904 | −5.9 | −5.6 | 0.3 | 1.3 | 6.0 |
| BrF3–C2H4 | 2.848 | −6.4 | −5.9 | 0.5 | 1.8 | 8.6 |
| BrF3–C6H6 | 2.845 | −8.8 | −8.3 | 0.4 | 3.2 | 10.5 |
| BrF5–H2 | 3.197 | −0.6 | −0.6 | 0.0 | 0.4 | 0.2 |
| BrF5–C2H2 | 3.218 | −3.7 | −3.6 | 0.1 | 1.1 | 1.2 |
| BrF5–C2H4 | 3.204 | −3.9 | −3.8 | 0.1 | 1.5 | 1.6 |
| BrF5–C6H6 | 2.911 | −9.1 | −8.7 | 0.4 | 3.5 | 2.8 |
| Complex | ΔEPauli | ΔEelstat | ΔEorb | ΔEdisp | ΔEint | ΔEelstat/ΔEorb |
|---|---|---|---|---|---|---|
| BrF3–H2 | 2.6 | −1.4 | −1.3 | −1.0 | −1.1 | 1.0 |
| BrF3–C2H2 | 16.4 | −10.6 | −10.3 | −2.1 | −6.5 | 1.0 |
| BrF3–C2H4 | 21.3 | −12.4 | −13.6 | −2.9 | −7.6 | 0.9 |
| BrF3–C6H6 | 23.0 | −12.1 | −13.3 | −5.7 | −8.1 | 0.9 |
| BrF5–H2 | 1.9 | −0.9 | −0.7 | −1.0 | −0.7 | 1.3 |
| BrF5–C2H2 | 5.9 | −5.4 | −7.1 | −2.5 | −9.1 | 0.8 |
| BrF5–C2H4 | 9.3 | −6.2 | −4.7 | −3.5 | −5.0 | 1.3 |
| BrF5–C6H6 | 18.9 | −10.8 | −8.4 | −7.8 | −8.2 | 1.3 |
| Complex | ρBCP | 2ρBCP | GBCP | VBCP | HBCP |
|---|---|---|---|---|---|
| BrF3–H2 | 0.008 | 0.027 | 0.006 | −0.005 | 0.001 |
| BrF3–C2H2 | 0.021 | 0.055 | 0.013 | −0.013 | 0.000 |
| BrF3–C2H4 | 0.024 | 0.053 | 0.014 | −0.014 | −0.001 |
| BrF3–C6H6 | 0.022 | 0.056 | 0.014 | −0.014 | 0.000 |
| BrF5–H2 | 0.005 | 0.019 | 0.004 | −0.003 | 0.001 |
| BrF5–C2H2 | 0.013 | 0.036 | 0.008 | −0.007 | 0.001 |
| BrF5–C2H4 | 0.013 | 0.034 | 0.008 | −0.007 | 0.001 |
| BrF5–C6H6 | 0.013 | 0.042 | 0.009 | −0.008 | 0.001 |
| Complex | TR NBO | QBr 1 | TRH | POL% |
|---|---|---|---|---|
| BrF3–H2 | −0.002 | 1.505 | −0.009 | 1.5 |
| BrF3–C2H2 | −0.059 | 1.500 | −0.088 | 8.5 |
| BrF3–C2H4 | −0.089 | 1.479 | −0.113 | 9.2 |
| BrF3–C6H6 | −0.070 | 1.494 | −0.114 | - |
| BrF5–H2 | −0.004 | 2.445 | −0.003 | 0.5 |
| BrF5–C2H2 | −0.012 | 2.461 | −0.040 | 3.3 |
| BrF5–C2H4 | −0.020 | 2.457 | −0.049 | 3.5 |
| BrF5–C6H6 | −0.019 | 2.465 | −0.064 | 4.1 |
© 2017 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grabowski, S.J. New Type of Halogen Bond: Multivalent Halogen Interacting with π- and σ-Electrons. Molecules 2017, 22, 2150. https://doi.org/10.3390/molecules22122150
Grabowski SJ. New Type of Halogen Bond: Multivalent Halogen Interacting with π- and σ-Electrons. Molecules. 2017; 22(12):2150. https://doi.org/10.3390/molecules22122150
Chicago/Turabian StyleGrabowski, Sławomir J. 2017. "New Type of Halogen Bond: Multivalent Halogen Interacting with π- and σ-Electrons" Molecules 22, no. 12: 2150. https://doi.org/10.3390/molecules22122150





