Structural and Functional Properties Changes of β-Conglycinin Exposed to Hydroxyl Radical-Generating Systems
Abstract
:1. Introduction
2. Results and Discussion
2.1. Characterization of Oxidative Markers of Oxidized β-Conglycinin
2.2. Changes in Secondary Structure of Oxidized β-Conglycinin
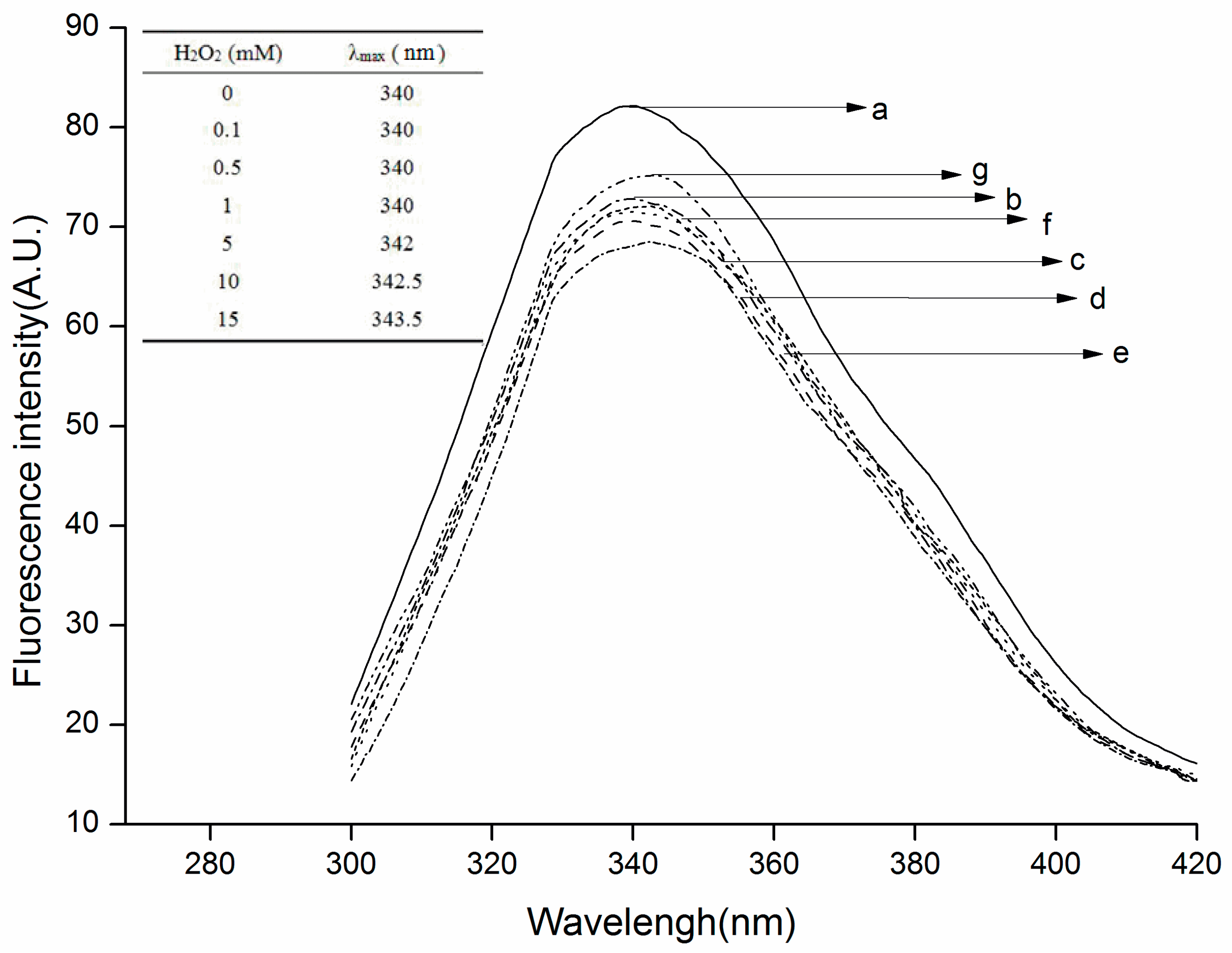
2.3. Changes in Surface Hydrophobicity and Intrinsic Fluorescence of Oxidized β-Conglycinin
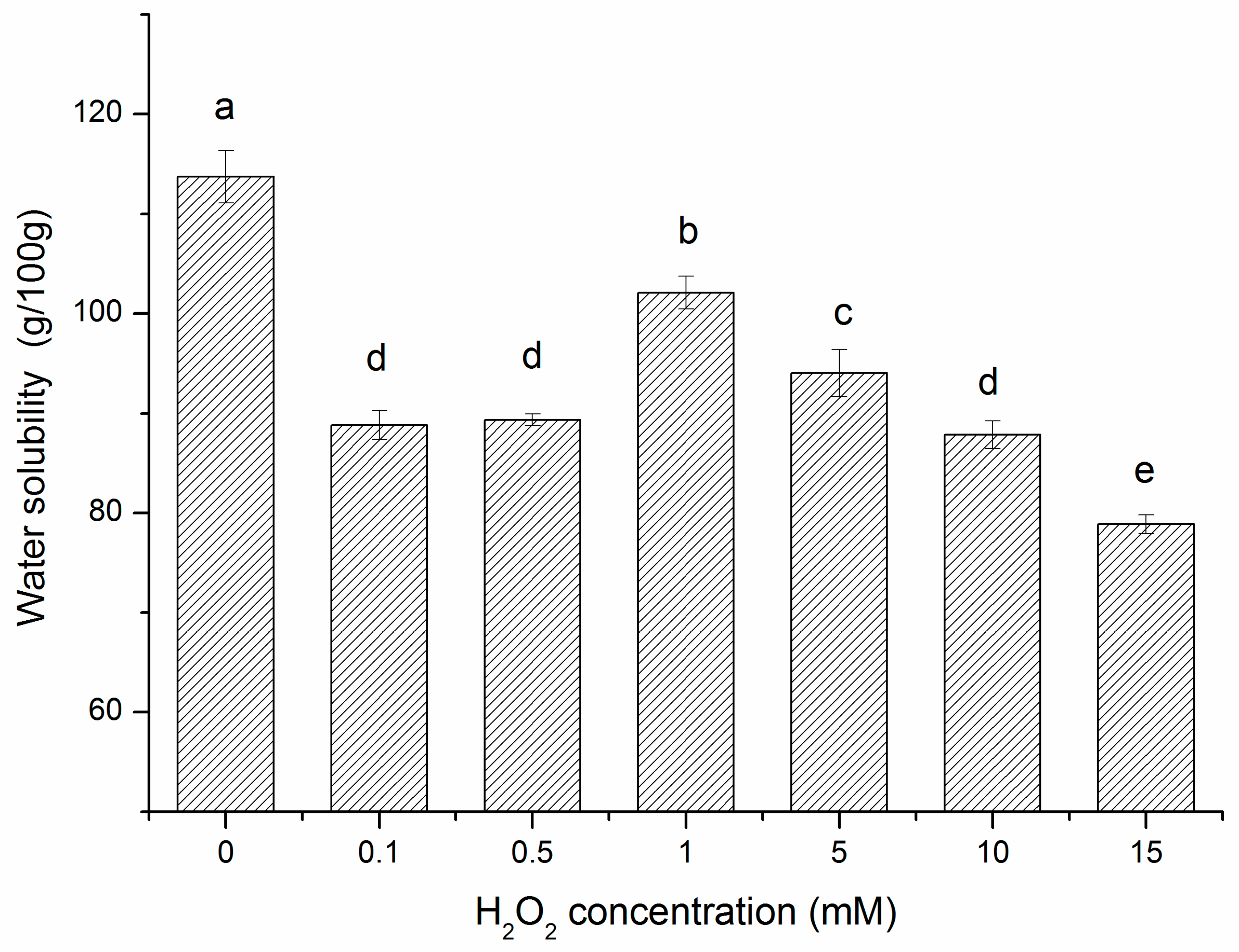
2.4. Changes in Solubility of Oxidized β-Conglycinin
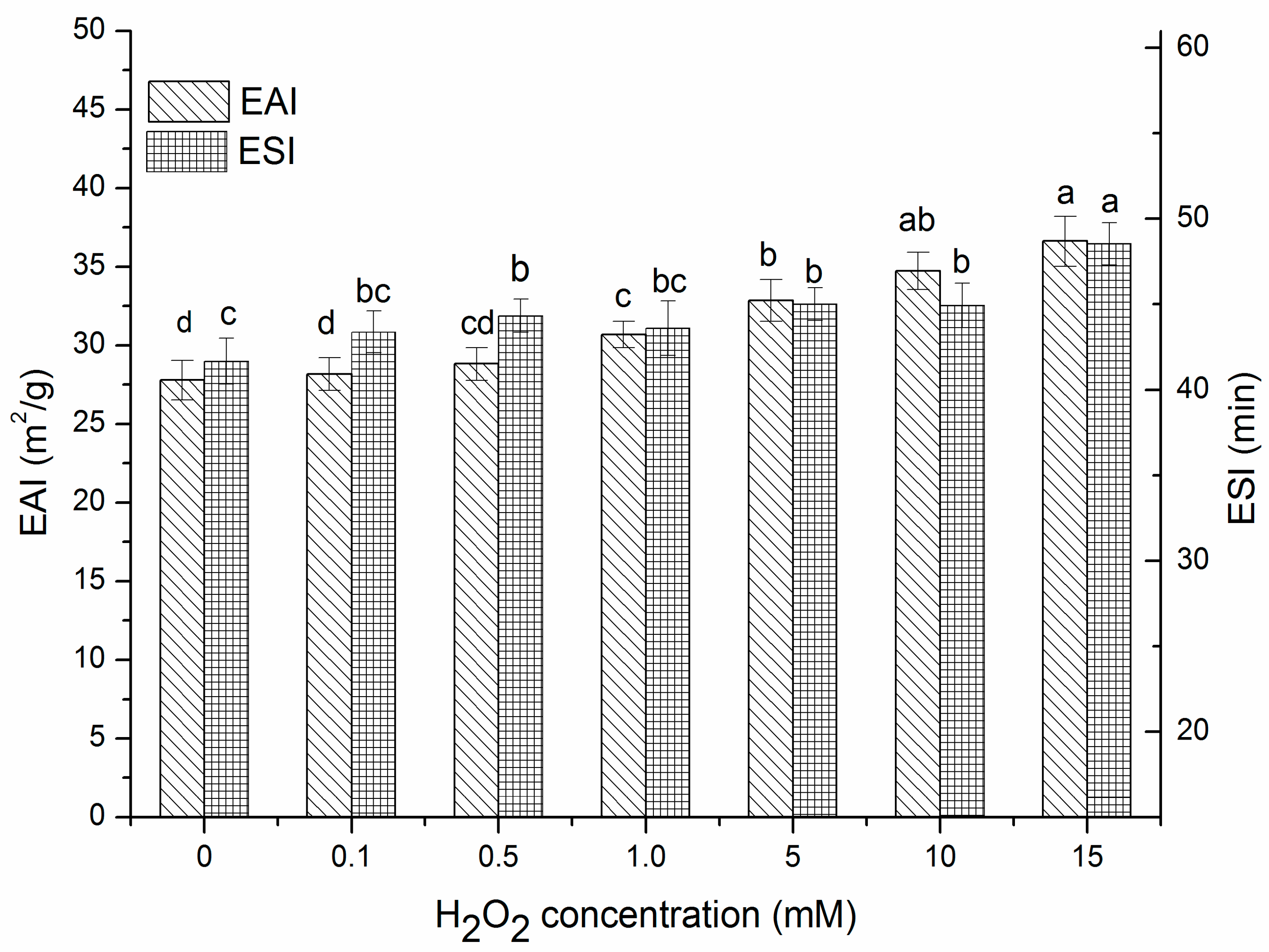
2.5. Changes in Emulsifying Properties of Oxidized β-Conglycinin
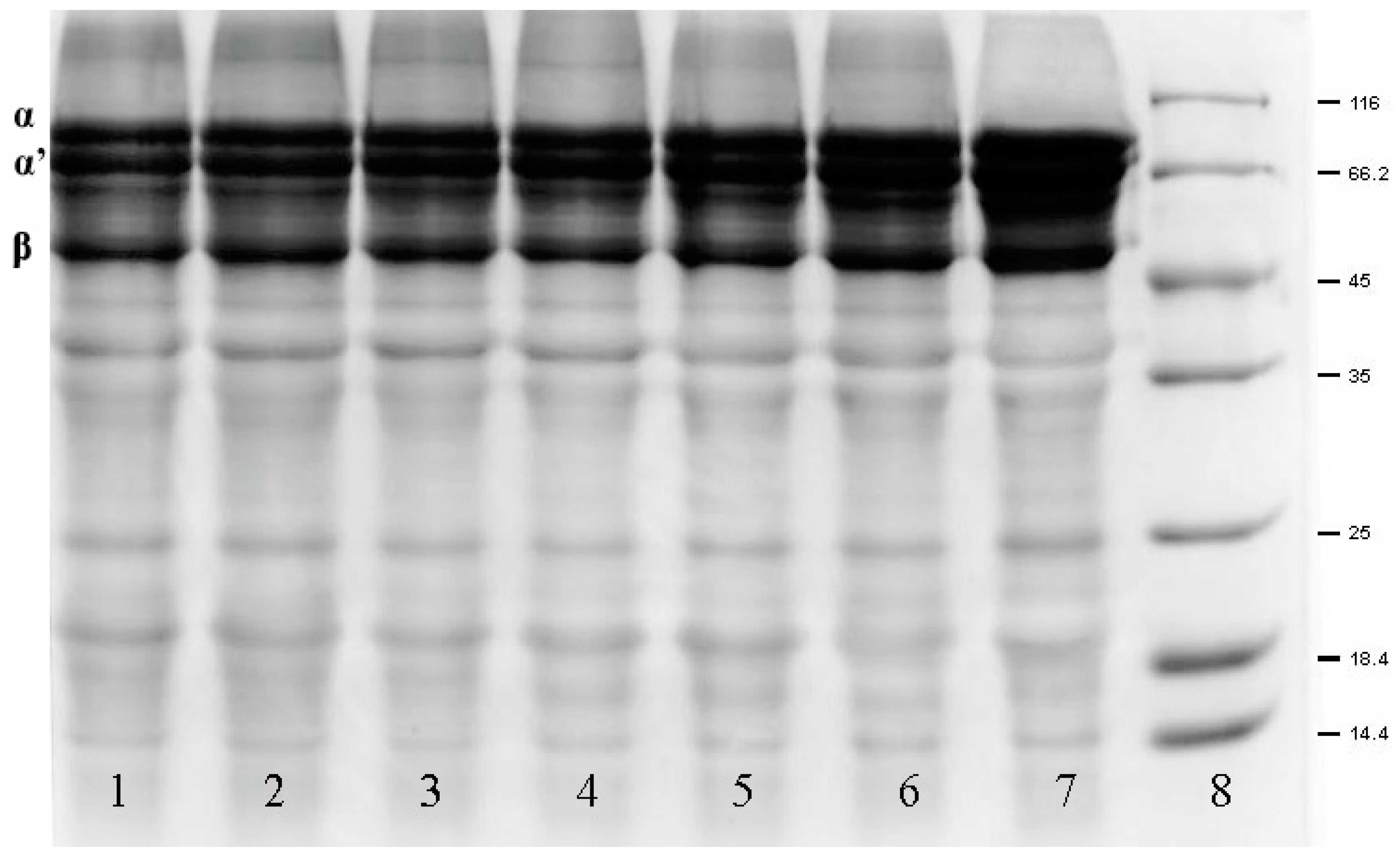
2.6. Changes in the Subunits and Allergenicity of Oxidized β-Conglycinin
3. Materials and Methods
3.1. Materials
3.2. Preparation of β-Conglycinin
3.3. Preparation of Oxidized β-Conglycinin
3.4. Measurement of Chemical and Structural Changes
3.4.1. Protein Carbonyls
3.4.2. Amino Acid Analysis
3.4.3. Available Lysine
3.4.4. Free Sulfhydryl and Disulfide Content
3.4.5. Fourier Transform Infrared Spectra (FTIR)
3.4.6. Surface Hydrophobicity (H0)
3.4.7. Fluorescence Measurement
3.4.8. Electrophoresis
3.5. Measurement of Functional Properties Changes
3.5.1. Protein Solubility
3.5.2. Determination of emulsifying Properties
3.5.3. Allergenicity Measurement
3.6. Statistical Analysis
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Natarajan, S.; Luthria, D.; Bae, H.; Lakshman, D.; Mitra, A. Transgenic Soybeans and Soybean Protein Analysis: An Overview. J. Agric. Food Chem. 2013, 61, 11736–11743. [Google Scholar] [CrossRef] [PubMed]
- Moure, A.; Sineiro, J.; Dominguez, H.; Parajo, J.C. Functionality of oilseed protein products: A review. Food Res. Int. 2006, 39, 945–963. [Google Scholar] [CrossRef]
- Cavallieri, A.L.F.; Garcez, M.M.; Takeuchi, K.P.; da Cunha, R.L. Heat-induced gels of soy protein and kappa-carrageenan at different pH values. Int. J. Food Sci. Technol. 2010, 45, 1130–1137. [Google Scholar] [CrossRef]
- Kim, K.M.; Weller, C.L.; Hanna, M.A.; Gennadios, A. Heat curing of soy protein films at selected temperatures and pressures. LWT—Food Sci. Technol. 2002, 35, 140–145. [Google Scholar] [CrossRef]
- Harel, S.; Kanner, J. Hydrogen peroxide generation in ground muscle tissues. J. Agric. Food Chem. 1985, 33, 629–636. [Google Scholar] [CrossRef]
- Shacter, E. Quantification and significance of protein oxidation in biological samples. Drug Metab. Rev. 2000, 32, 307–326. [Google Scholar] [CrossRef] [PubMed]
- Davies, M.J. The oxidative environment and protein damage. BBA Protein Proteom. 2005, 1703, 93–109. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Zhang, C.M.; Kong, X.Z.; Hua, Y.F. Oxidative modification of soy protein by peroxyl radicals. Food Chem. 2009, 116, 295–301. [Google Scholar] [CrossRef]
- Maqsood, S.; Abushelaibi, A.; Manheem, K.; Rashedi, A.; Kadim, I. Lipid oxidation, protein degradation, microbial and sensorial quality of camel meat as influenced by phenolic compounds. LWT—Food Sci. Technol. 2015, 63, 953–959. [Google Scholar] [CrossRef]
- Zhou, F.; Zhao, M.; Cui, C.; Sun, W. Influence of linoleic acid-induced oxidative modifications on physicochemical changes and in vitro digestibility of porcine myofibrillar proteins. LWT—Food Sci. Technol. 2015, 61, 414–421. [Google Scholar] [CrossRef]
- Chanarat, S.; Benjakul, S.; Xiong, Y.L. Physicochemical changes of myosin and gelling properties of washed tilapia mince as influenced by oxidative stress and microbial transglutaminase. J. Food Sci. Technol. 2015, 52, 3824–3836. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.Q.; Kong, B.H.; Xia, X.F.; Liu, Q.; Diao, X.P. Structural changes of the myofibrillar proteins in common carp (Cyprinuscarpio) muscle exposed to a hydroxyl radical-generating system. Process Biochem. 2013, 48, 863–870. [Google Scholar] [CrossRef]
- Liu, Q.; Lu, Y.; Han, J.C.; Chen, Q.; Kong, B.H. Structure-modification by moderate oxidation in hydroxyl radical-generating systems promote the emulsifying properties of soy protein isolate. Food Struct. 2015, 6, 21–28. [Google Scholar] [CrossRef]
- Kong, B.H.; Xiong, Y.L.; Cui, X.H.; Zhao, X.H. Hydroxyl Radical-Stressed Whey Protein Isolate: Functional and Rheological Properties. Food Bioprocess Technol. 2013, 6, 169–176. [Google Scholar] [CrossRef]
- Wu, W.; Hua, Y.F.; Lin, Q.L.; Xiao, H.X. Effects of oxidative modification on thermal aggregation and gel properties of soy protein by peroxyl radicals. Int. J. Food Sci. Technol. 2011, 46, 1891–1897. [Google Scholar] [CrossRef]
- Chen, N.; Zhao, M.; Sun, W.; Ren, J.; Cui, C. Effect of oxidation on the emulsifying properties of soy protein isolate. Food Res. Int. 2013, 52, 26–32. [Google Scholar] [CrossRef]
- Kinsella, J.E. Functional properties of soy proteins. J. Am. Oil Chem. Soc. 1979, 56, 242–258. [Google Scholar] [CrossRef]
- Fukushima, D. Recent progress in research and technology on soybeans. Food Sci. Technol. Res. 2001, 7, 8–16. [Google Scholar] [CrossRef]
- Hashidume, T.; Kato, A.; Tanaka, T.; Miyoshi, S.; Itoh, N.; Nakata, R.; Inoue, H.; Oikawa, A.; Nakai, Y.; Shimizu, M.; et al. Single ingestion of soy β-conglycinin induces increased postprandial circulating FGF21 levels exerting beneficial health effects. Sci. Rep. 2016, 6, 28183. [Google Scholar] [CrossRef] [PubMed]
- Kimura, A.; Fukuda, T.; Zhang, M.; Motoyama, S.; Maruyama, N.; Utsumi, S. Comparison of physicochemical properties of 7S and 11S globulins from pea, fava bean, cowpea, and French bean with those of soybean-French bean 7S globulin exhibits excellent properties. J. Agric. Food Chem. 2008, 56, 10273–10279. [Google Scholar] [CrossRef] [PubMed]
- Meinlschmidt, P.; Schweiggert-Weisz, U.; Eisner, P. Soy protein hydrolysates fermentation: Effect of debittering and degradation of major soy allergens. LWT—Food Sci. Technol. 2016, 71, 202–212. [Google Scholar] [CrossRef]
- Uchida, K.; Kanematsu, M.; Sakai, K.; Matsuda, T.; Hattori, N.; Mizuno, Y.; Suzuki, D.; Miyata, T.; Noguchi, N.; Niki, E.; et al. Protein-bound acrolein: Potential markers for oxidative stress. Proc. Natl. Acad. Sci. USA 1998, 95, 4882–4887. [Google Scholar] [CrossRef] [PubMed]
- Sante-Lhoutellier, V.; Aubry, L.; Gatellier, P. Effect of oxidation on in vitro digestibility of skeletal muscle myofibrillar proteins. J. Agric. Food Chem. 2007, 55, 5343–5348. [Google Scholar] [CrossRef] [PubMed]
- Stadtman, E.R. Metal ion-catalyzed oxidation of proteins: Biochemical mechanism and biological consequences. Free Radic. Biol. Med. 1990, 9, 315–325. [Google Scholar] [CrossRef]
- Zhang, W.G.; Xiao, S.; Ahn, D.U. Protein Oxidation: Basic Principles and Implications for Meat Quality. Crit. Rev. Food Sci. Nutr. 2013, 53, 1191–1201. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Wu, X.J.; Hua, Y.F. Structural modification of soy protein by the lipid peroxidation product acrolein. LWT—Food Sci. Technol. 2010, 43, 133–140. [Google Scholar] [CrossRef]
- Ye, L.; Liao, Y.; Zhao, M.; Sun, W. Effect of protein oxidation on the conformational properties of peanut protein isolate. J. Chem. 2013, 2013, 1–6. [Google Scholar] [CrossRef]
- Liu, G.; Xiong, Y.L.; Butterfield, D.A. Chemical, Physical, and Gel-forming properties of oxidized myofibrils and whey- and soy-protein isolates. J. Food Sci. 2000, 65, 811–818. [Google Scholar] [CrossRef]
- Kozarova, A.; Sliskovic, I.; Mutus, B. Identification of redox sensitive thiols of protein disulfide isomerase using isotope coded affinity technology and mass spectrometry. J. Am. Soc. Mass Spectrom. 2007, 18, 260–269. [Google Scholar] [CrossRef] [PubMed]
- Eaton, P. Protein thiol oxidation in health and disease: Techniques for measuring disulfides and related modifications in complex protein mixtures. Free Radic. Biol. Med. 2006, 40, 1889–1899. [Google Scholar] [CrossRef] [PubMed]
- Segat, A.; Misra, N.N.; Fabbro, A.; Buchini, F.; Lippe, G.; Cullen, P.J.; Innocente, N. Effects of ozone processing on chemical, structural and functional properties of whey protein isolate. Food Res. Int. 2014, 66, 365–372. [Google Scholar] [CrossRef]
- Wang, Y.T.; Wang, Z.J.; Handa, C.L.; Xu, J. Effects of ultrasound pre-treatment on the structure of β-conglycinin and glycincin and the antioxidant activity of their hydrolysates. Food Chem. 2017, 218, 165–172. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Hackman, R.M.; Li, C.; Xu, X.; Zhou, G.; Feng, X. Different physicochemical, structural and digestibility characteristics of myofibrillar protein from PSE and normal pork before and after oxidation. Meat Sci. 2016, 121, 228–237. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.; Li, C.; Ullah, N.; Cao, J.; Lan, Y.; Ge, W.; Hackman, R.M.; Li, Z.; Chen, L. Susceptibility of whey protein isolate to oxidation and changes in physicochemical, structural, and digestibility characteristics. J. Dairy Sci. 2015, 98, 7602–7613. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Hou, L.; Zhang, C.M.; Kong, X.Z.; Hua, Y.F. Structural modification of soy protein by 13-hydroperoxyoctadecadienoic acid. Eur. Food Res. Technol. 2009, 229, 771–778. [Google Scholar] [CrossRef]
- Levine, R.L.; Berlett, B.S.; Moskovitz, J.; Mosoni, L.; Stadtman, E.R. Methionine residues may protect proteins from critical oxidative damage. Mech. Ageing Dev. 1999, 107, 323–332. [Google Scholar] [CrossRef]
- Wu, W.; Zhang, C.M.; Hua, Y.F. Structural modification of soy protein by the lipid peroxidation product malondialdehyde. J. Sci. Food Agric. 2009, 89, 1416–1423. [Google Scholar] [CrossRef]
- Tang, C.H.; Sun, X.; Foegeding, E.A. Modulation of physicochemical and conformational properties of kidney bean vicilin (phaseolin) by glycation with glucose: Implications for structure–function relationships of legume vicilins. J. Agric. Food Chem. 2011, 59, 10114–10123. [Google Scholar] [CrossRef] [PubMed]
- Jamdar, S.N.; Rajalakshmi, V.; Pednekar, M.D.; Juan, F.; Yardi, V.; Sharma, A. Influence of degree of hydrolysis onfunctional properties, antioxidant activity and ACE inhibitoryactivity of peanut protein hydrolysate. Food Chem. 2010, 121, 178–184. [Google Scholar] [CrossRef]
- Xiong, Y.L.; Agyare, K.K.; Addo, K. Hydrolyzed wheat gluten suppresses transglutaminase-mediated gelation but improves emulsification of pork myofibrillar protein. Meat Sci. 2008, 80, 535–544. [Google Scholar] [CrossRef] [PubMed]
- Nishinari, K.; Fang, Y.; Guo, S.; Phillips, G.O. Soy proteins: A review on composition, aggregation and emulsification. Food Hydrocoll. 2014, 39, 301–318. [Google Scholar] [CrossRef]
- Jonathan, O.S.; Jack, B.; Michael, P.; Richard, G.; Ian, N. Comparative assessment of the effect of ultrasound treatment on protein functionality pre- and post-emulsification. Colloids Surf. A Physicochem. Eng. Asp. 2015, 484, 89–98. [Google Scholar]
- Cui, X.H.; Xiong, Y.L.; Kong, B.H.; Zhao, X.H.; Liu, N. Hydroxyl radical-stressed whey protein isolate: Chemical and structural properties. Food Bioprocess Technol. 2012, 5, 2454–2461. [Google Scholar] [CrossRef]
- L’Hocine, L.; Boye, J.I.; Munyana, C. Detection and quantification of soy allergens in food: Study of two commercial enzyme-linked immunosorbent assays. J. Food Sci. 2007, 72, C145–C153. [Google Scholar] [CrossRef] [PubMed]
- Shreffler, W.G.; Beyer, K.; Chu, T.T.; Burks, A.W.; Sampson, H.A. Microarray immunoassay: Association of clinical history, in vitro IgE function, and heterogeneity of allergenic peanut epitopes. J. Allergy Clin. Immunol. 2004, 113, 776–782. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, H.B.; Kim, W.; Jang, S.; Kerley, M.S. All these subunits of soybean β-conglycinin are potential food allergens. J. Agric. Food Chem. 2009, 57, 938–943. [Google Scholar] [CrossRef] [PubMed]
- Matsuo, H.; Yokooji, T.; Taogoshi, T. Common food allergens and their IgE-binding epitopes. Allergol. Int. 2015, 64, 332–343. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Li, Z.; Lin, H.; Du, S.; Hao, Z.; Lin, H.; Zhu, Z. Effect of malondialdehyde treatment on the IgE binding capacity and conformational structure of shrimp tropomyosin. Food Chem. 2015, 175, 374–380. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Lu, Z.; Khan, M.N.; Lin, H.; Zhang, L. Protein carbonylation during electron beam irradiation may be responsible for changes in IgE binding to turbot parvalbumin. Food Chem. Toxicol. 2014, 69, 32–37. [Google Scholar] [CrossRef] [PubMed]
- Benjakul, S.; Morrissey, M.T. Protein Hydrolysates from Pacific Whiting Solid Wastes. J. Agric. Food Chem. 1997, 45, 3243–3430. [Google Scholar] [CrossRef]
- Huang, Y.R.; Hua, Y.F.; Qiu, A.Y. Soybean protein aggregation induced by lipoxygenase catalyzed linoleic acid oxidation. Food Res. Int. 2006, 39, 240–249. [Google Scholar] [CrossRef]
- Pearce, K.N.; Kinsella, J.E. Emulsifying properties of protein: Evaluation of turbidmetric technique. J. Agric. Food Chem. 1978, 26, 716–723. [Google Scholar] [CrossRef]
- Laemmli, U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Li, L.; Yuan, D.; Zhao, X.; Cui, S.; Hu, J.; Wang, J. Reduction of the allergenic protein in soybeanmeal by enzymatic hydrolysis. Food Agric. Immunol. 2014, 25, 301–310. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds β-conglycinin and oxidized β-conglycinin are available from the authors. |


| H2O2 (mmol/L) | Carbonyls (nmol/mg) | Histidine (g/100 g) | Lysine (g/100 g) | Available Lysine (g/100 g) | Free SH (nmol/mg) | Disulfide Groups (nmol/mg) |
|---|---|---|---|---|---|---|
| 0 | 8.02 ± 0.29 e | 1.17 ± 0.01 a | 2.91 ± 0.02 a | 6.23 ± 0.08 a | 8.66 ± 0.04 a | 84.89 ± 2.28 bc |
| 0.1 | 8.14 ± 0.17 e | 1.16 ± 0.02 a | 2.89 ± 0.03 a | 5.96 ± 0.08 b | 8.58 ± 0.01 b | 81.42 ± 3.17 cd |
| 0.5 | 8.36 ± 0.27 e | 1.15 ± 0.01 ab | 2.82 ± 0.02 b | 5.44 ± 0.14 c | 7.42 ± 0.03 c | 79.25 ± 1.77 d |
| 1 | 9.18 ± 0.26 d | 1.13 ± 0.01 b | 2.79 ± 0.03 b | 5.06 ± 0.11 d | 5.18 ± 0.02 d | 88.44 ± 3.36 b |
| 5 | 10.95 ± 0.20 c | 1.10 ± 0.02 c | 2.71 ± 0.03 c | 4.29 ± 0.17 e | 1.36 ± 0.05 e | 89.13 ± 3.39 b |
| 10 | 13.73 ± 0.25 b | 1.08 ± 0.03 c | 2.62 ± 0.04 d | 4.09 ± 0.06 f | 1.01 ± 0.07 f | 95.18 ± 1.31 a |
| 15 | 14.45 ± 0.18 a | 1.04 ± 0.01 d | 2.54 ± 0.03 e | 3.68 ± 0.13 g | 0.95 ± 0.04 g | 96.24 ± 3.21 a |
| H2O2 (mM) | α-Helix (%) | β-Sheet (%) | β-Turn (%) | Random Coil (%) |
|---|---|---|---|---|
| 0 | 16.20 ± 0.18 a | 40.74 ± 0.19 a | 26.56 ± 0.18 c | 16.50 ± 0.11 d |
| 0.1 | 15.89 ± 0.19 bc | 40.89 ± 0.12 a | 26.39 ± 0.17 c | 16.83 ± 0.13 c |
| 0.5 | 16.07 ± 0.17 ab | 39.58 ± 0.23 b | 27.47 ± 0.22 b | 16.88 ± 0.24 c |
| 1 | 15.71 ± 0.20 cd | 39.21 ± 0.16 c | 27.64 ± 0.11 b | 17.44 ± 0.17 b |
| 5 | 15.85 ± 0.14 bc | 38.69 ± 0.21 d | 28.04 ± 0.15 a | 17.42 ± 0.12 b |
| 10 | 15.48 ± 0.15 de | 37.93 ± 0.12 e | 28.22 ± 0.11 a | 18.37 ± 0.20 a |
| 15 | 15.39 ± 0.15 e | 37.95 ± 0.15 e | 28.20 ± 0.19 a | 18.46 ± 0.15 a |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, J.; Chen, Z.; Han, D.; Li, Y.; Sun, X.; Wang, Z.; Jin, H. Structural and Functional Properties Changes of β-Conglycinin Exposed to Hydroxyl Radical-Generating Systems. Molecules 2017, 22, 1893. https://doi.org/10.3390/molecules22111893
Xu J, Chen Z, Han D, Li Y, Sun X, Wang Z, Jin H. Structural and Functional Properties Changes of β-Conglycinin Exposed to Hydroxyl Radical-Generating Systems. Molecules. 2017; 22(11):1893. https://doi.org/10.3390/molecules22111893
Chicago/Turabian StyleXu, Jing, Zijing Chen, Dong Han, Yangyang Li, Xiaotong Sun, Zhongjiang Wang, and Hua Jin. 2017. "Structural and Functional Properties Changes of β-Conglycinin Exposed to Hydroxyl Radical-Generating Systems" Molecules 22, no. 11: 1893. https://doi.org/10.3390/molecules22111893