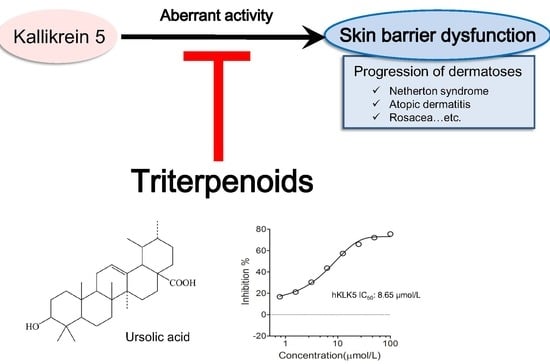
Inhibition of Human Kallikrein 5 Protease by Triterpenoids from Natural Sources
Abstract
:1. Introduction
2. Results
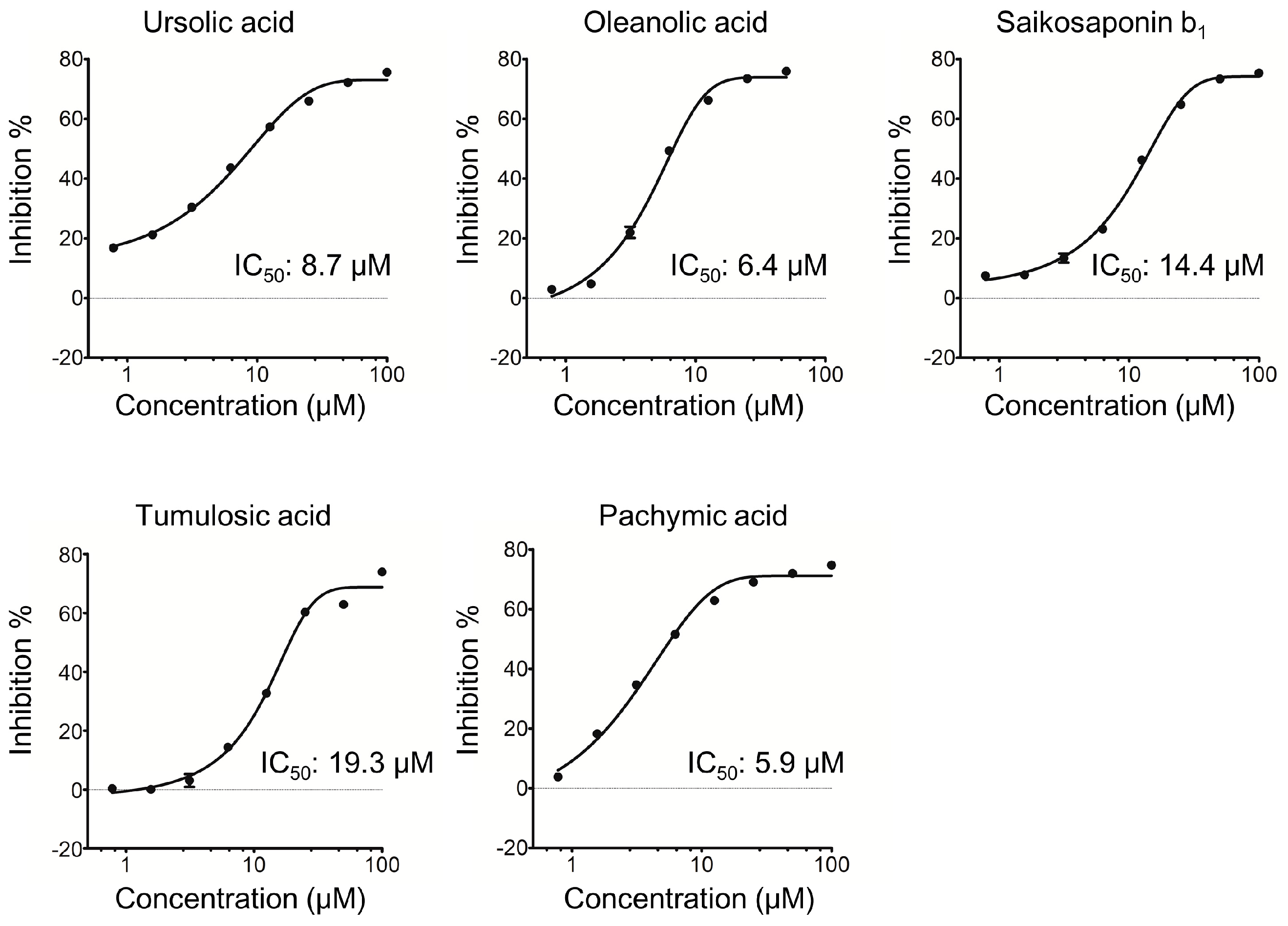
2.1. Triterpenoids Inhibit KLK5 Protease Activity
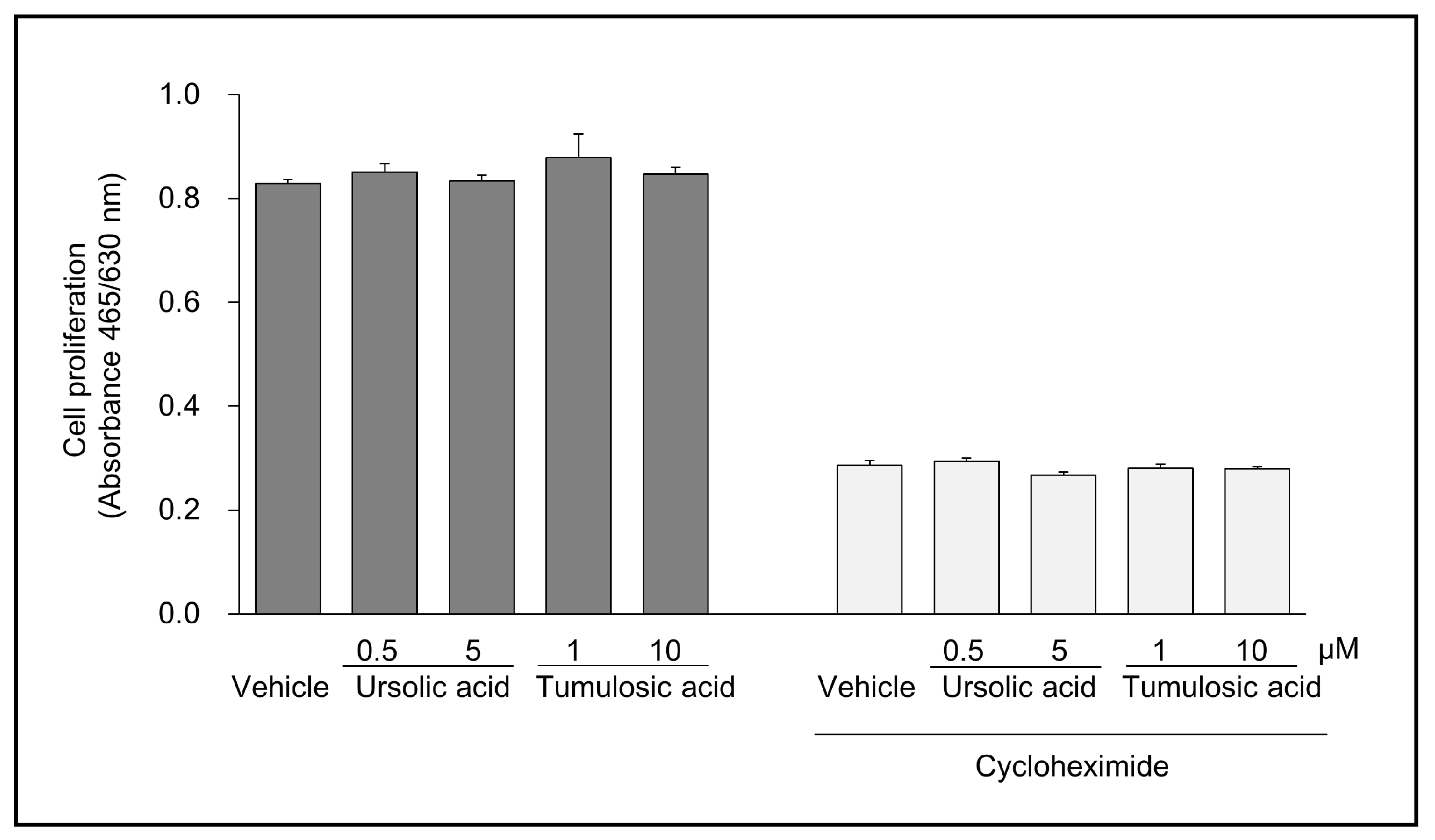
2.2. Ursolic Acid and Tumulosic Acid Decreased LL-37 Production in Epidermal Keratinocytes
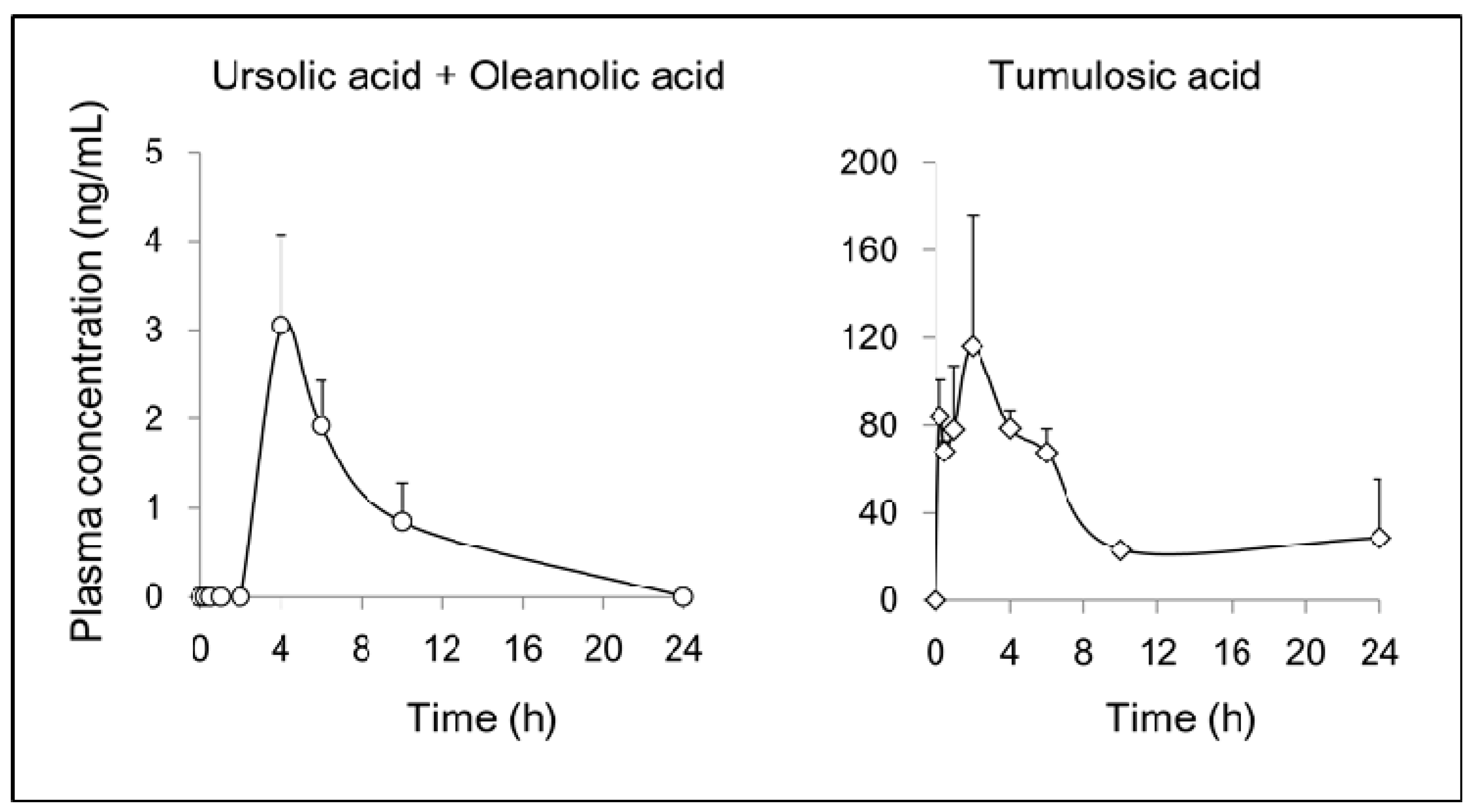
2.3. Pharmacokinetics of Triterpenoids in Rat after Oral Administration of JHT
3. Discussion
3.1. New Findings and Enzyme Selectivity
3.2. Structure–Activity Relationship
3.3. Effect of Proteolytic Activity on LL-37 Production
3.4. Potential of Triterpenoids as Internal Drugs
3.5. Summary: Possible Effects of Triterpenoids on Dermatoses
4. Materials and Methods
4.1. Tested Triterpenoids and Reference Regents
4.2. Measurements of Each Enzymatic Activity
4.3. Detection of LL-37 by Immunoprecipitation
4.4. Cell Growth Test
4.5. Pharmacokinetic Analysis of Triterpenoids in Rats
4.6. Statistical Analysis
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Brattsand, M.; Stefansson, K.; Lundh, C.; Haasum, Y.; Egelrud, T. A proteolytic cascade of kallikreins in the stratum corneum. J. Investig. Dermatol. 2005, 124, 198–203. [Google Scholar] [CrossRef] [PubMed]
- Caubet, C.; Jonca, N.; Brattsand, M.; Guerrin, M.; Bernard, D.; Schmidt, R.; Egelrud, T.; Simon, M.; Serre, G. Degradation of corneodesmosome proteins by two serine proteases of the kallikrein family, SCTE/KLK5/hK5 and SCCE/KLK7/hK7. J. Investig. Dermatol. 2004, 122, 1235–1244. [Google Scholar] [CrossRef] [PubMed]
- Schechter, N.M.; Choi, E.J.; Wang, Z.M.; Hanakawa, Y.; Stanley, J.R.; Kang, Y.; Clayman, G.L.; Jayakumar, A. Inhibition of human kallikreins 5 and 7 by the serine protease inhibitor lympho-epithelial Kazal-type inhibitor (LEKTI). Biol. Chem. 2005, 386, 1173–1184. [Google Scholar] [CrossRef] [PubMed]
- Furio, L.; Pampalakis, G.; Michael, I.P.; Nagy, A.; Sotiropoulou, G.; Hovnanian, A. KLK5 inactivation reverses cutaneous hallmarks of netherton syndrome. PLoS Genet. 2015, 11, e1005389. [Google Scholar] [CrossRef] [PubMed]
- Yamasaki, K.; Schauber, J.; Coda, A.; Lin, H.; Dorschner, R.A.; Schechter, N.M.; Bonnart, C.; Descargues, P.; Hovnanian, A.; Gallo, R.L. Kallikrein-mediated proteolysis regulates the antimicrobial effects of cathelicidins in skin. FASEB J. 2006, 20, 2068–2080. [Google Scholar] [CrossRef] [PubMed]
- Yamasaki, K.; Di Nardo, A.; Bardan, A.; Murakami, M.; Ohtake, T.; Coda, A.; Dorschner, R.A.; Bonnart, C.; Descargues, P.; Hovnanian, A.; et al. Increased serine protease activity and cathelicidin promotes skin inflammation in rosacea. Nat. Med. 2007, 13, 975–980. [Google Scholar] [CrossRef] [PubMed]
- Yamasaki, K.; Kanada, K.; Macleod, D.T.; Borkowski, A.W.; Morizane, S.; Nakatsuji, T.; Cogen, A.L.; Gallo, R.L. TLR2 expression is increased in rosacea and stimulates enhanced serine protease production by keratinocytes. J. Investig. Dermatol. 2011, 131, 688–697. [Google Scholar] [CrossRef] [PubMed]
- Yamasaki, K.; Gallo, R.L. Rosacea as a disease of cathelicidins and skin innate immunity. J. Investig. Dermatol. Symp. Proc. 2011, 15, 12–15. [Google Scholar] [CrossRef] [PubMed]
- Komatsu, N.; Saijoh, K.; Kuk, C.; Liu, A.C.; Khan, S.; Shirasaki, F.; Takehara, K.; Diamandis, E.P. Human tissue kallikrein expression in the stratum corneum and serum of atopic dermatitis patients. Exp. Dermatol. 2007, 16, 513–519. [Google Scholar] [CrossRef] [PubMed]
- Briot, A.; Deraison, C.; Lacroix, M.; Bonnart, C.; Robin, A.; Besson, C.; Dubus, P.; Hovnanian, A. Kallikrein 5 induces atopic dermatitis-like lesions through PAR2-mediated thymic stromal lymphopoietin expression in Netherton syndrome. J. Exp. Med. 2009, 206, 1135–1147. [Google Scholar] [CrossRef] [PubMed]
- Goettig, P.; Magdolen, V.; Brandstetter, H. Natural and synthetic inhibitors of kallikrein-related peptidases (KLKs). Biochimie 2010, 92, 1546–1567. [Google Scholar] [CrossRef] [PubMed]
- Safayhi, H.; Rall, B.; Sailer, E.R.; Ammon, H.P. Inhibition by boswellic acids of human leukocyte elastase. J. Pharmacol. Exp. Ther. 1997, 281, 460–463. [Google Scholar] [PubMed]
- Feng, L.; Liu, X.; Zhu, W.; Guo, F.; Wu, Y.; Wang, R.; Chen, K.; Huang, C.; Li, Y. Inhibition of human neutrophil elastase by pentacyclic triterpenes. PLoS ONE 2013, 8, e82794. [Google Scholar] [CrossRef] [PubMed]
- Rajic, A.; Akihisa, T.; Ukiya, M.; Yasukawa, K.; Sandeman, R.M.; Chandler, D.S.; Polya, G.M. Inhibition of trypsin and chymotrypsin by anti-inflammatory triterpenoids from Compositae flowers. Planta Med. 2001, 67, 599–604. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.W.; Hong, S.P.; Jeong, S.W.; Kim, B.; Bak, H.; Ryoo, H.C.; Lee, S.H.; Ahn, S.K. Simultaneous effect of ursolic acid and oleanolic acid on epidermal permeability barrier function and epidermal keratinocyte differentiation via peroxisome proliferator-activated receptor-alpha. J. Dermatol. 2007, 34, 625–634. [Google Scholar] [CrossRef] [PubMed]
- Cuellar, M.J.; Giner, R.M.; Recio, M.C.; Just, M.J.; Manez, S.; Rios, J.L. Effect of the basidiomycete Poria cocos on experimental dermatitis and other inflammatory conditions. Chem. Pharm. Bull. 1997, 45, 492–494. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.K.; Oh, H.M.; Lee, S.; Park, J.W.; Khang, D.; Lee, S.W.; Lee, W.S.; Rho, M.C.; Kim, S.H. Oleanolic acid acetate inhibits atopic dermatitis and allergic contact dermatitis in a murine model. Toxicol. Appl. Pharmacol. 2013, 269, 72–80. [Google Scholar] [CrossRef] [PubMed]
- Higaki, S.; Toyomoto, T.; Morohashi, M. Seijo-bofu-to, Jumi-haidoku-to and Toki-shakuyaku-san suppress rashes and incidental symptoms in acne patients. Drugs Exp. Clin. Res. 2002, 28, 193–196. [Google Scholar] [PubMed]
- Mizawa, M.; Makino, T.; Inami, C.; Shimizu, T. Jumihaidokuto (Shi-Wei-Ba-Du-Tang), a kampo formula, decreases the disease activity of palmoplantar pustulosis. Dermatol. Res. Pract. 2016, 2016, 4060673. [Google Scholar] [CrossRef] [PubMed]
- Sekiguchi, K.; Koseki, J.; Tsuchiya, K.; Matsubara, Y.; Iizuka, S.; Imamura, S.; Matsumoto, T.; Watanabe, J.; Kaneko, A.; Aiba, S.; et al. Suppression of Propionibacterium acnes-induced dermatitis by a traditional Japanese medicine, jumihaidokuto, modifying macrophage functions. Evid. Based Complent. Alternat. Med. 2015, 2015, 439258. [Google Scholar] [CrossRef]
- Nose, M.; Sakushima, J.; Harada, D.; Ogihara, Y. Comparison of immunopharmacological actions of 8 kinds of kampo-hozais clinically used in atopic dermatitis on delayed-type hypersensitivity in mice. Biol. Pharm. Bull. 1999, 22, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Ying, Q.L.; Rinehart, A.R.; Simon, S.R.; Cheronis, J.C. Inhibition of human leucocyte elastase by ursolic acid. Evidence for a binding site for pentacyclic triterpenes. Biochem. J. 1991, 277, 521–526. [Google Scholar] [CrossRef] [PubMed]
- Yoshikawa, M.; Matsuda, H. Antidiabetogenic activity of oleanolic acid glycosides from medicinal foodstuffs. Biofactors 2000, 13, 231–237. [Google Scholar] [CrossRef] [PubMed]
- Yamasaki, K.; Gallo, R.L. Antimicrobial peptides in human skin disease. Eur. J. Dermatol. 2008, 18, 11–21. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Feng, Y.; Ding, A. The research on the chemical components of Schizonepeta tenuifolia Briq. J. Chin. Med. Mater. 2001, 24, 183–184. [Google Scholar]
- Rios, J.L. Chemical constituents and pharmacological properties of Poria cocos. Planta Med. 2011, 77, 681–691. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.Y.; Kim, M.H.; Kim, J.H.; Jung, H.S.; Sohn, Y.; Choi, Y.J.; Hwang, M.K.; Kim, S.H.; Kim, J.; Yang, W.M. Schizonepeta tenuifolia inhibits the development of atopic dermatitis in mice. Phytother. Res. 2013, 27, 1131–1135. [Google Scholar] [CrossRef] [PubMed]
- Tohda, C.; Kakihara, Y.; Komatsu, K.; Kuraishi, Y. Inhibitory effects of methanol extracts of herbal medicines on substance P-induced itch-scratch response. Biol. Pharm. Bull. 2000, 23, 599–601. [Google Scholar] [CrossRef] [PubMed]
- Fung, D.; Lau, C.B. Schizonepeta tenuifolia: Chemistry, pharmacology, and clinical applications. J. Clin. Pharmacol. 2002, 42, 30–36. [Google Scholar] [CrossRef] [PubMed]
- Nukaya, H.; Yamashiro, H.; Fukazawa, H.; Ishida, H.; Tsuji, K. Isolation of inhibitors of TPA-induced mouse ear edema from Hoelen, Poria cocos. Chem. Pharm. Bull. 1996, 44, 847–849. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Luo, S.; Zhang, Y.; Chen, Z. Development of a liquid chromatography-mass spectrometry method for the determination of ursolic acid in rat plasma and tissue: Application to the pharmacokinetic and tissue distribution study. Anal. Bioanal. Chem. 2011, 399, 2877–2884. [Google Scholar] [CrossRef] [PubMed]
- Jeong, D.W.; Kim, Y.H.; Kim, H.H.; Ji, H.Y.; Yoo, S.D.; Choi, W.R.; Lee, S.M.; Han, C.K.; Lee, H.S. Dose-linear pharmacokinetics of oleanolic acid after intravenous and oral administration in rats. Biopharm. Drug Dispos. 2007, 28, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Matsubara, Y.; Matsumoto, T.; Sekiguchi, K.; Koseki, J.; Kaneko, A.; Yamaguchi, T.; Kurihara, Y.; Kobayashi, H. Oral administration of the Japanese traditional medicine keishibukuryogan-ka-yokuinin decreases reactive oxygen metabolites in rat plasma: Identification of chemical constituents contributing to antioxidant activity. Molecules 2017, 22, 256. [Google Scholar] [CrossRef] [PubMed]
- Lv, C.; Li, Q.; Zhang, Y.; Sui, Z.; He, B.; Xu, H.; Yin, Y.; Chen, X.; Bi, K. A UFLC-MS/MS method with a switching ionization mode for simultaneous quantitation of polygalaxanthone III, four ginsenosides and tumulosic acid in rat plasma: Application to a comparative pharmacokinetic study in normal and Alzheimer’s disease rats. J. Mass Spectrom. 2013, 48, 904–913. [Google Scholar] [CrossRef] [PubMed]
- Xiao, F.; Li, Q.; Liang, K.; Zhao, L.; He, B.; Ji, W.; Chen, X.; Wang, Z.; Bi, K.; Jia, Y. Comparative pharmacokinetics of three triterpene acids in rat plasma after oral administration of Poria extract and its formulated herbal preparation: GuiZhi-FuLing capsule. Fitoterapia 2012, 83, 117–124. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Not available. |
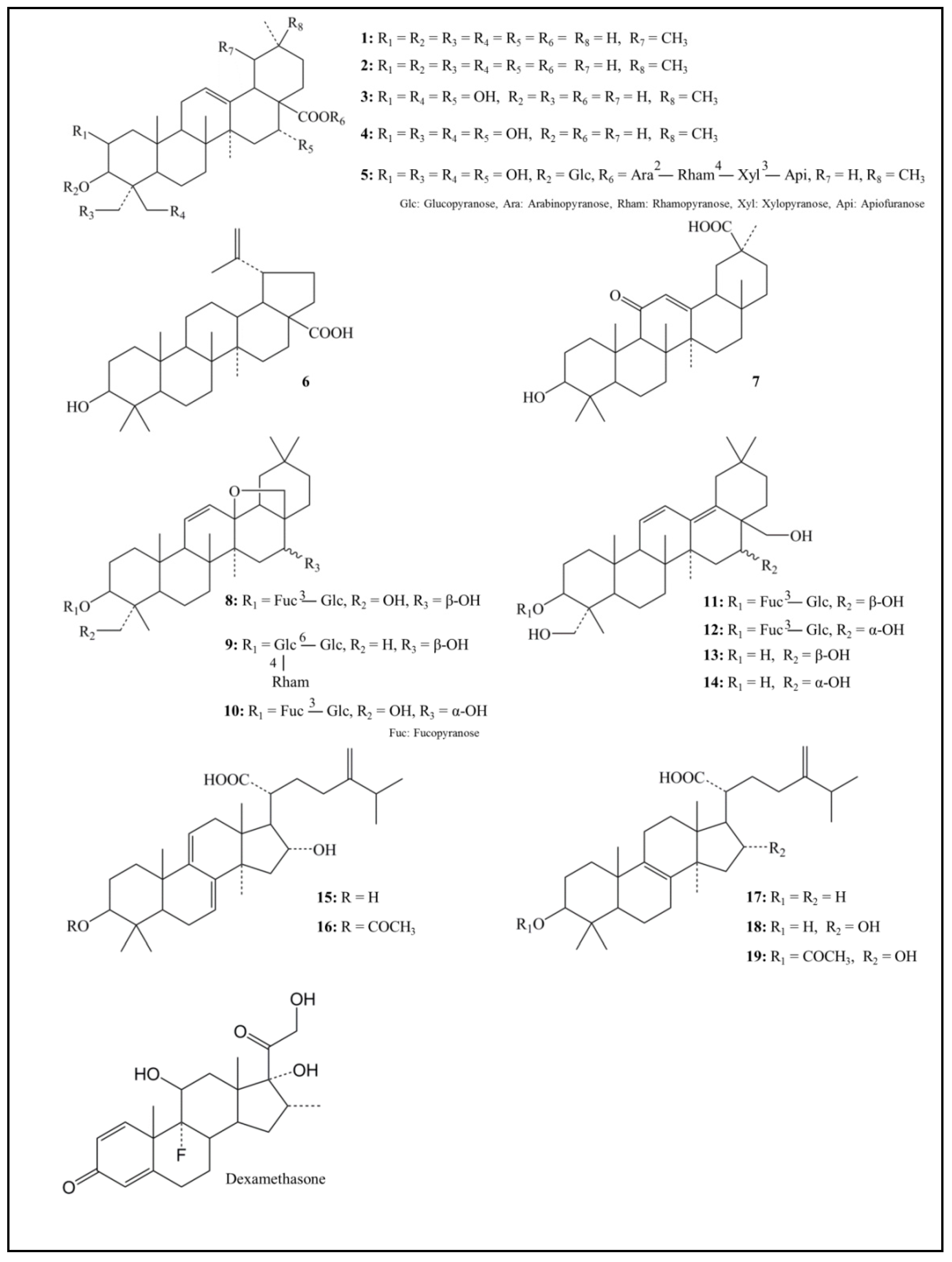

| Structure | Compound | Anti-KLK5 Activity % Inhibition |
|---|---|---|
| Pentacyclic triterpenoid | ||
| 1 Ursolic acid | 54.6 ± 1.3 | |
| 2 Oleanolic acid | 51.6 ± 1.8 | |
| 3 Polygalacic acid | 5.5 ± 2.3 | |
| 4 Platycodigenin | 8.8 ± 2.0 | |
| 5 Platycodin D | −6.0 ± 2.9 | |
| 6 Betulinic acid | 5.5 ± 2.5 | |
| 7 18β-Glycyrrhetinic acid | 16.0 ± 1.2 | |
| 8 Saikosaponin a | 4.3 ± 0.7 | |
| 9 Saikosaponin c | 4.1 ± 1.6 | |
| 10 Saikosaponin d | −10.4 ± 3.5 | |
| 11 Saikosaponin b1 | 39.6 ± 1.6 | |
| 12 Saikosaponin b2 | 21.1 ± 1.4 | |
| 13 Saikogenin A | 24.3 ± 2.6 | |
| 14 Saikogenin D | −1.5 ± 3.6 | |
| Tetracyclic triterpenoid | ||
| 15 Dehydrotumulosic acid | −46.2 ± 2.7 | |
| 16 Dehydropachymic acid | 33.9 ± 3.7 | |
| 17 Eburicoic acid | −51.0 ± 3.0 | |
| 18 Tumulosic acid | 36.0 ± 1.9 | |
| 19 Pachymic acid | 56.2 ± 1.5 | |
| Reference | ||
| Dexamethasone | −1.8 ± 2.8 | |
| Leupeptin hemisulfate | 96.9 ± 0.1 |
| Ursolic Acid | Tumulosic Acid | |||
|---|---|---|---|---|
| IC50 (μM) | Inhibition % at 100 μM | IC50 (μM) | Inhibition % at 100 μM | |
| Kallikrein 5 | 5.8 | 74.5 ± 0.2 | 14.8 | 58.7 ± 0.2 |
| Kallikrein 7 | >100 | 38.4 ± 1.6 | >100 | 19.5 ± 1.5 |
| Trypsin | 14.8 | 76.9 ± 3.5 | 45.3 | 73.2 ± 1.6 |
| Chymotrypsin C | >100 | 35.3 ± 2.9 | >100 | 4.1 ± 5.2 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matsubara, Y.; Matsumoto, T.; Koseki, J.; Kaneko, A.; Aiba, S.; Yamasaki, K. Inhibition of Human Kallikrein 5 Protease by Triterpenoids from Natural Sources. Molecules 2017, 22, 1829. https://doi.org/10.3390/molecules22111829
Matsubara Y, Matsumoto T, Koseki J, Kaneko A, Aiba S, Yamasaki K. Inhibition of Human Kallikrein 5 Protease by Triterpenoids from Natural Sources. Molecules. 2017; 22(11):1829. https://doi.org/10.3390/molecules22111829
Chicago/Turabian StyleMatsubara, Yosuke, Takashi Matsumoto, Junichi Koseki, Atsushi Kaneko, Setsuya Aiba, and Kenshi Yamasaki. 2017. "Inhibition of Human Kallikrein 5 Protease by Triterpenoids from Natural Sources" Molecules 22, no. 11: 1829. https://doi.org/10.3390/molecules22111829