Synthesis, Structure, Surface and Antimicrobial Properties of New Oligomeric Quaternary Ammonium Salts with Aromatic Spacers
Abstract
:1. Introduction
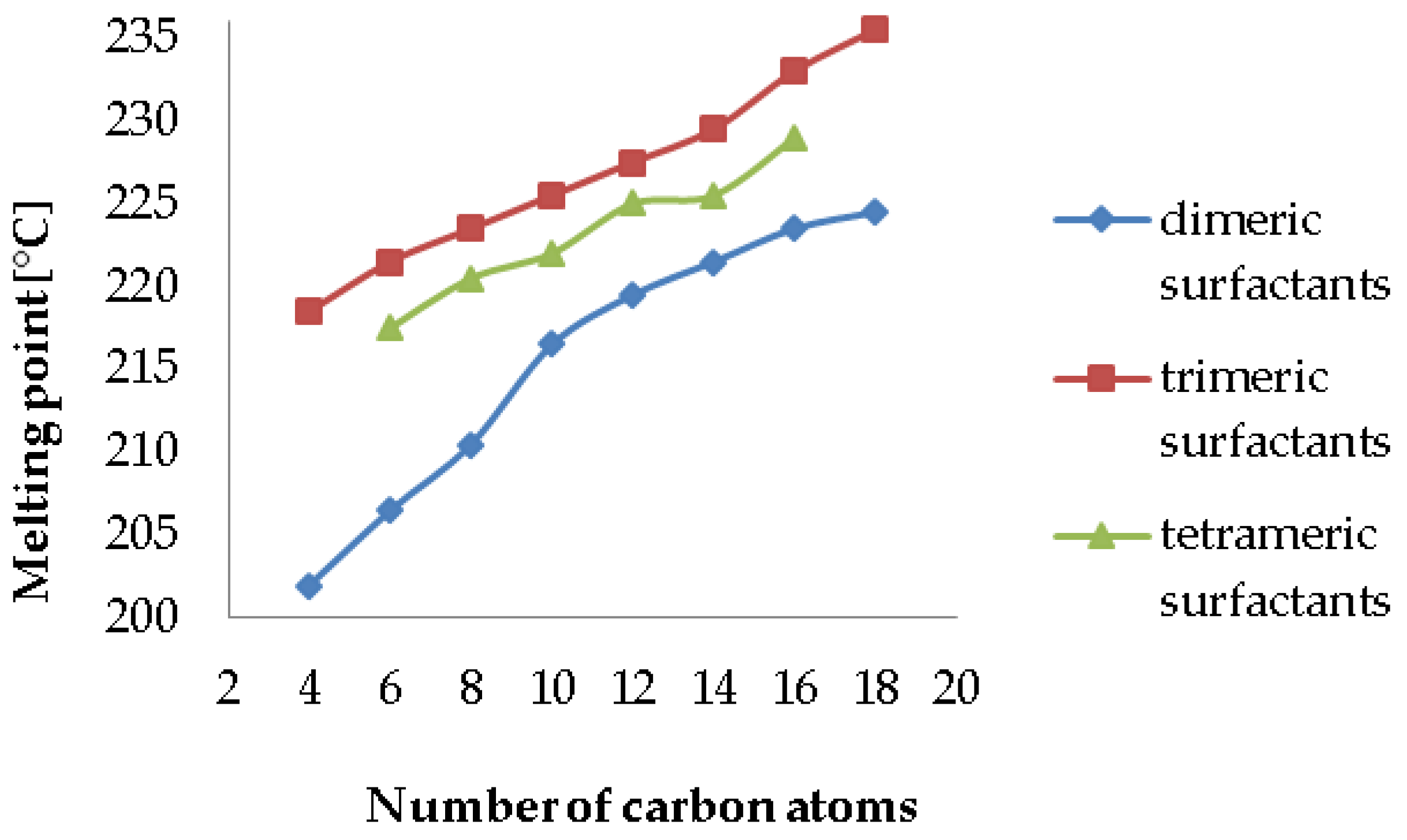
2. Results and Discussion
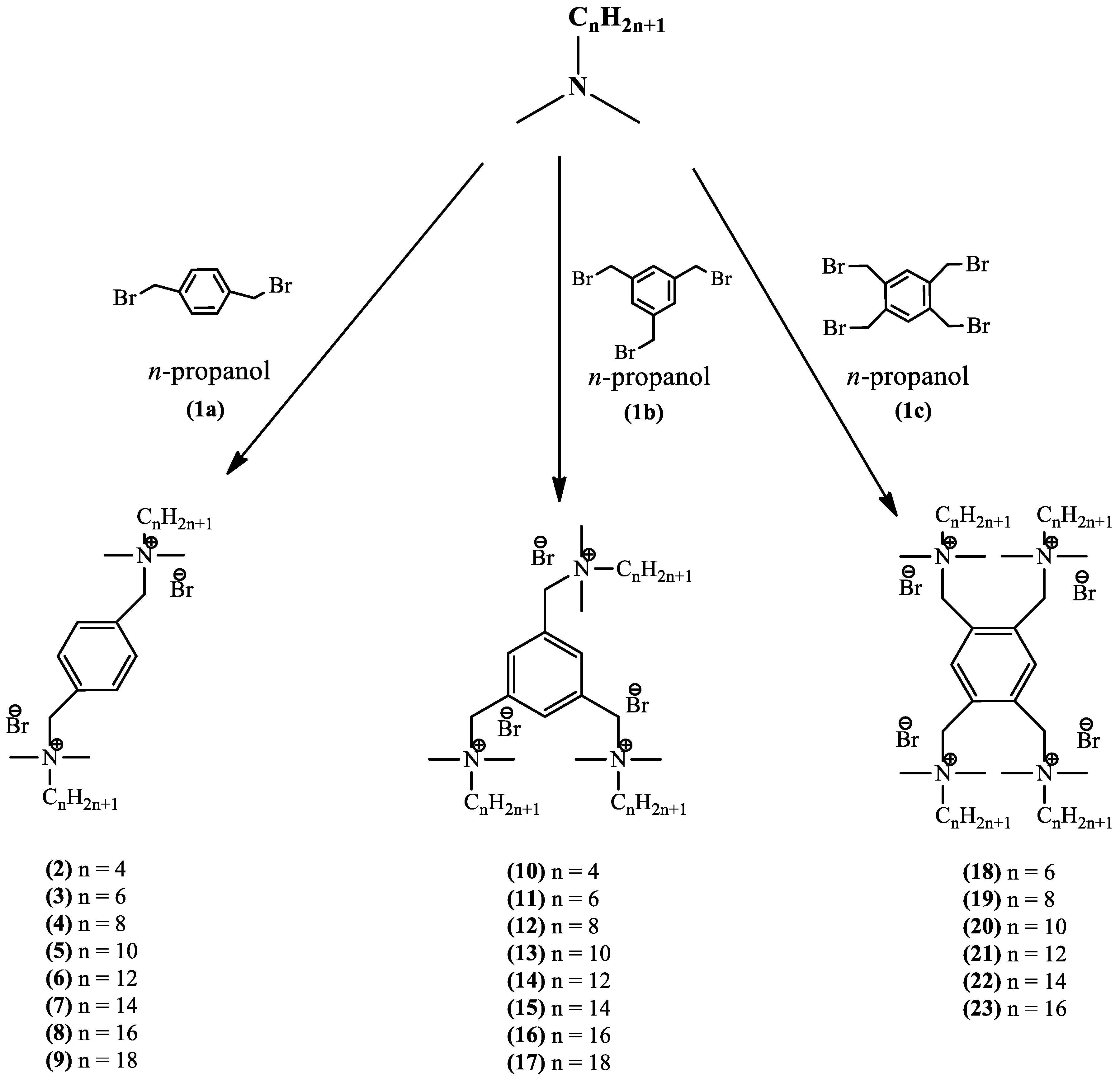
2.1. Synthesis
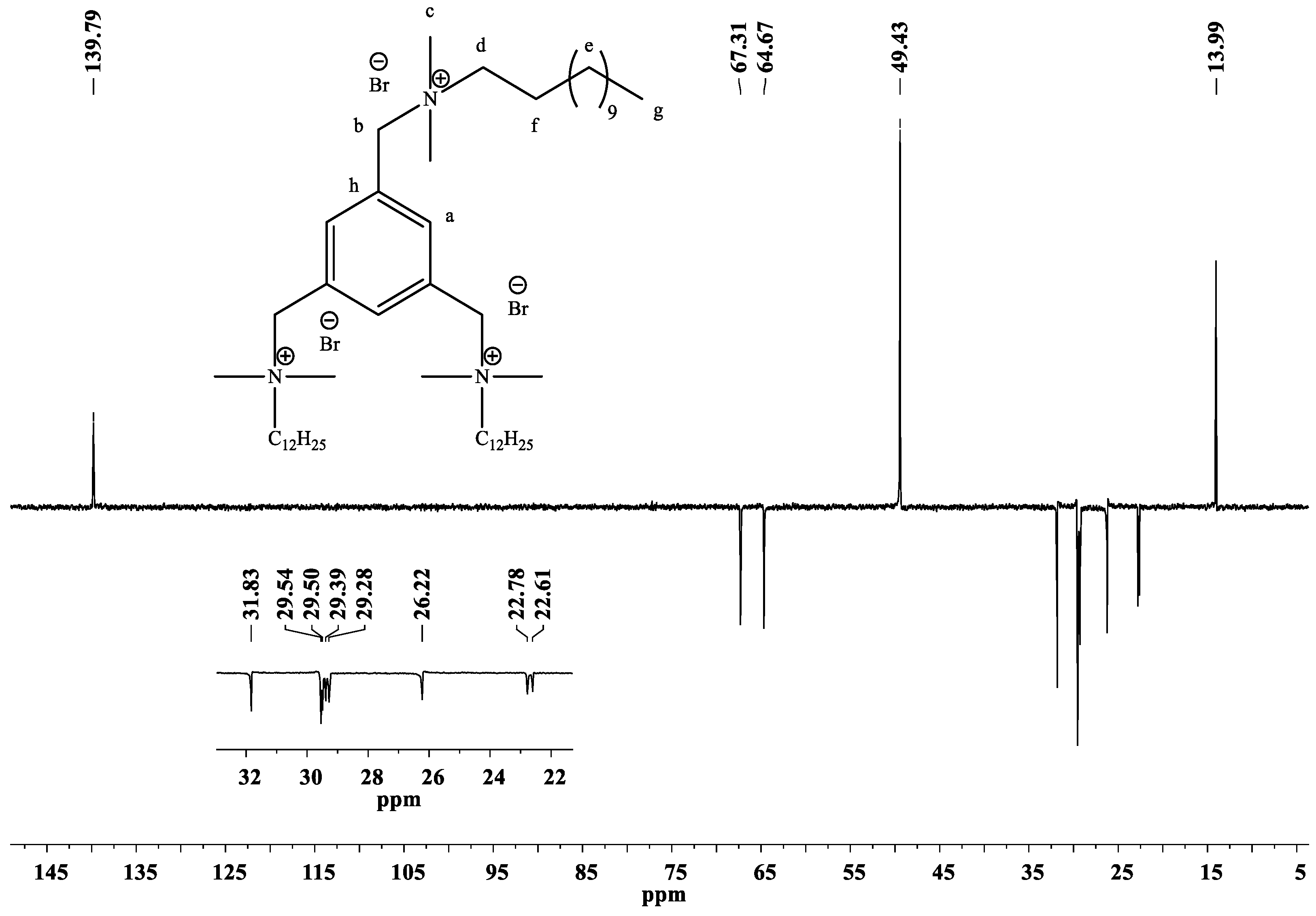
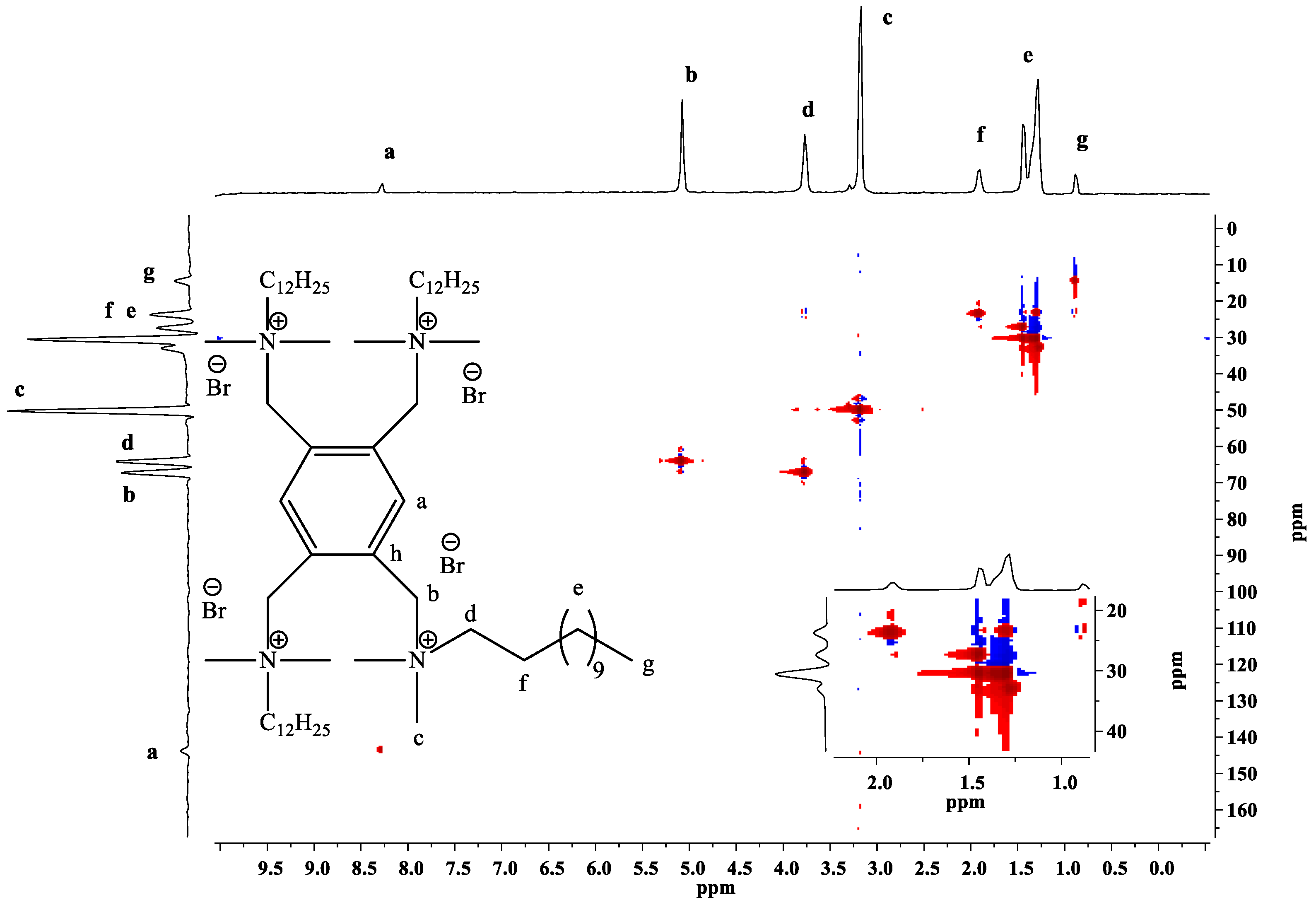
2.2. Spectroscopic Study
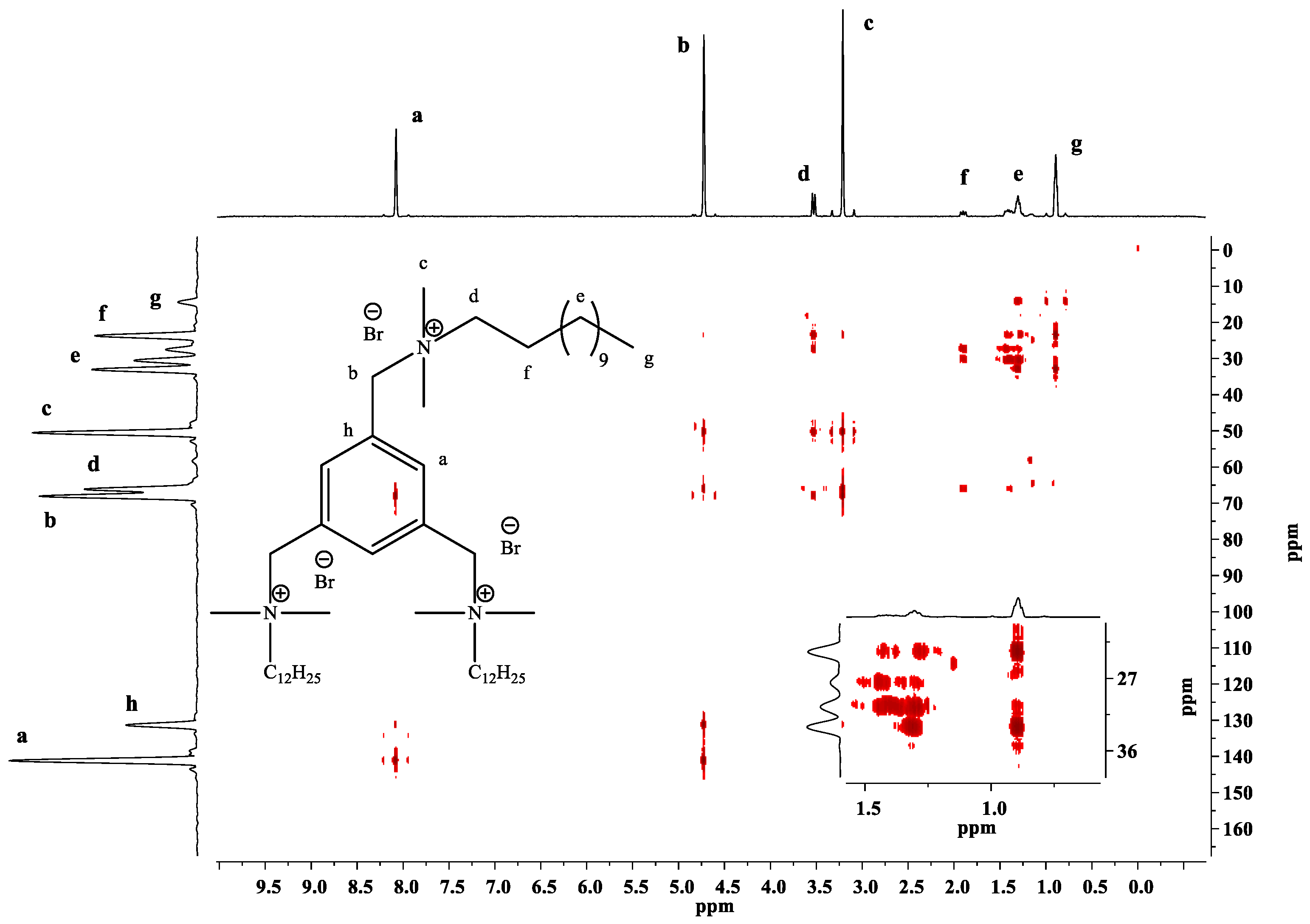
2.2.1. 1H-NMR, 13C-NMR and 2D-NMR Spectra
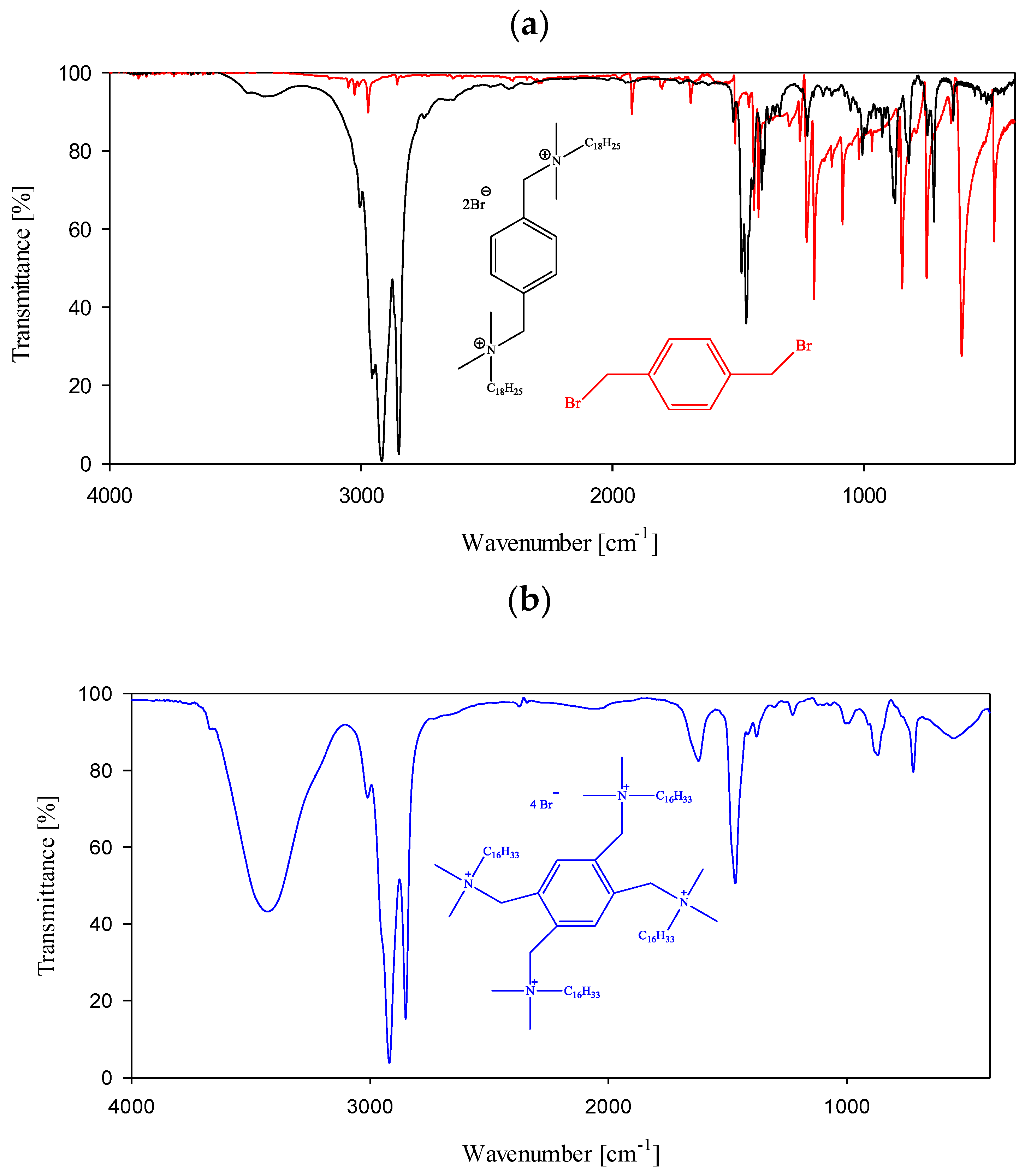
2.2.2. FT-IR Spectra
2.2.3. PM5 Calculations
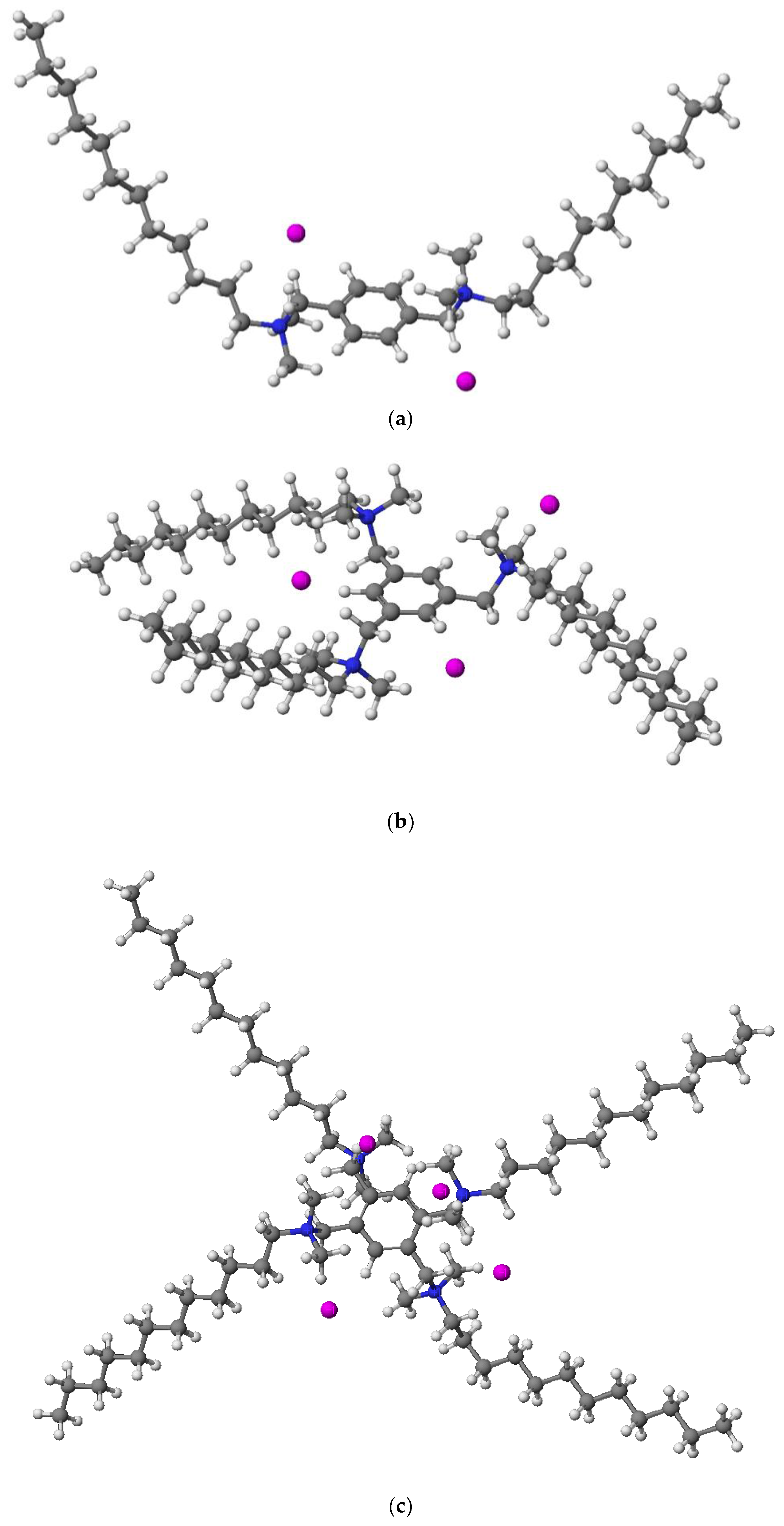
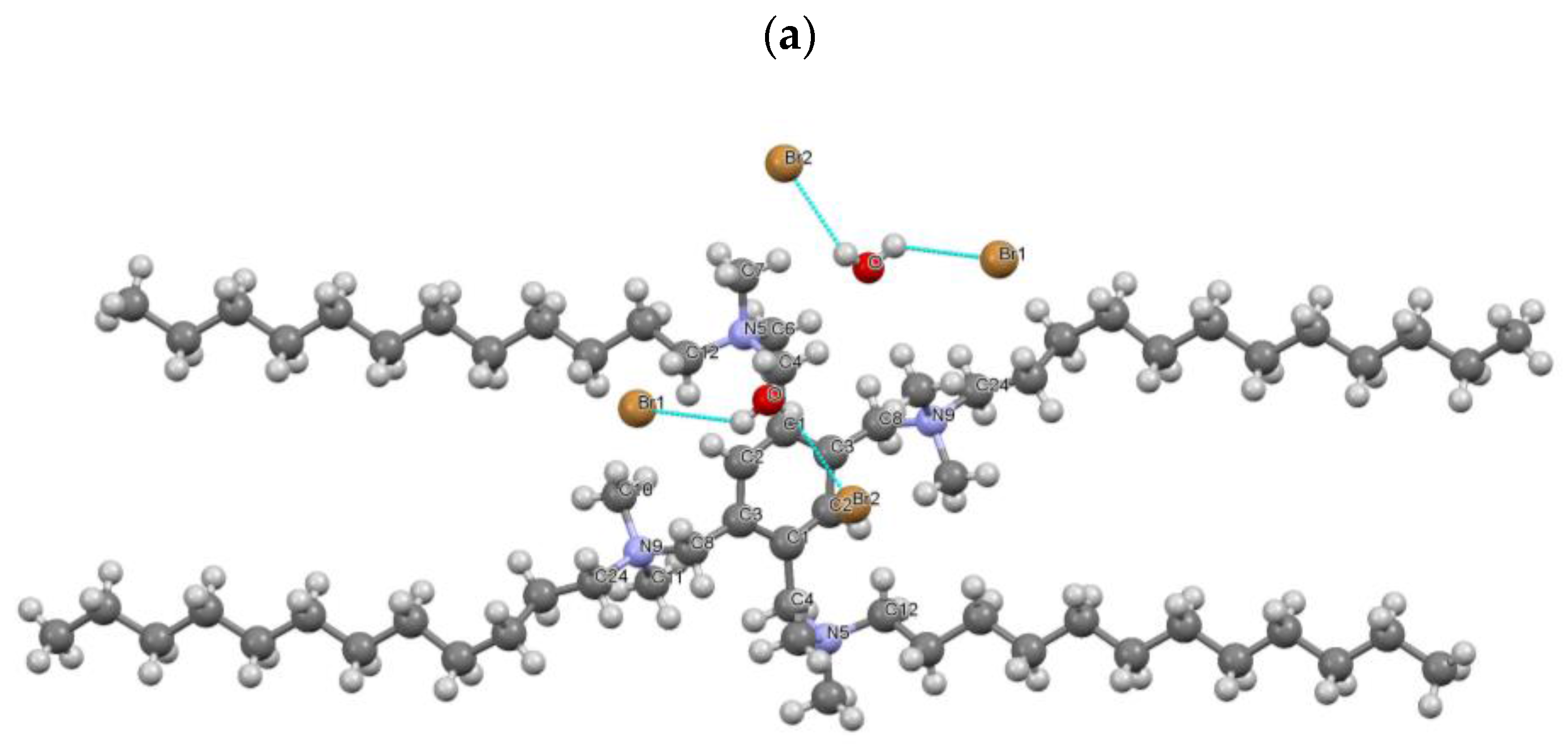
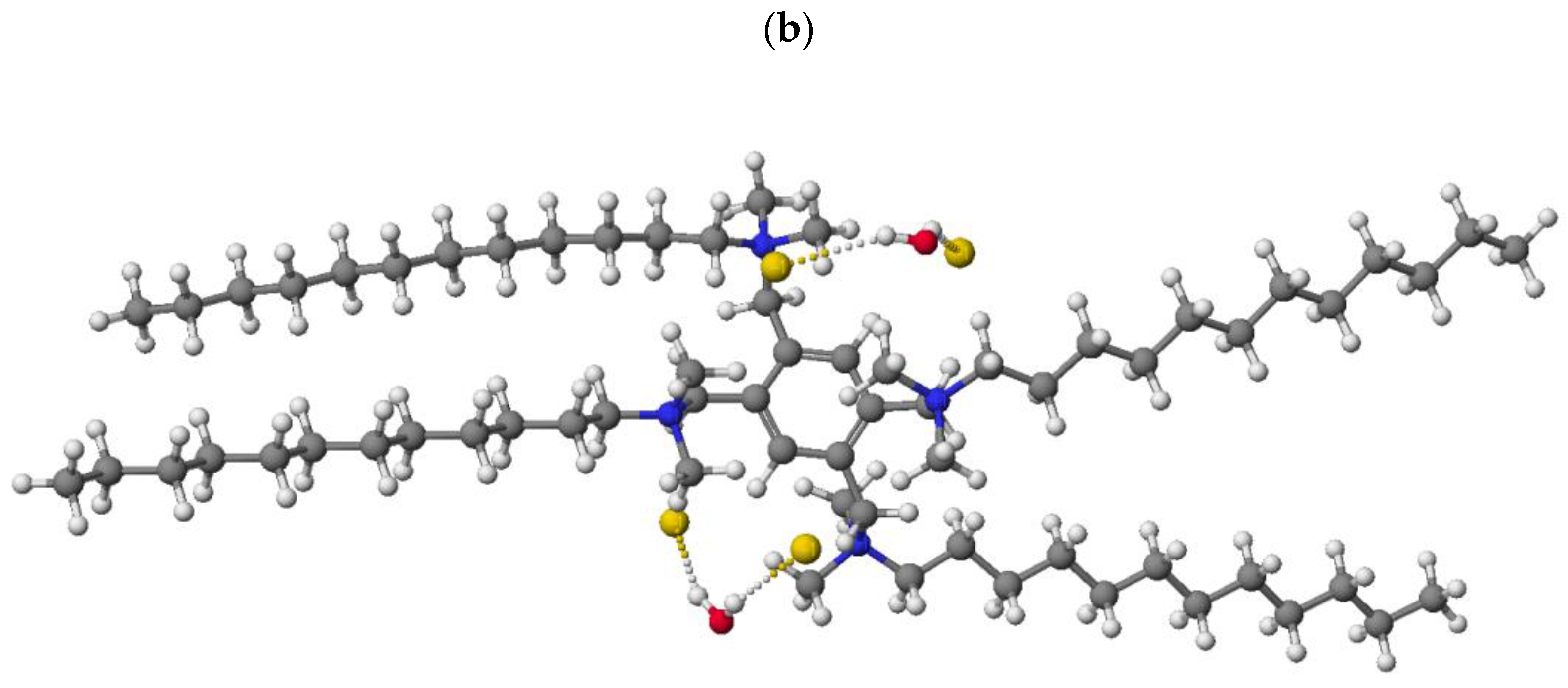
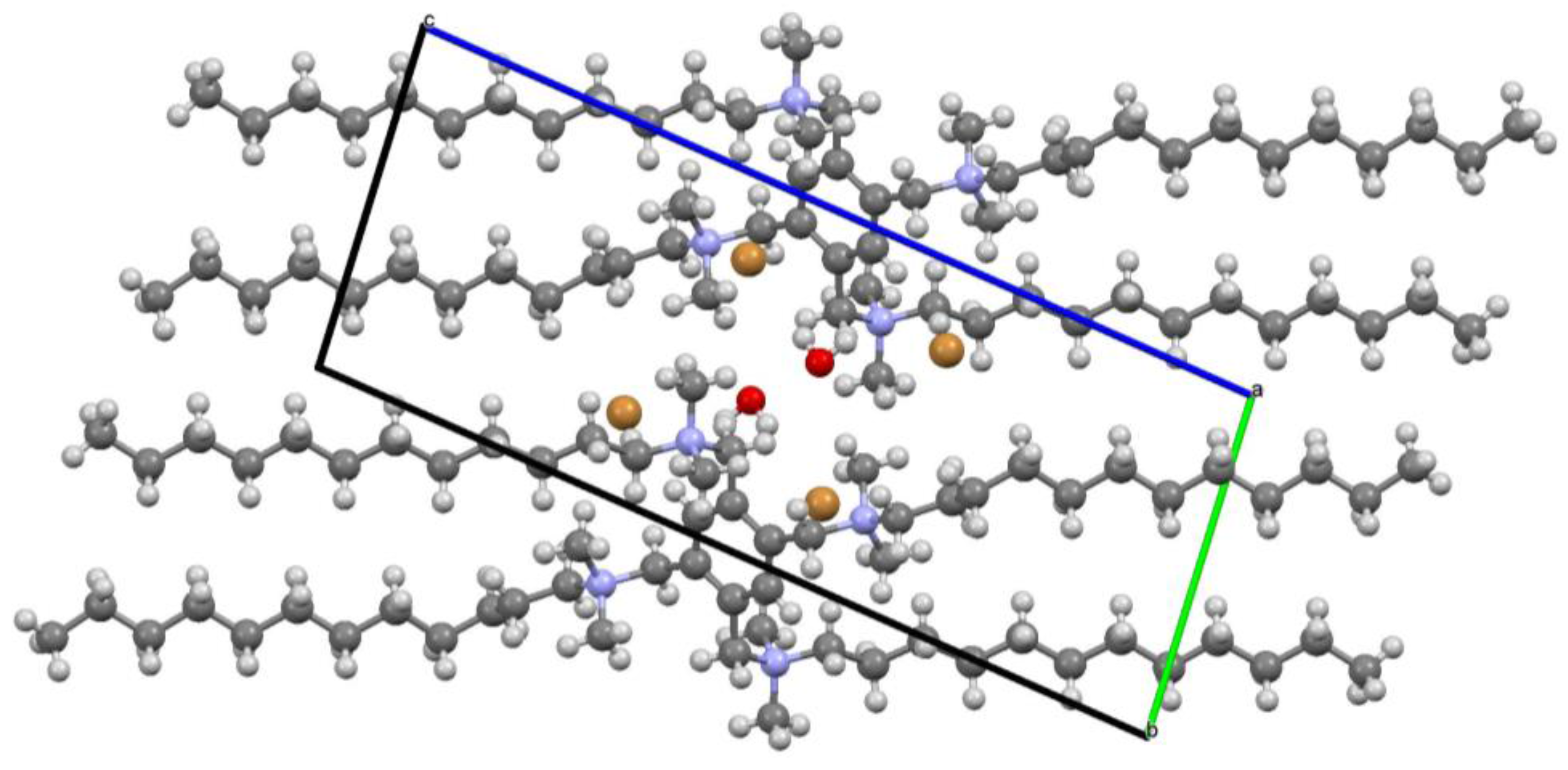
2.2.4. X-ray Analysis
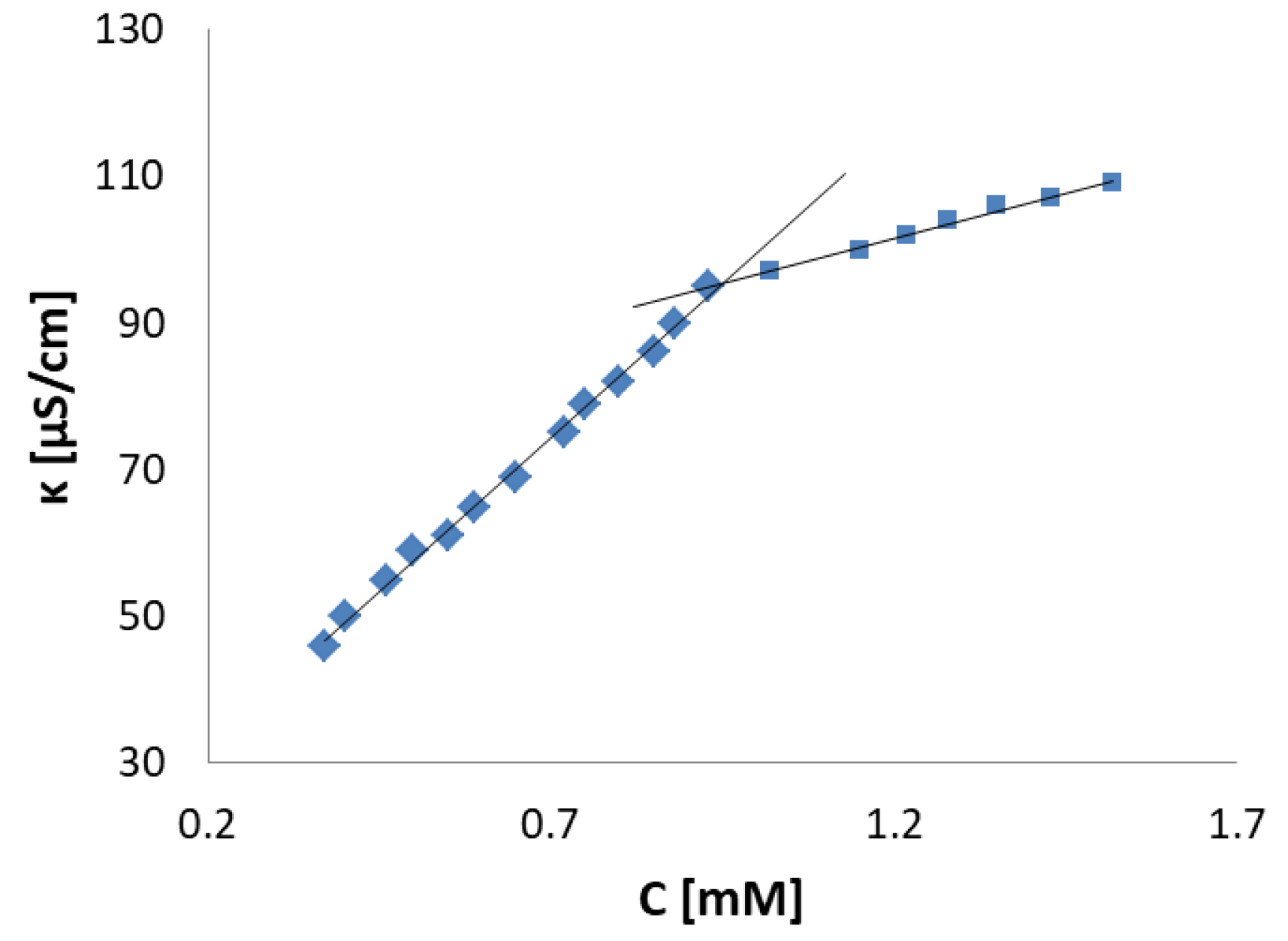
2.2.5. Aggregation Behavior
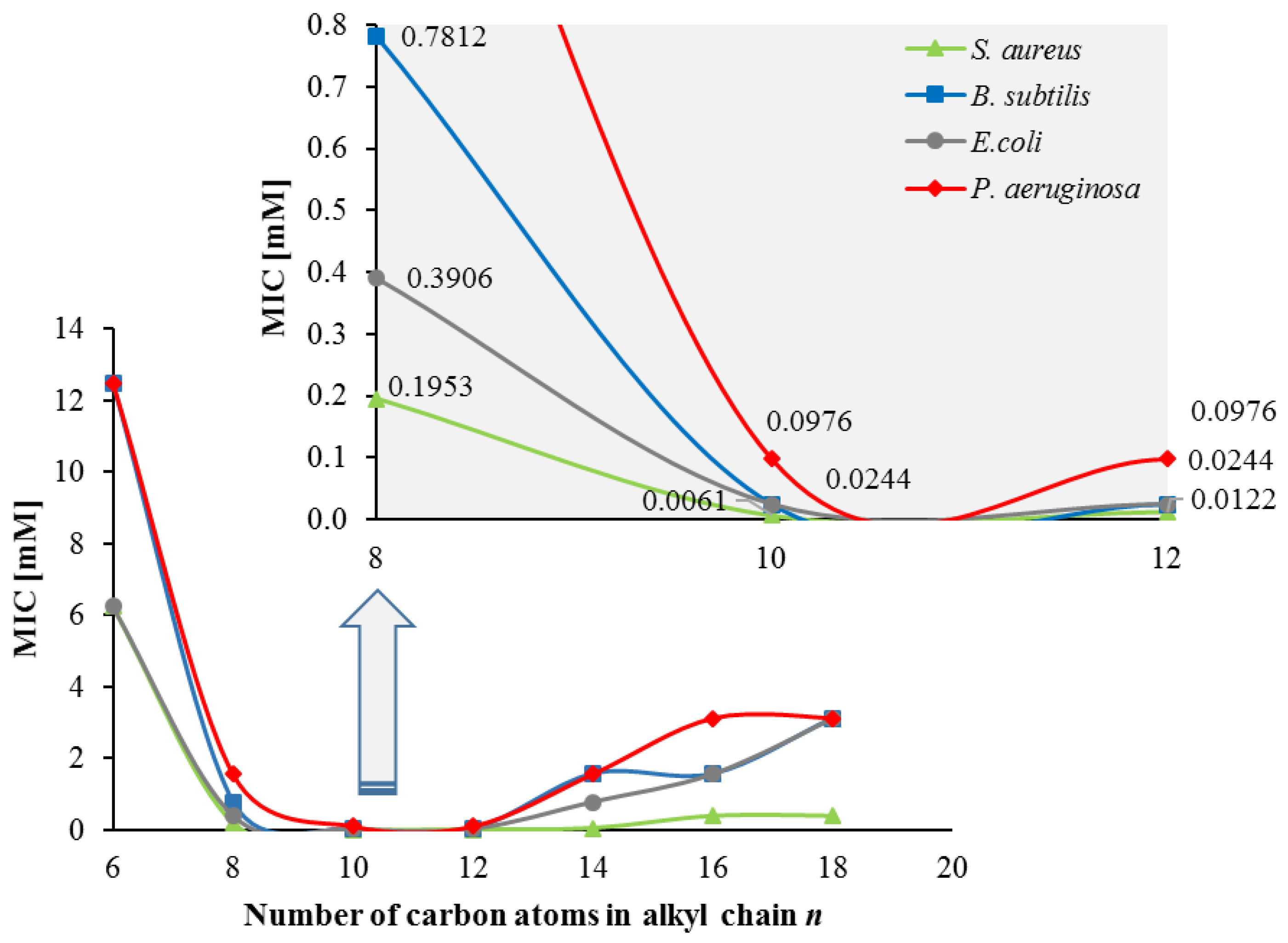
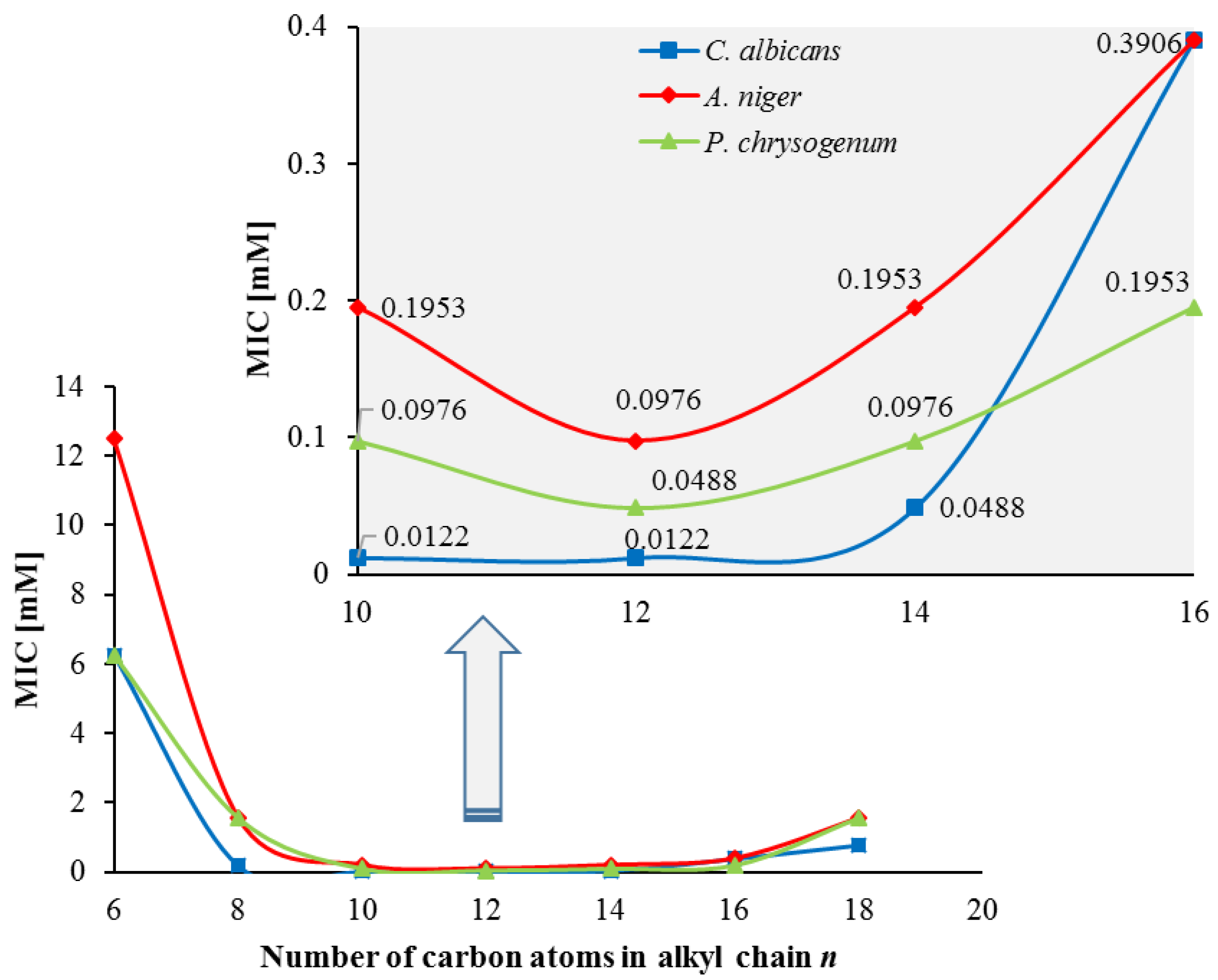
2.2.6. Antimicrobial Properties
3. Materials and Methods
3.1. General Information
3.2. Synthesis
Series of dimeric quaternary ammonium salts (2–9).
Series of trimeric quaternary ammonium salts (10–17).
Series of tetrameric quaternary ammonium salts (18–23).
3.3. Critical Micellization Concentration
3.4. Antimicrobial Properties
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Myers, D. Surfactant Science and Technology, 3rd ed.; Wiley: Hoboken, NJ, USA; New York, NY, USA, 2006. [Google Scholar]
- Rhein, L.D. Surfactants in Personal Care Products and Decorative Cosmetics, 3rd ed.; CRC Press: Boca Raton, FL, USA, 2007. [Google Scholar]
- Karsa, R.D. Surfactants in Polymers, Coatings, Inks, and Adhesives, 1st ed.; Blackwell: Oxford, UK, 2003. [Google Scholar]
- Schramm, L.L.; Stasiuk, E.N.; Marangoni, D.G. Surfactants and their applications. Annu. Rep. Prog. Chem. Sect. C Phys. Chem. 2003, 99, 3–48. [Google Scholar] [CrossRef]
- Tehrani-Bagha, A.; Holmberg, K. Solubilization of Hydrophobic Dyes in Surfactant Solutions. Materials 2013, 6, 580–608. [Google Scholar] [CrossRef] [PubMed]
- Siedenbiedel, F.; Tiller, J.C. Antimicrobial Polymers in Solution and on Surfaces: Overview and Functional Principles. Polymers 2012, 4, 46–71. [Google Scholar] [CrossRef]
- Russell, H.A. Principles and Practice of Disinfection, Preservation, and Sterilization, 5th ed.; John Wiley & Sons: Chichester, West Sussex, UK, 2012. [Google Scholar]
- Zhou, C.; Wang, F.; Chen, H.; Li, M.; Qiao, F.; Liu, Z. Selective Antimicrobial Activities and Action Mechanism of Micelles Self-Assembled by Cationic Oligomeric Surfactants. ACS Appl. Mater. Interfaces 2016, 8, 4242–4249. [Google Scholar] [CrossRef] [PubMed]
- John, V.T.; Simmons, B.; McPherson, G.L.; Bose, A. Recent developments in materials synthesis in surfactant systems. Curr. Opin. Colloid Interface Sci. 2002, 7, 288–295. [Google Scholar] [CrossRef]
- Shinde, P.V.; Kategaonkar, A.H.; Shingate, B.B.; Shingare, M.S. Surfactant catalyzed convenient and greener synthesis of tetrahydrobenzo[a]xanthene-11-ones at ambient temperature. Beilstein J. Org. Chem. 2011, 7, 53–58. [Google Scholar] [CrossRef] [PubMed]
- Akbar, J.R. Pharmaceutical Applications of Gemini Surfactants. 2011. Available online: https://uwspace.uwaterloo.ca/handle/10012/5700 (accessed on 15 March 2017).
- Sivaramakrishnan, C.N. The use of surfactants in the finishing of technical textiles. In Advances in the Dyeing and Finishing of Technical Textiles; Elsevier: Philadelphia, PA, USA, 2013; pp. 199–235. Available online: http://linkinghub.elsevier.com/retrieve/pii/B9780857094339500092 (accessed on 20 January 2017).
- Le Marechal, A.M.; Križanec, B.; Vajnhandl, S.; Valh, J.V. Textile finishing industry as an important source of organic pollutants. In Organic Pollutants Ten Years after the Stockholm Convention-Environmental and Analytical Update; InTech: Rijeka, Croatia, 2012; Available online: http://www.intechopen.com/source/pdfs/29368/InTech-Textile_finishing_industry_as_an_important_source_of_organic_pollutants.pdf (accessed on 12 September 2017).
- Castro, M.J.L.; Ojeda, C.; Cirelli, A.F. Advances in surfactants for agrochemicals. Environ. Chem. Lett. 2014, 12, 85–95. [Google Scholar] [CrossRef]
- Shen, A.Q.; Gleason, B.; McKinley, G.H.; Stone, H.A. Fiber coating with surfactant solutions. Phys. Fluids 2002, 14, 4055–4068. [Google Scholar] [CrossRef]
- Ma, J.; Tang, J.; Cheng, Q.; Zhang, H.; Shinya, N.; Qin, L.-C. Effects of surfactants on spinning carbon nanotube fibers by an electrophoretic method. Sci. Technol. Adv. Mater. 2010, 11, 065005. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Qiao, Y.; Cheng, X.; Yan, Y.; Li, Z.; Huang, J. Hydrotropic salt promotes anionic surfactant self-assembly into vesicles and ultralong fibers. J. Colloid Interface Sci. 2012, 369, 238–244. [Google Scholar] [CrossRef] [PubMed]
- Pritchard, G. Plastics Additives; Brewis, D., Briggs, D., Eds.; Polymer Science and Technology Series; Springer: Dordrecht, The Netherlands, 1998; Volume 1, Available online: http://link.springer.com/10.1007/978-94-011-5862-6 (accessed on 20 February 2017).
- Kralova, I.; Sjöblom, J. Surfactants Used in Food Industry: A Review. J. Dispers. Sci. Technol. 2009, 30, 1363–1383. [Google Scholar] [CrossRef]
- Nitschke, M.; Silva, S.S. Recent Food Applications of Microbial Surfactants. Crit. Rev. Food Sci. Nutr. 2016, 20, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Raffa, P.; Broekhuis, A.A.; Picchioni, F. Polymeric surfactants for enhanced oil recovery: A review. J. Pet. Sci. Eng. 2016, 145, 723–733. [Google Scholar] [CrossRef]
- Maithufi, M.N.; Joubert, D.J.; Klumperman, B. Application of Gemini Surfactants as Diesel Fuel Wax Dispersants. Energy Fuels 2011, 25, 162–171. [Google Scholar] [CrossRef]
- Kumar, N.; Tyagi, R. Industrial Applications of Dimeric Surfactants: A Review. J. Dispers. Sci. Technol. 2014, 35, 205–214. [Google Scholar] [CrossRef]
- Athas, J.C.; Jun, K.; McCafferty, C.; Owoseni, O.; John, V.T.; Raghavan, S.R. An Effective Dispersant for Oil Spills Based on Food-Grade Amphiphiles. Langmuir 2014, 30, 9285–9294. [Google Scholar] [CrossRef] [PubMed]
- Song, D. Development of High Efficient and Low Toxic Oil Spill Dispersants based on Sorbitol Derivants Nonionic Surfactants and Glycolipid Biosurfactants. J. Environ. Prot. 2013, 4, 16–22. [Google Scholar] [CrossRef]
- Lopes, L.R.B.; Soares, V.L.P.; Barcellos, M.T.C.; Mansur, C.R.E. Desenvolvimento de surfatantes para aplicação na indústria de explosivos. Polímeros 2014, 24, 474–477. [Google Scholar] [CrossRef]
- Brown, P.; Butts, C.P.; Eastoe, J. Stimuli-responsive surfactants. Soft Matter 2013, 9, 2365–2374. [Google Scholar] [CrossRef]
- Surfactants Market Rising at 4.20% CAGR from 2015–2023 due to Rising Demand for Detergents and Personal Care Products: Transparency Market Research. Available online: https://globenewswire.com/news-release/2016/01/29/805884/0/en/Surfactants-Market-Rising-at-4-20-CAGR-from-2015-2023-due-to-Rising-Demand-for-Detergents-and-Personal-Care-Products-Transparency-Market-Research.html (accessed on 16 January 2017).
- Zana, R.; Xia, J. Gemini Surfactants. Synthesis, Interfacial and Solution-Phase Behavior, and Applications; Marcel Dekker: New York, NY, USA, 2003. [Google Scholar]
- Abd El-Lateef, H.M.; Abo-Riya, M.A.; Tantawy, A.H. Empirical and quantum chemical studies on the corrosion inhibition performance of some novel synthesized cationic gemini surfactants on carbon steel pipelines in acid pickling processes. Corros. Sci. 2016, 108, 94–110. [Google Scholar] [CrossRef]
- Brycki, B.; Kowalczyk, I.; Szulc, A.; Kaczerewska, O.; Pakiet, M. Multifunctional Gemini Surfactants—Structure, Synthesis, Properties and Applications, Surfactants and Characterization of Surfactants; Najjar, R., Ed.; Intech: Rijeka, Croatia, 2017; pp. 97–155. [Google Scholar]
- Abdallah, M.; Eltass, H.M.; Hegazy, M.A.; Ahmed, H. Adsorption and inhibition effect of novel cationic surfactant for pipelines carbon steel in acidic solution. Prot. Met. Phys. Chem. Surf. 2016, 52, 721–730. [Google Scholar] [CrossRef]
- Heakal, F.E.-T.; Elkholy, A.E. Gemini surfactants as corrosion inhibitors for carbon steel. J. Mol. Liq. 2017, 230, 395–407. [Google Scholar] [CrossRef]
- Hegazy, M.A.; Abd El-Rehim, S.S.; Badr, E.A.; Kamel, W.M.; Youssif, A.H. Mono-, Di- and Tetra-Cationic Surfactants as Carbon Steel Corrosion Inhibitors. J. Surfactants Deterg. 2015, 18, 1033–1042. [Google Scholar] [CrossRef]
- Kaczerewska, O.; Leiva-Garcia, R.; Akid, R.; Brycki, B. Efficiency of cationic gemini surfactants with 3-azamethylpentamethylene spacer as corrosion inhibitors for stainless steel in hydrochloric acid. J. Mol. Liq. 2017, 247, 6–13. [Google Scholar] [CrossRef]
- Labena, A.; Hegazy, M.A.; Horn, H.; Muller, E. Cationic Gemini Surfactant as a Corrosion Inhibitor and a Biocide for High Salinity Sulfidogenic Bacteria Originating from an Oil-Field Water Tank. J. Surfactants Deterg. 2014, 17, 419–431. [Google Scholar] [CrossRef]
- Mobin, M.; Aslam, R.; Zehra, S.; Ahmad, M. Bio-/Environment-Friendly Cationic Gemini Surfactant as Novel Corrosion Inhibitor for Mild Steel in 1 M HCl Solution. J. Surfactants Deterg. 2017, 20, 57–74. [Google Scholar] [CrossRef]
- McCullough, D.H., III; Janout, V.; Li, J.; Hsu, J.T.; Troung, Q.; Wilusz, E.; Regen, S.L. Glued Langmuir—Blodgett Bilayers from porous versus Nonporous Surfactants. J. Am. Chem. Soc. 2004, 126, 9916–9917. [Google Scholar] [CrossRef] [PubMed]
- Laschewsky, A.; Lunkenheimer, K.; Rakotoaly, R.H.; Wattebled, L. Spacer Effects in Dimeric Cationic Surfactants. Colloid Polym. Sci. 2005, 283, 469–479. [Google Scholar] [CrossRef]
- Song, L.D.; Rosen, M.J. Surface Properties, Micellization, and Premicellar Aggregation of Gemini Surfactants with Rigid and Flexible Spacers. Langmuir 1996, 12, 1149–1153. [Google Scholar] [CrossRef]
- CAChe Version 5.04. UserGuide; Fujitsu: Chiba, Japan, 2003. [Google Scholar]
- Stewart, J.J.P. Optimization of parameters for semiempirical methods. III. Extension of PM3 to Be, Mg, Zn, Ga, Ge, As, Se, Cd, In, Sn, Sb, Te, Hg, Tl, Pb, and Bi. J. Comput. Chem. 1991, 12, 320–341. [Google Scholar] [CrossRef]
- Stewart, J.J.P. Optimization of parameters for semiempirical methods. I. Method. J. Comput. Chem. 1989, 10, 209–220. [Google Scholar] [CrossRef]
- Deng, S.-R.; Wu, L.; Wang, H.; Zhou, B.; Wong, W.-K.; Guo, J.-P.; Li, Z.-Y.; Zhou, Y.-H. Synthesis and Structure of a Novel Disulfide-Containing Aniline. Synth. Commun. 2005, 35, 129–135. [Google Scholar] [CrossRef]
- Pospieszny, T.; Koenig, H.; Brycki, B. Synthesis and spectroscopic studies of new quasi podands from bile acid derivatives. Tetrahedron Lett. 2013, 54, 4700–4704. [Google Scholar] [CrossRef]
- Pospieszny, T.; Koenig, H.; Kowalczyk, I.; Brycki, B. Synthesis, spectroscopic and theoretical studies of new quasi-podands from bile acid derivatives linked by 1,2,3-triazole rings. Molecules 2014, 19, 2557–2570. [Google Scholar] [CrossRef] [PubMed]
- Macrae, C.F.; Bruno, I.J.; Chisholm, J.A.; Edgington, P.R.; McCabe, P.; Pidcock, E.; Rodriguez-Monge, L.; Taylor, R.; van de Streek, J.; Wood, P.A. Mercury CSD 2.0—New Features for the Visualization and Investigation of Crystal Structures. J. Appl. Crystallogr. 2008, 41, 466–470. [Google Scholar]
- Gilbert, P.; Moore, L.E. Cationic antiseptics: Diversity of action under a common epithet. J. Appl. Microbiol. 2005, 99, 703–715. [Google Scholar] [CrossRef] [PubMed]
- Fazlara, A.; Ekhtelat, M. The Disinfectant Effects of Benzalkonium Chloride on Some Important Foodborne Pathogens. Am. Eurasian J. Agric. Environ. Sci. 2012, 12, 23–29. [Google Scholar]
- Feder-Kubis, J.; Tomczuk, K. The effect of the cationic structures of chiral ionic liquids on their antimicrobial activities. Tetrahedron 2013, 69, 4190–4198. [Google Scholar] [CrossRef]
- Merianos, J.J. Surface-active agents. In Disinfection, Sterilization and Preservations, 4th ed.; Block, S.S., Ed.; Lea & Febiger: Gainesville, FL, USA, 1991. [Google Scholar]
- El Hage, S.; Lajoie, B.; Stigliani, J.-L.; Furiga-Chusseau, A.; Roques, C.; Baziard, G. Synthesis, antimicrobial activity and physic-chemical properties of some n-alkyldimethyl-benzylammonium halides. J. Appl. Biomed. 2014, 12, 245–253. [Google Scholar] [CrossRef]
- Koziróg, A.; Brycki, B. Monomeric and gemini surfactants as antimicrobial agents—Influence on environmental and reference strains. Acta Biochim. Pol. 2015, 62, 879–883. [Google Scholar] [CrossRef] [PubMed]
- Brycki, B.; Małecka, I.; Koziróg, A.; Otlewska, A. Synthesis, Structure and Antimicrobial Properties of Novel Benzalkonium Chloride Analogues with Pyridine Rings. Molecules 2017, 22, 130. [Google Scholar] [CrossRef] [PubMed]
- Xcalibur CCD System, CrysAlisPro Software System. 2014. Available online: www.aqilent.com/chem (accessed on 16 January 2017).
- Sheldrick, G.M. A short history of SHELX. Acta Crystallogr. Sect. A Found. Crystallogr. 2007, 64, 112–122. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds 2–23 are available from the authors. |
| Compound | Heat of Formation [kcal/mol] | ΔHOF [kcal/mol] |
|---|---|---|
| 1a | 10.6060 | – |
| 1b | 5.6268 | – |
| 1c | 3.0680 | – |
| 2 | −2.1145 | 8.4915 |
| 3 | −24.5953 | −13.9893 |
| 4 | −46.7459 | −36.1399 |
| 5 | −68.8809 | −58.2749 |
| 6 | −91.0872 | −80.4812 |
| 7 | −113.3524 | −102.7464 |
| 8 | −135.6207 | −125.0147 |
| 9 | −158.0240 | −147.4180 |
| 10 | −34.9103 | −5.6268 |
| 11 | −68.3429 | −62.7161 |
| 12 | −101.8142 | −107.4410 |
| 30 | −135.2579 | −140.8847 |
| 14 | −155.2349 | −160.8617 |
| 15 | −213.0509 | −218.6777 |
| 16 | −233.2146 | −238.8414 |
| 17 | −266.9034 | −272.5302 |
| 18 | −94.7088 | −97.7768 |
| 19 | −130.8176 | −133.8856 |
| 20 | −184.1519 | −187.2199 |
| 21 | −228.7916 | −231.8596 |
| 22 | −280,4156 | −283,4836 |
| 23 | −325,1616 | −328,2296 |
| Empirical Formula | C66H138Br4N4O2 |
|---|---|
| Formula weight | 1339,44 |
| Temperature | 293(2) K |
| Wavelength | 0.71073 Å |
| Crystal system, space group | Triclinic, P-1 |
| Unit cell dimensions | a: 8.6269(17) b: 9.2619(13) c: 23.787(4) α: 96.864(13) β: 99.006(15) γ: 90.157(14) |
| Volume | 1863.3(6) Å3 |
| Z; Dx | 2; 1194 mg/mm3 |
| µ | 2200 mm−1 |
| F(000) | 718 |
| θ Range for data collection | 6.354–53.284 |
| Limiting indices | −10 ≤ h ≤ 10, −11 ≤ k ≤ 11, −29 ≤ l ≤ 29 |
| Reflections collected/unique | 27738/7298 (0,0111) 0.927 |
| R(F) I > 2σ (I) | 0.1306 |
| wR(F2) | 0.1437 |
| max/min Δρ | 0.45/−0.40 e/Ǻ−3 |
| Compound | CMC [mM] | ΔG°mic [kJ/mol] | β |
|---|---|---|---|
| 3 | 12.38 | −18.12 | 0.19 |
| 4 | 11.67 | −19.15 | 0.22 |
| 5 | 7.27 | −19.25 | 0.16 |
| 6 | 1.2 | −39.01 | 0.53 |
| 7 | 0.92 | −43.88 | 0.62 |
| 8 | 0.48 | −38.81 | 0.40 |
| 9 | 0.29 | −28.79 | 0.15 |
| Compound | S. aureus | B. subtilis | E. coli | P. aeruginosa | C. albicans | A. niger | P. chrysogenum |
|---|---|---|---|---|---|---|---|
| 2 | >12.5 | >12.5 | >12.5 | >12.5 | >12.5 | >12.5 | >12.5 |
| 3 | 6.2500 | 12.5000 | 6.2500 | 12.5000 | 6.2500 | 12.5000 | 6.2500 |
| 4 | 0.1953 | 0.7812 | 0.3906 | 1.5625 | 0.1953 | 1.5625 | 1.5625 |
| 5 | 0.0061 | 0.0244 | 0.0244 | 0.0976 | 0.0122 | 0.1953 | 0.0976 |
| 6 | 0.0122 | 0.0244 | 0.0244 | 0.0976 | 0.0122 | 0.0976 | 0.0488 |
| 7 | 0.0488 | 1.5625 | 0.7812 | 1.5625 | 0.0488 | 0.1953 | 0.0976 |
| 8 | 0.3906 | 1.5625 | 1.5625 | 3.1250 | 0.3906 | 0.3906 | 0.1953 |
| 9 | 0.3906 | 6.2500 | 6.2500 | 3.1250 | 0.3906 | 0.7812 | 0.7812 |
| Chemical Substance | Alkyl Chain | Microorganisms | Literature | ||
|---|---|---|---|---|---|
| S. aureus | P. aeruginosa | A. niger | |||
| BAC | 10 | 0.0048 ** | 1.25 * | 0.625 * | |
| 12 | 0.0024 ** | 0.312 * | 0.0781 * | El Hage et al. [52] | |
| 14 | 0.0006 ** | 0.0781 * | 0.0195 * | ||
| 16 | 0.0006 ** | 0.156 * | 0.156 * | ||
| 10 | 1.44 * | 3.85 | nt | ||
| 12 | 0.13 * | 0.35 | Merianos [51] | ||
| 14 | 0.04 * | 0.11 | |||
| 16 | 0.076 * | 0.5 | |||
| 12 | 0.122 * | 0.183 | nt | Fazlara et al. [49] | |
| 12 | 0.0028 | 0.175 | nt | Feder-Kubis et al. [50] | |
| N,N-dimethyl-N-(4-methylpyridyl)-N-alkyl-ammonium chlorides | 10 | 1.5625 * | 1.5625 | 12.499 * | |
| 12 | 0.1953 * | 0.7812 | 1.5625 * | ||
| 14 | 0.0488 * | 0.1953 | 0.3906 * | Brycki et al. [54] | |
| 16 | 0.0244 * | 0.0976 | 0.1953 * | ||
| Benzyl-N-alkyl-dimethyl-ammonium bromide | 10 | 0.0195 ** | 1.25 * | 0.625 * | |
| 12 | 0.0048 ** | 0.156 * | 0.0781 * | El Hage et al. [52] | |
| 14 | 0.0024 ** | 0.078 * | 0.0195 * | ||
| 16 | 0.0012 ** | 0.312 * | 0.0781 * | ||
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brycki, B.; Koziróg, A.; Kowalczyk, I.; Pospieszny, T.; Materna, P.; Marciniak, J. Synthesis, Structure, Surface and Antimicrobial Properties of New Oligomeric Quaternary Ammonium Salts with Aromatic Spacers. Molecules 2017, 22, 1810. https://doi.org/10.3390/molecules22111810
Brycki B, Koziróg A, Kowalczyk I, Pospieszny T, Materna P, Marciniak J. Synthesis, Structure, Surface and Antimicrobial Properties of New Oligomeric Quaternary Ammonium Salts with Aromatic Spacers. Molecules. 2017; 22(11):1810. https://doi.org/10.3390/molecules22111810
Chicago/Turabian StyleBrycki, Bogumił, Anna Koziróg, Iwona Kowalczyk, Tomasz Pospieszny, Paulina Materna, and Jędrzej Marciniak. 2017. "Synthesis, Structure, Surface and Antimicrobial Properties of New Oligomeric Quaternary Ammonium Salts with Aromatic Spacers" Molecules 22, no. 11: 1810. https://doi.org/10.3390/molecules22111810
APA StyleBrycki, B., Koziróg, A., Kowalczyk, I., Pospieszny, T., Materna, P., & Marciniak, J. (2017). Synthesis, Structure, Surface and Antimicrobial Properties of New Oligomeric Quaternary Ammonium Salts with Aromatic Spacers. Molecules, 22(11), 1810. https://doi.org/10.3390/molecules22111810







