Preparation and Bioactivity Assessment of Chitosan-1-Acetic Acid-5-Flurouracil Conjugates as Cancer Prodrugs
Abstract
:1. Introduction
2. Experimental
2.1. Materials and Methods
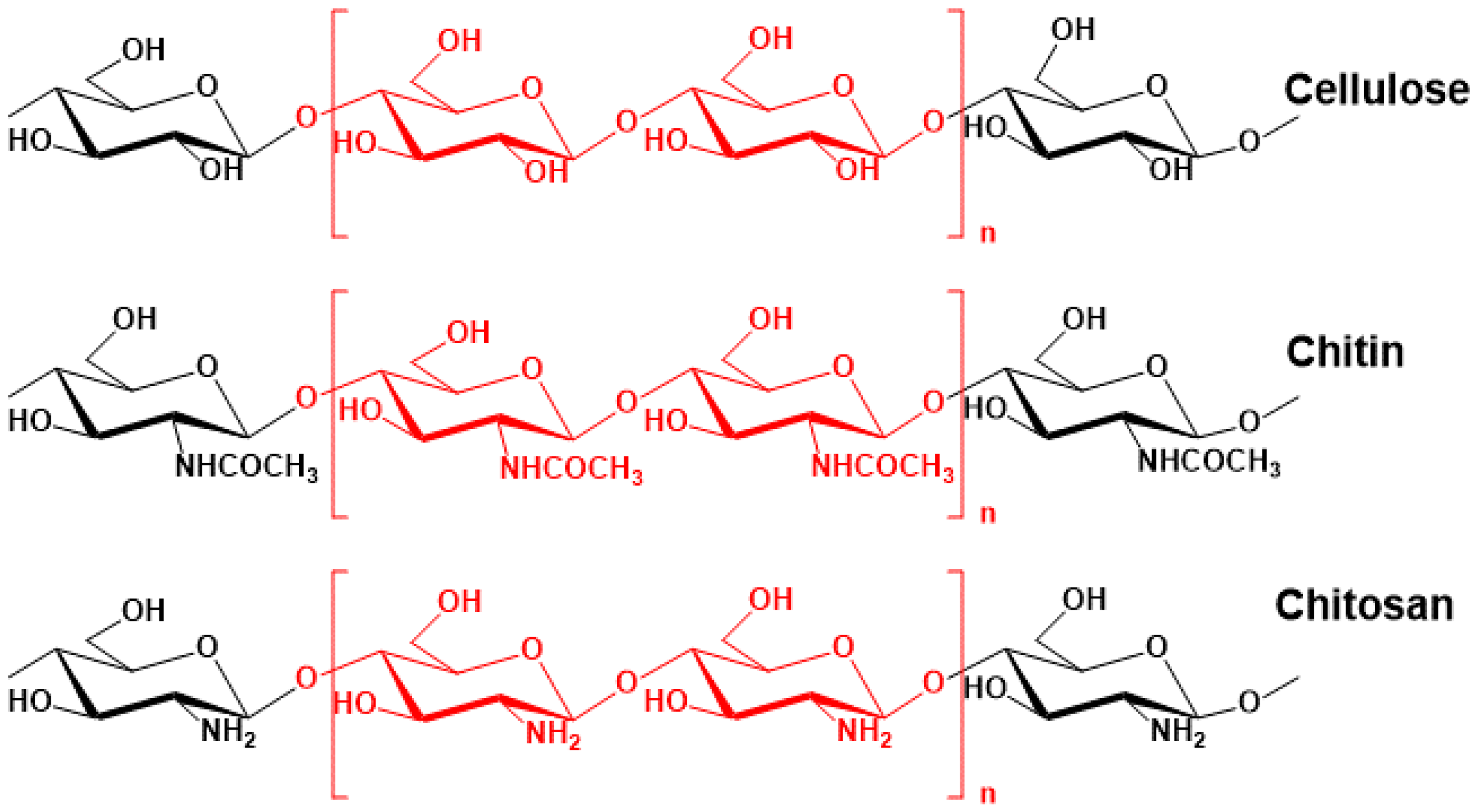
2.2. Extraction of Chitin and Chitosan from Fish Scales
2.3. Synthesis of CS-FUAC Conjugates
2.3.1. Preparation of 1-Acetic acid-5-Fluorouracil (FUAC)
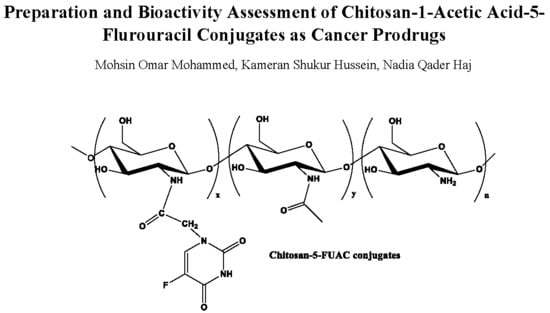
2.3.2. Preparation of CS-FUAC Conjugate
2.4. Chemical Structure Characterization
2.5. The Percentage of Drug Content in the Conjugates
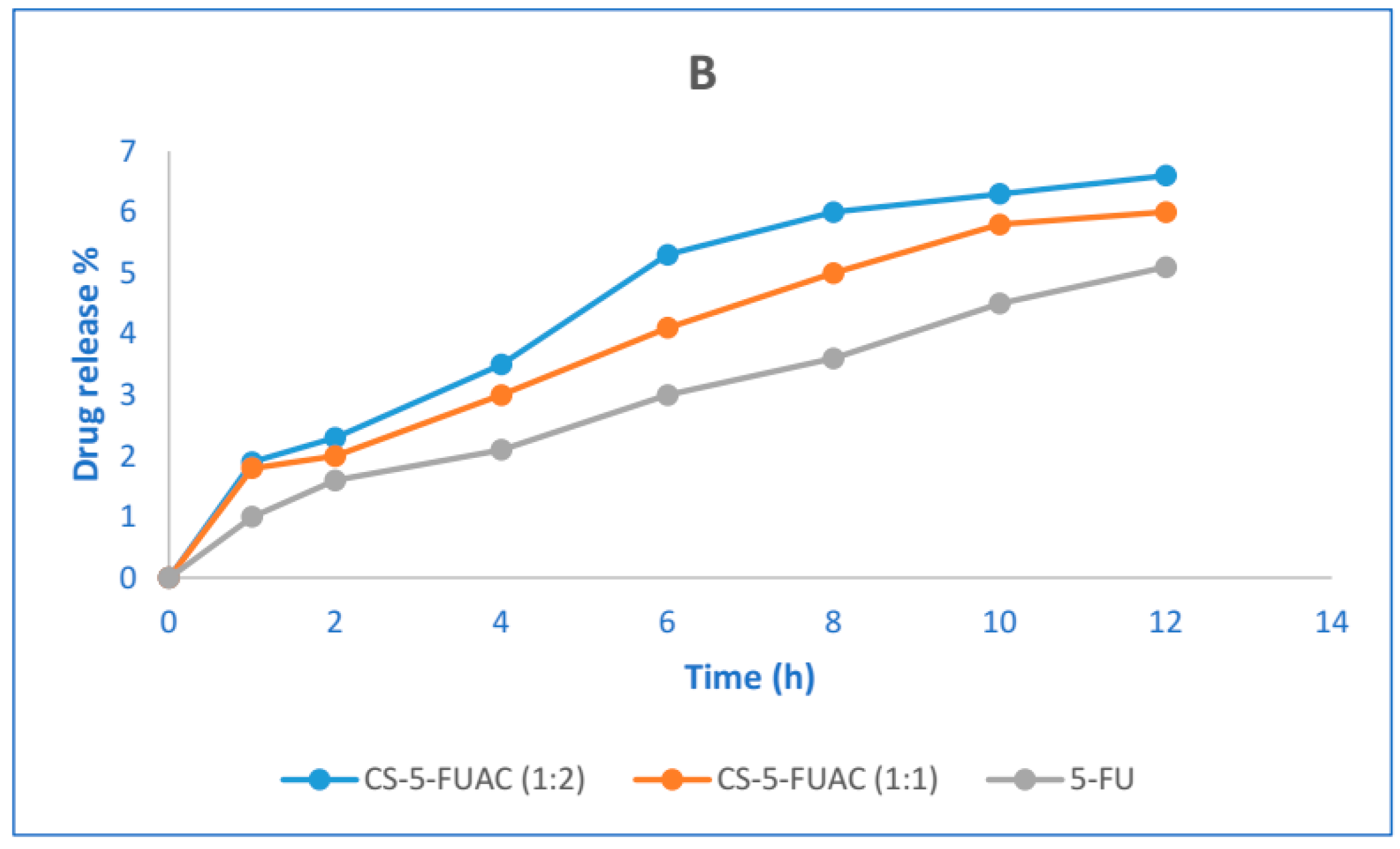
2.6. The In Vitro Drug Release Test
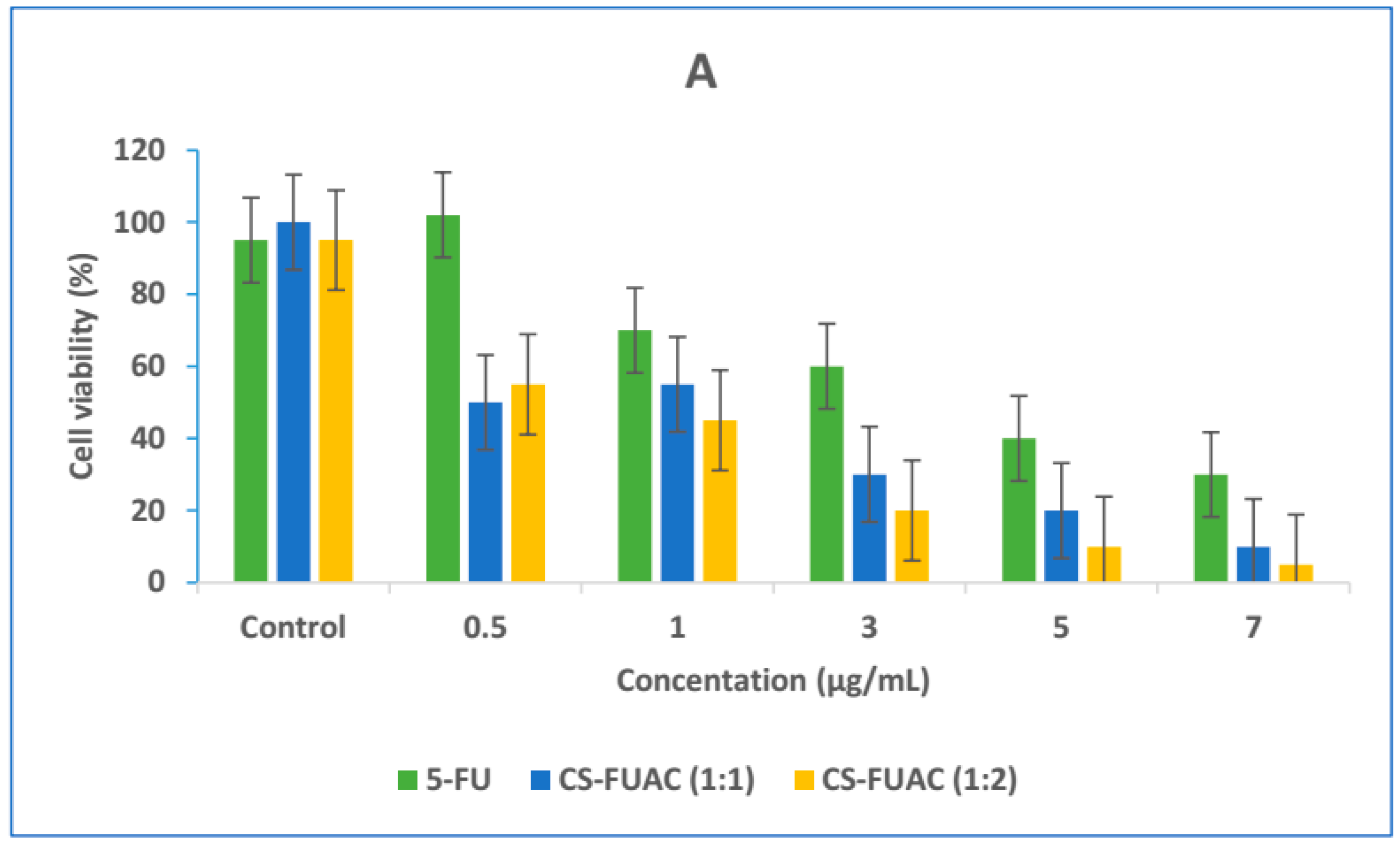
2.7. In Vitro Cytotoxicity Study
2.8. Hemolytic Activity
3. Results and Discussion
3.1. The Chemical Structure Characterization of CS
3.2. Preparation and Characterization of CS-FUAC
3.3. The percentage of Drug Content in CS-FUAC Conjugates
3.4. The Chemical Stability of CS-FUAC Conjugates
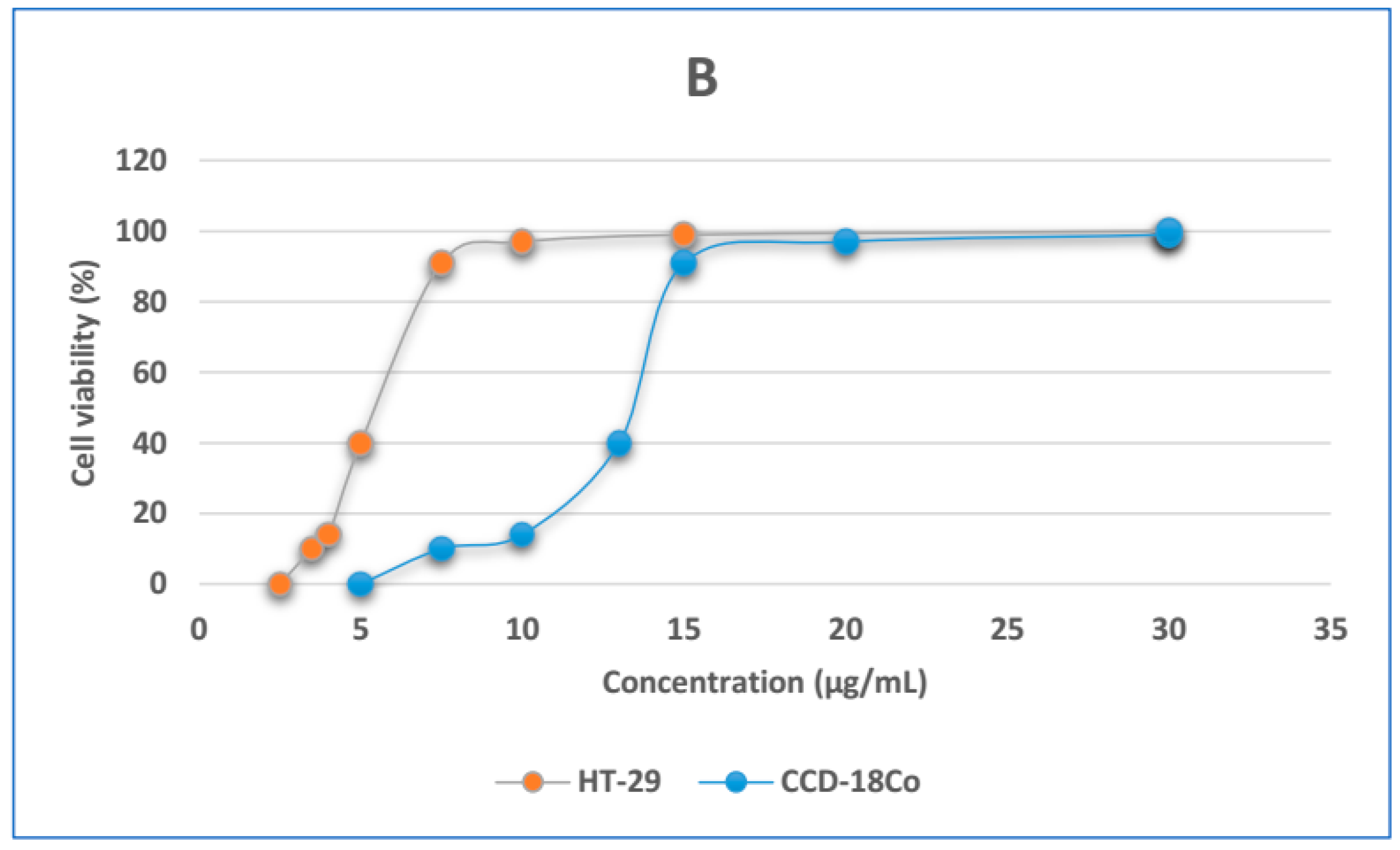
3.5. In Vitro Cytotoxicity
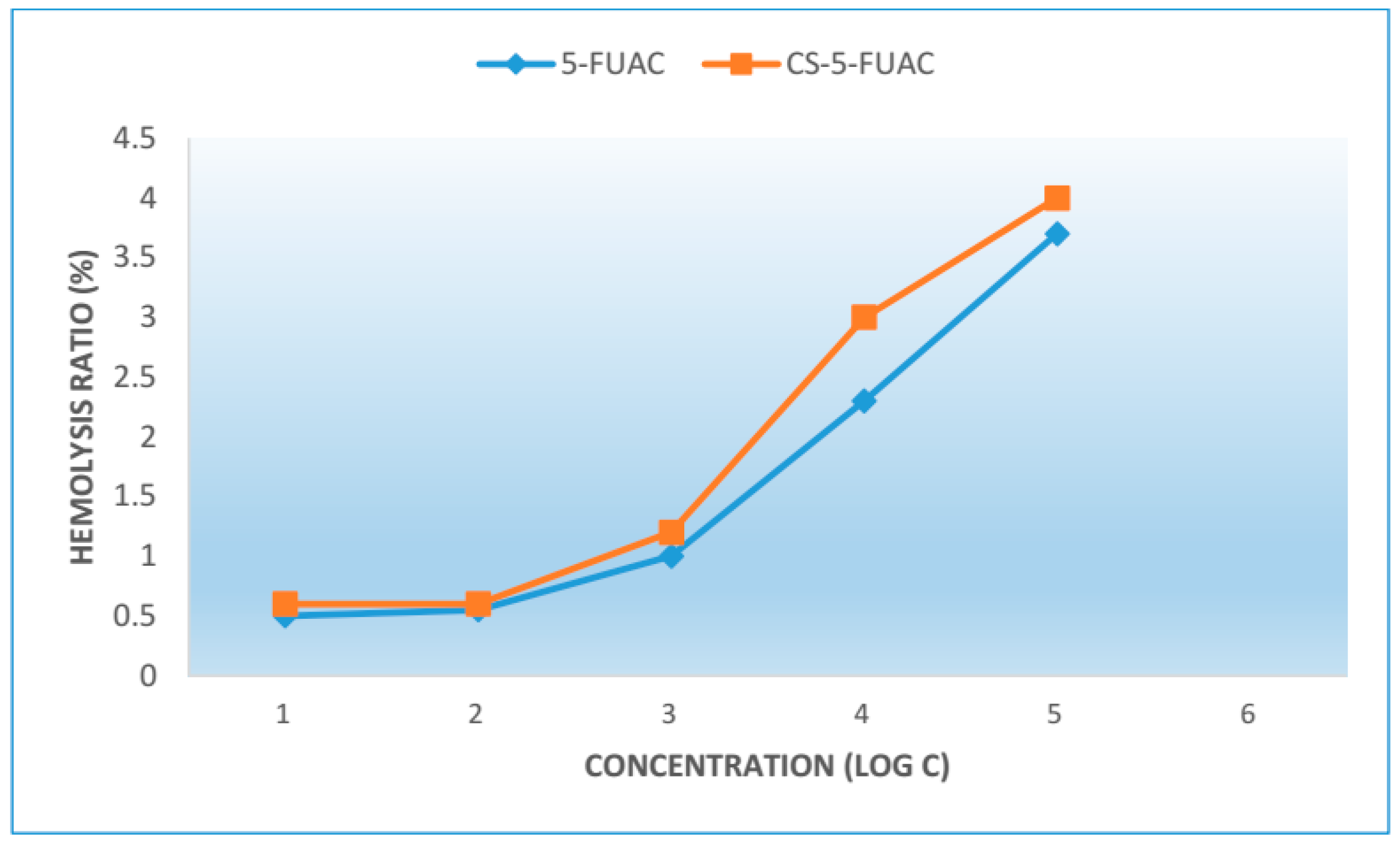
3.6. Hemolytic Activity
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Sharma, R.; Rawal, R.K.; Gaba, T.; Singla, N.; Malhotra, M.; Matharoo, S.; Bhardwaj, T.R. Design, synthesis and ex vivo evaluation of colon-specific azo based prodrugs of anticancer agents. Bioorg. Med. Chem. Lett. 2013, 23, 5332–5338. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.; Rawal, R.K.; Malhotra, M.; Sharma, A.K.; Bhardwaj, T.R. Design, synthesis and ex vivo release studies of colon-specific polyphosphazene-anticancer drug conjugates. Bioorg. Med. Chem. 2014, 22, 1104–1114. [Google Scholar] [CrossRef] [PubMed]
- Hua, S.; Marks, E.; Schneider, J.J.; Keely, S. Advances in oral nano-delivery systems for colon targeted drug delivery in inflammatory bowel disease: Selective targeting to diseased versus healthy tissue. Nanomed. Nanotechnol. Biol. Med. 2015, 11, 1117–1132. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Mishra, B. Colon Targeted Drug Delivery Systems—An Overview. Curr. Drug Deliv. 2008, 5, 186–198. [Google Scholar] [CrossRef] [PubMed]
- Chourasia, M.K.; Jain, S.K. Pharmaceutical approaches to colon targeted drug delivery systems. J. Pharm. Pharm. Sci. 2003, 6, 33–66. [Google Scholar] [PubMed]
- Longley, D.B.; Harkin, D.P.; Johnston, P.G. 5-Fluorouracil: Mechanisms of action and clinical strategies. Nat. Rew. Cancer 2003, 3, 330–338. [Google Scholar] [CrossRef] [PubMed]
- Cameron, D.A.; Gabra, H.; Leonard, R.C. Continuous 5-fluorouracil in the treatment of breast cancer. Br. J. Cancer 1994, 70, 120–124. [Google Scholar] [CrossRef] [PubMed]
- Arias, J.L. Novel strategies to improve the anticancer action of 5-fluorouracil by using drug delivery systems. Molecules 2008, 13, 2340–2369. [Google Scholar] [CrossRef] [PubMed]
- Laquintana, V.; Denora, N.; Lopalco, A.; Lopedota, A.; Cutrignelli, A.; Lasorsa, F.M.; Agostino, G.; Franco, M. Translocator protein ligand-plga conjugated nanoparticles for 5-fluorouracil delivery to glioma cancer cells. Mol. Pharm. 2014, 11, 859–871. [Google Scholar] [CrossRef] [PubMed]
- Gu, C.; Le, V.; Lang, M.; Liu, J. Preparation of polysaccharide derivates chitosan-graft-poly(e-caprolactone) amphiphilic copolymer micelles for 5-fluorouracil drug delivery. Colloids Surf. B Biointerfaces 2014, 116, 745–750. [Google Scholar] [CrossRef] [PubMed]
- Puga, A.M.; Lima, A.C.; Mano, J.F.; Concheiro, A.; Alvarez-Lorenzo, C. Pectin-coated chitosan microgels crosslinked on superhydrophobic surfaces for 5-fluorouracil encapsulation. Carbohydr. Polym. 2013, 98, 331–340. [Google Scholar] [CrossRef] [PubMed]
- Ashwanikumar, N.; Kumar, N.A.; Asha Nair, S.; Vinod Kumar, G.S. Methacrylic-based nanogels for the pH-sensitive delivery of 5-Fluorouracil in the colon. Int. J. Nanomed. 2012, 7, 5769–5779. [Google Scholar]
- Zhu, K.; Ye, T.; Liu, J.; Peng, Z.; Xu, S.; Lei, J.; Deng, H.; Li, B. Nanogels fabricated by lysozyme and sodium carboxymethyl cellulose for 5-fluorouracil controlled release. Int. J. Pharm. 2013, 441, 721–727. [Google Scholar] [CrossRef] [PubMed]
- Vilaça, N.; Amorim, R.; Machado, A.F.; Parpot, P.; Pereira, M.F.R.; Sardo, M.; Rocha, J.; Fonseca, A.M.; Neves, I.C.; Baltazar, F. Potentiation of 5-fluorouracil encapsulated in zeolites as drug delivery systems for in vitro models of colorectal carcinoma. Colloids Surf. B Biointerfaces 2013, 112, 237–244. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giannakas, A.; Patsaoura, A.; Barkoula, N. A novel solution blending method for using olive oil and corn oil as plasticizers in chitosan based organoclay nanocomposites. Polymers 2017, 157, 550–557. [Google Scholar] [CrossRef] [PubMed]
- Zou, M.; Okamoto, H.; Cheng, G.; Hao, X.; Sun, J.; Cui, F.; Danjo, K. Synthesis and properties of polysaccharide prodrugs of 5-aminosalicylic acid as potential colon-specific delivery systems. Eur. J. Pharm. Biopharm. 2005, 59, 155–160. [Google Scholar] [CrossRef] [PubMed]
- Jung, Y.; Kim, H.H.; Kim, H.; Kong, H.; Choi, B.; Yang, Y.; Kim, Y. Evaluation of 5-aminosalicyltaurine as a colon-specific prodrug of 5-aminosalicylic acid for treatment of experimental colitis. Eur. J. Pharm. Sci. 2006, 28, 26–33. [Google Scholar] [CrossRef] [PubMed]
- Varshosaz, J.; Emami, J.; Tavakoli, N.; Fassihi, A.; Minaiyan, M.; Ahmadi, F.; Dorkoosh, F. Synthesis and evaluation of dextran-budesonide conjugates as colon specific prodrugs for treatment of ulcerative colitis. Int. J. Pharm. 2009, 365, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Hoste, K.; De Winne, K.; Schacht, E. Polymeric prodrugs. Int. J. Pharm. 2004, 277, 119–131. [Google Scholar] [CrossRef] [PubMed]
- Kumari, S.; Rath, P.K. Extraction and Characterization of Chitin and Chitosan from (Labeo rohit) Fish Scales. Procedia Mater. Sci. 2014, 6, 482–489. [Google Scholar] [CrossRef]
- Sun, Z.-J.; Sun, B.; Sun, C.-W.; Wang, L.-B.; Xie, X.; Ma, W.-C.; Lu, X.-L.; Dong, D.-L. A poly(glycerol-sebacate-(5-fluorouracil-1-acetic acid)) polymer with potential use for cancer therapy. J. Bioact. Compat. Polym. 2012, 27, 18–30. [Google Scholar] [CrossRef]
- Yang, H.; Bremner, D.H.; Tao, L.; Li, H.; Hu, J.; Zhu, L. Carboxymethyl chitosan-mediated synthesis of hyaluronic acid-targeted graphene oxide for cancer drug delivery. Carbohydr. Polym. 2016, 135, 72–78. [Google Scholar] [CrossRef] [PubMed]
- Conover, W. Buffer Solutions: The Basics (Beynon, R.J.; Easterby, J.S.). J. Chem. Educ. 1998, 75, 153. [Google Scholar] [CrossRef]
- Kumar, S.U.; Gopinath, P. Controlled delivery of bPEI-niclosamide complexes by PEO nanofibers and evaluation of its anti-neoplastic potentials. Colloids Surf. B Biointerfaces 2015, 131, 170–181. [Google Scholar] [CrossRef] [PubMed]
- Gul, S.; Aziz-ur-Rehman; Abbasi, M.A.; Khan, K.M.; Nafeesa, K.; Siddiqa, A.; Akhtar, M.N.; Shahid, M.; Subhani, Z. Synthesis, antimicrobial evaluation and hemolytic activity of 2-[[5-alkyl/aralkyl substituted-1,3,4-oxadiazol-2-yl]thio]-N-[4-(4-morpholinyl)phenyl]acetamide derivatives. J. Saudi Chem. Soc. 2017, 21, S425–S433. [Google Scholar] [CrossRef]
- Liebert, T.F.; Heinze, T. Tailored cellulose esters: Synthesis and structure determination. Biomacromolecules 2005, 6, 333–340. [Google Scholar] [CrossRef] [PubMed]
- Paradis, D.; Vallee, F.; Allard, S.; Bisson, C.; Daviau, N.; Drapeau, C.; Auger, F.; LeBel, M. Comparative study of pharmacokinetics and serum bactericidal activities of cefpirome, ceftazidime, ceftriaxone, imipenem, and ciprofloxacin. Antimicrob. Agents Chemother. 1992, 36, 2085–2092. [Google Scholar] [CrossRef] [PubMed]
- Helander, I.M.; Nurmiaho-Lassila, E.L.; Ahvenainen, R.; Rhoades, J.; Roller, S. Chitosan disrupts the barrier properties of the outer membrane of Gram-negative bacteria. Int. J. Food Microbiol. 2001, 71, 235–244. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds Chitosan and small amount from CS-FUAC are available from the authors. |






| Sample i | Drug loading (wt %) ii | Yield (%) |
|---|---|---|
| CS-FUAC (1:1) | 0.61 | 60 |
| CS-FUAC (1:2) | 0.75 | 68 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mohammed, M.O.; Hussain, K.S.; Haj, N.Q. Preparation and Bioactivity Assessment of Chitosan-1-Acetic Acid-5-Flurouracil Conjugates as Cancer Prodrugs. Molecules 2017, 22, 1629. https://doi.org/10.3390/molecules22111629
Mohammed MO, Hussain KS, Haj NQ. Preparation and Bioactivity Assessment of Chitosan-1-Acetic Acid-5-Flurouracil Conjugates as Cancer Prodrugs. Molecules. 2017; 22(11):1629. https://doi.org/10.3390/molecules22111629
Chicago/Turabian StyleMohammed, Mohsin O., Kameran S. Hussain, and Nadia Q. Haj. 2017. "Preparation and Bioactivity Assessment of Chitosan-1-Acetic Acid-5-Flurouracil Conjugates as Cancer Prodrugs" Molecules 22, no. 11: 1629. https://doi.org/10.3390/molecules22111629