Self-Nanoemulsifying Drug Delivery Systems Containing Plantago lanceolata—An Assessment of Their Antioxidant and Antiinflammatory Effects
Abstract
:1. Introduction
2. Results
2.1. Bioactive Compounds in Plantago lancolata Leaves
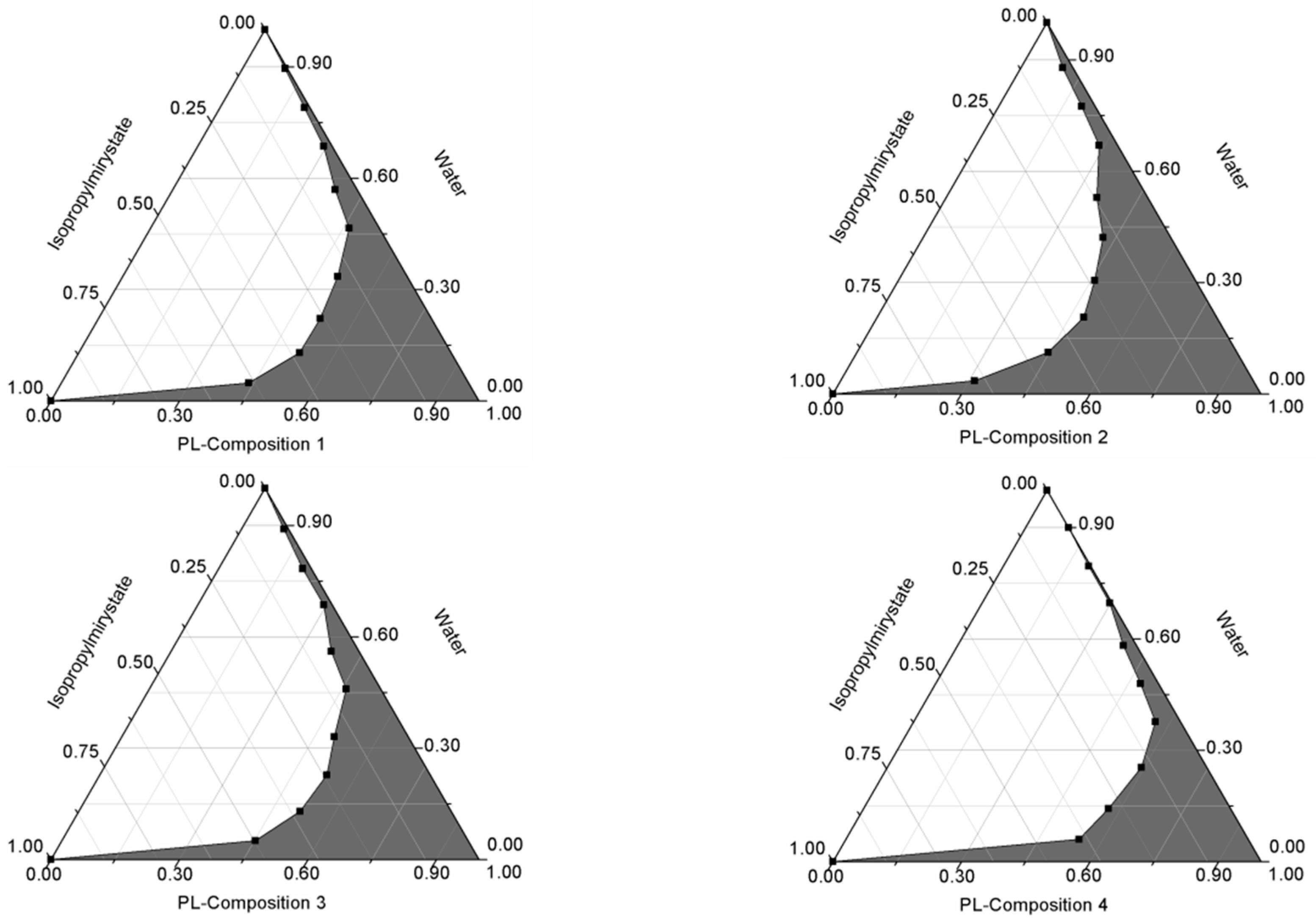
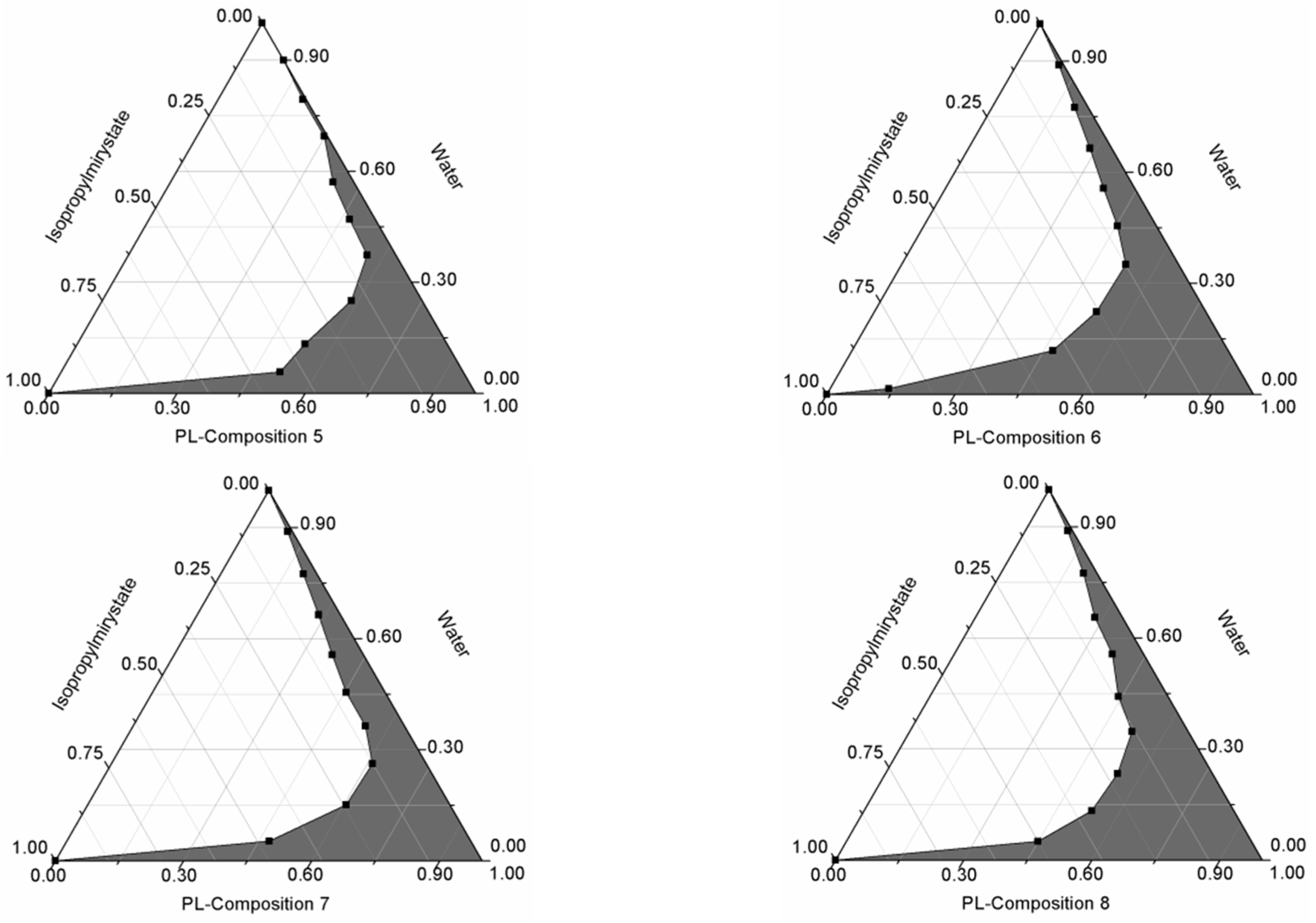
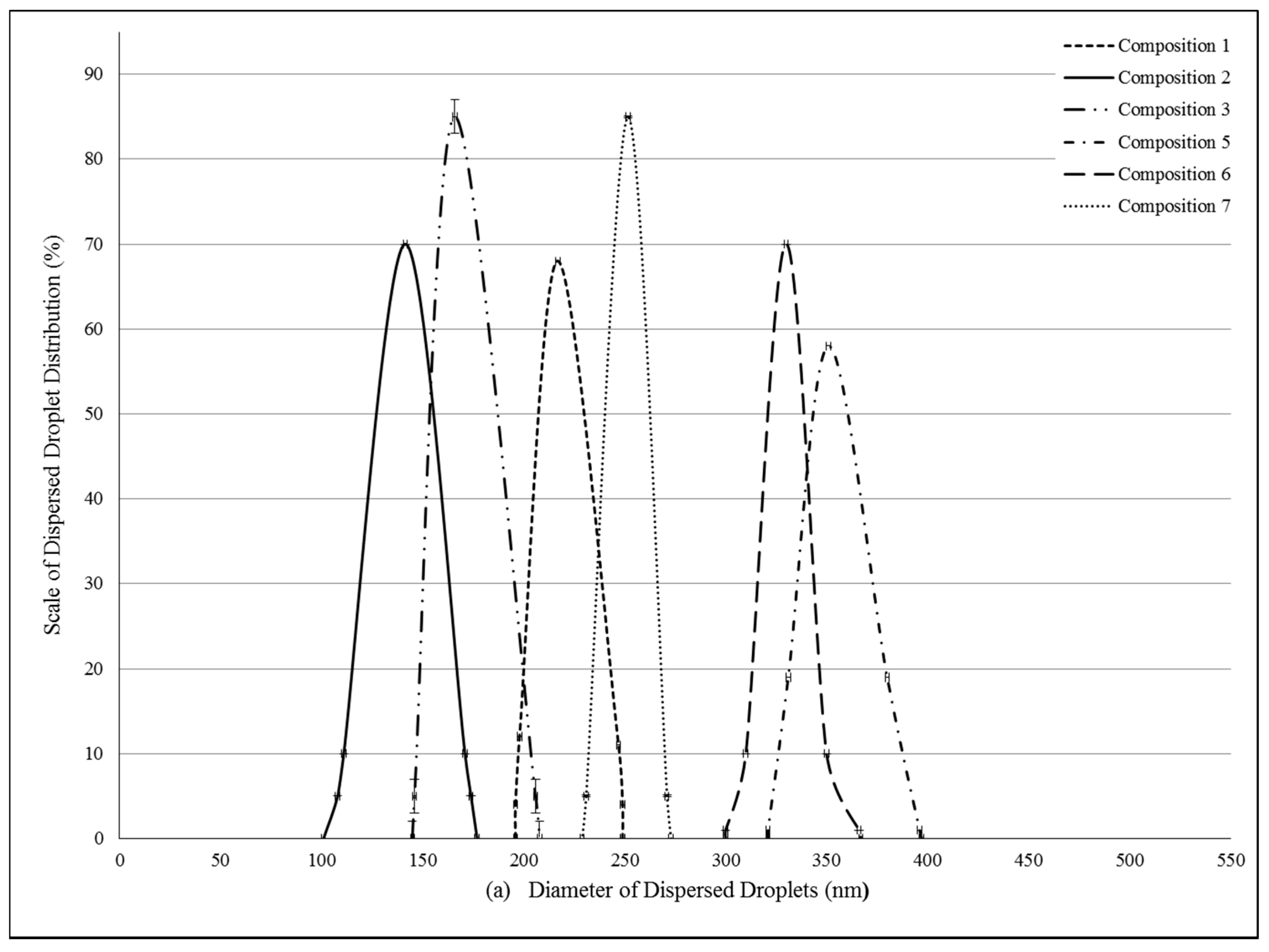
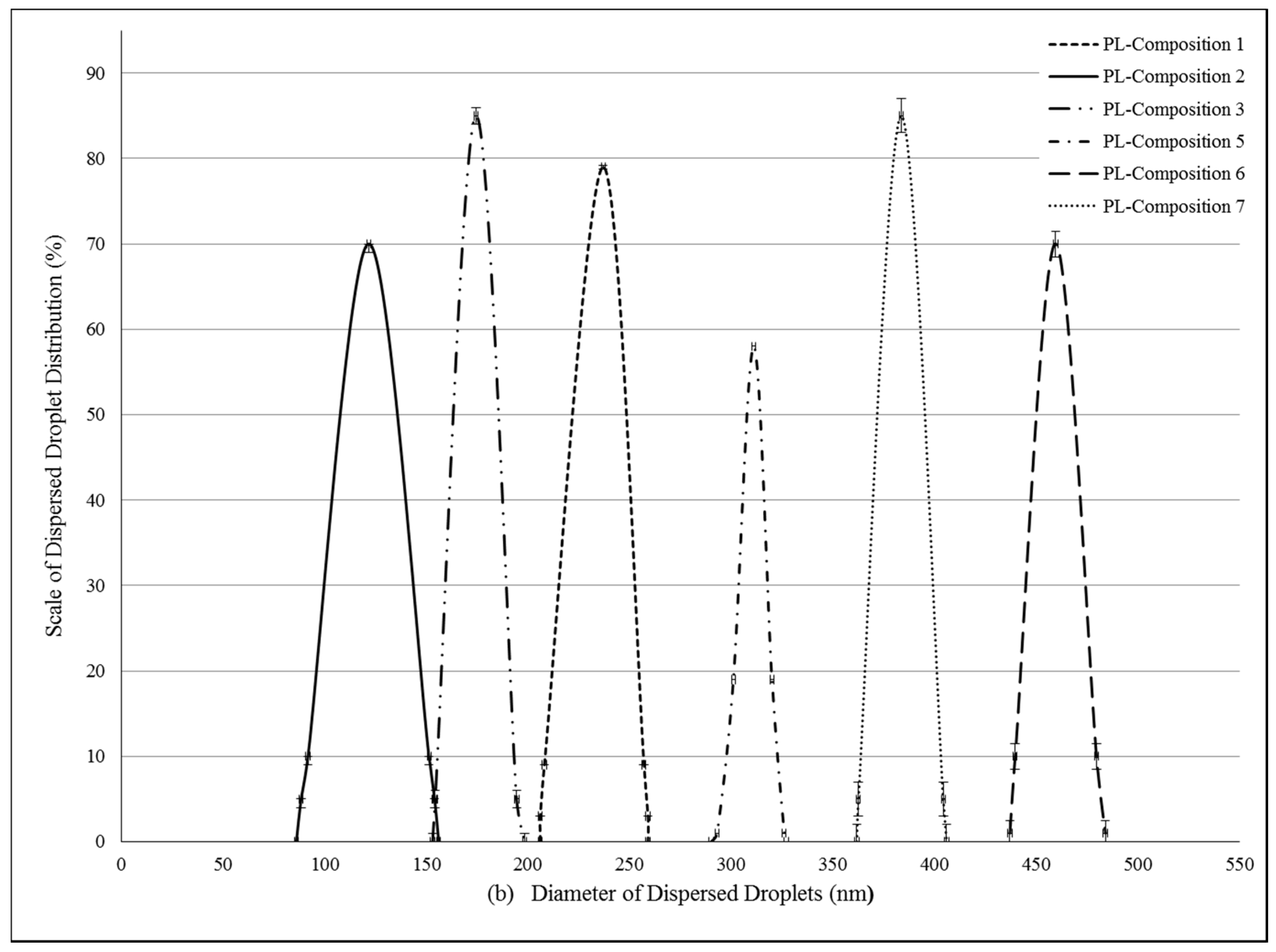
2.2. Formulation and Evaluation of Self-Nano-Emulsifying Drug Delivery Systems
2.3. Stability Studies of Self-Nano-Emulsifying Drug Delivery Systems
2.4. Toxicity Investigations
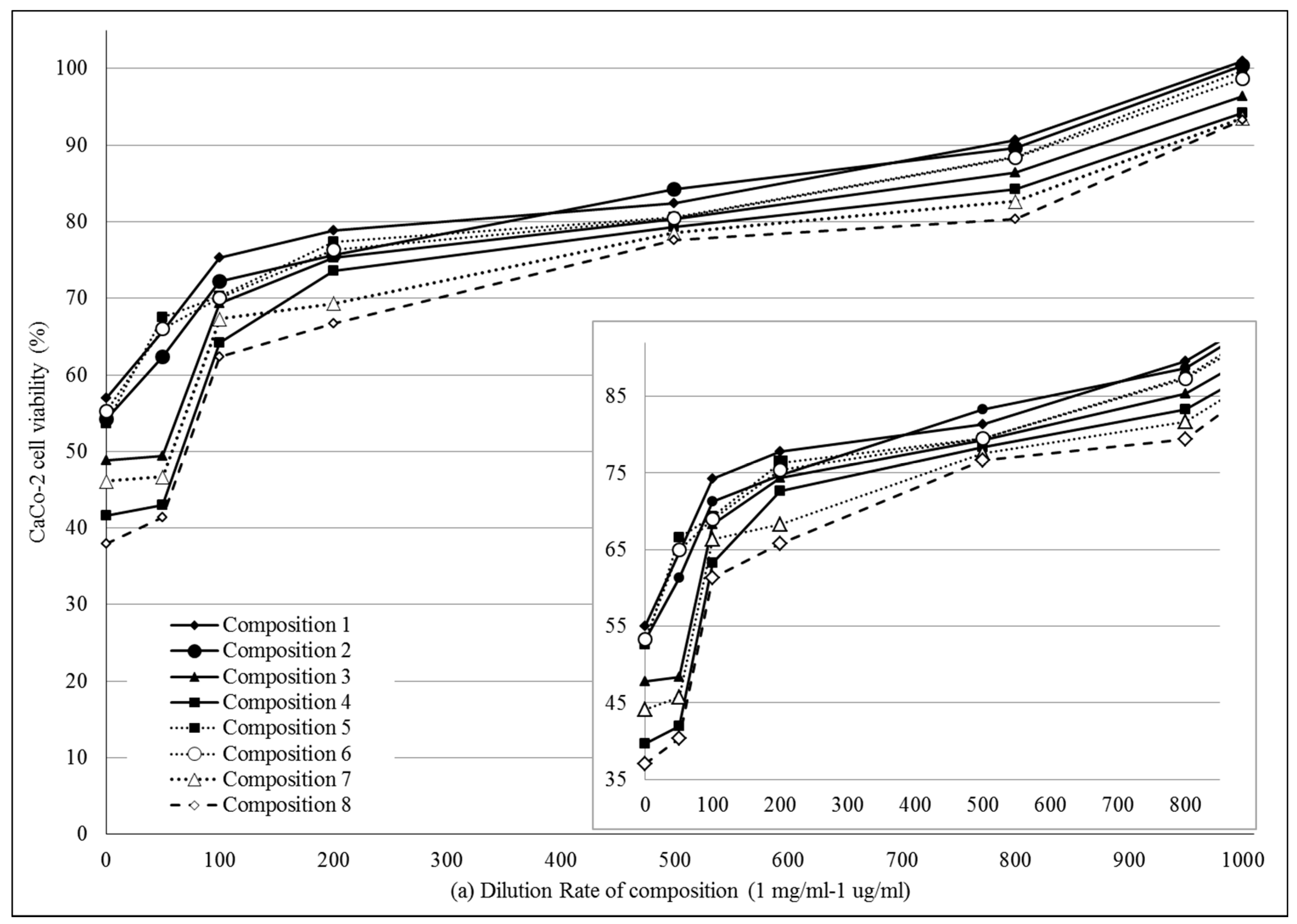
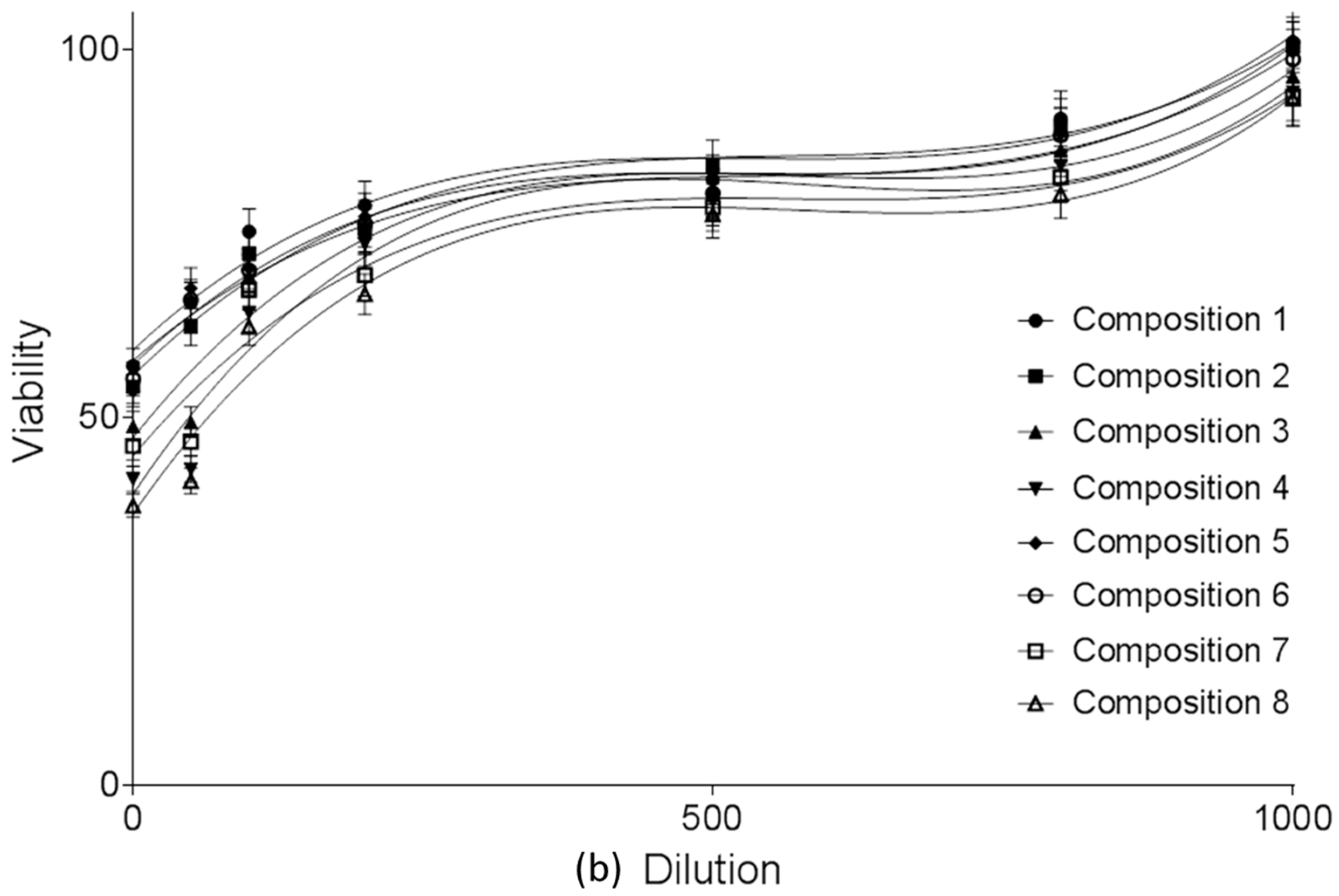
2.4.1. MTT Viability Assay on Caco-2 Cell Monolayers
2.4.2. Effect of PL-SNEDDS on Hepatic Function Markers
2.5. In Vitro Dissolution Study
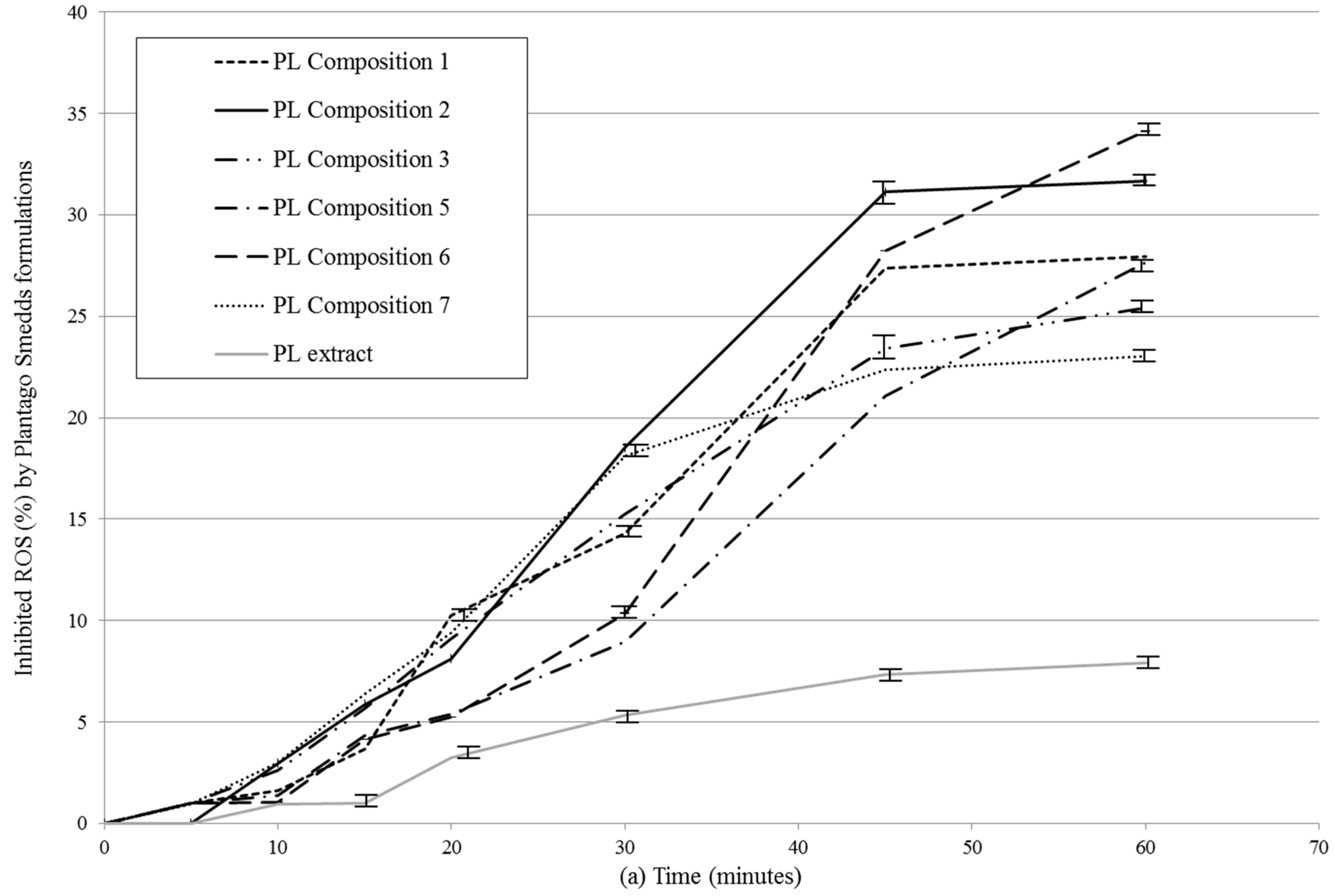
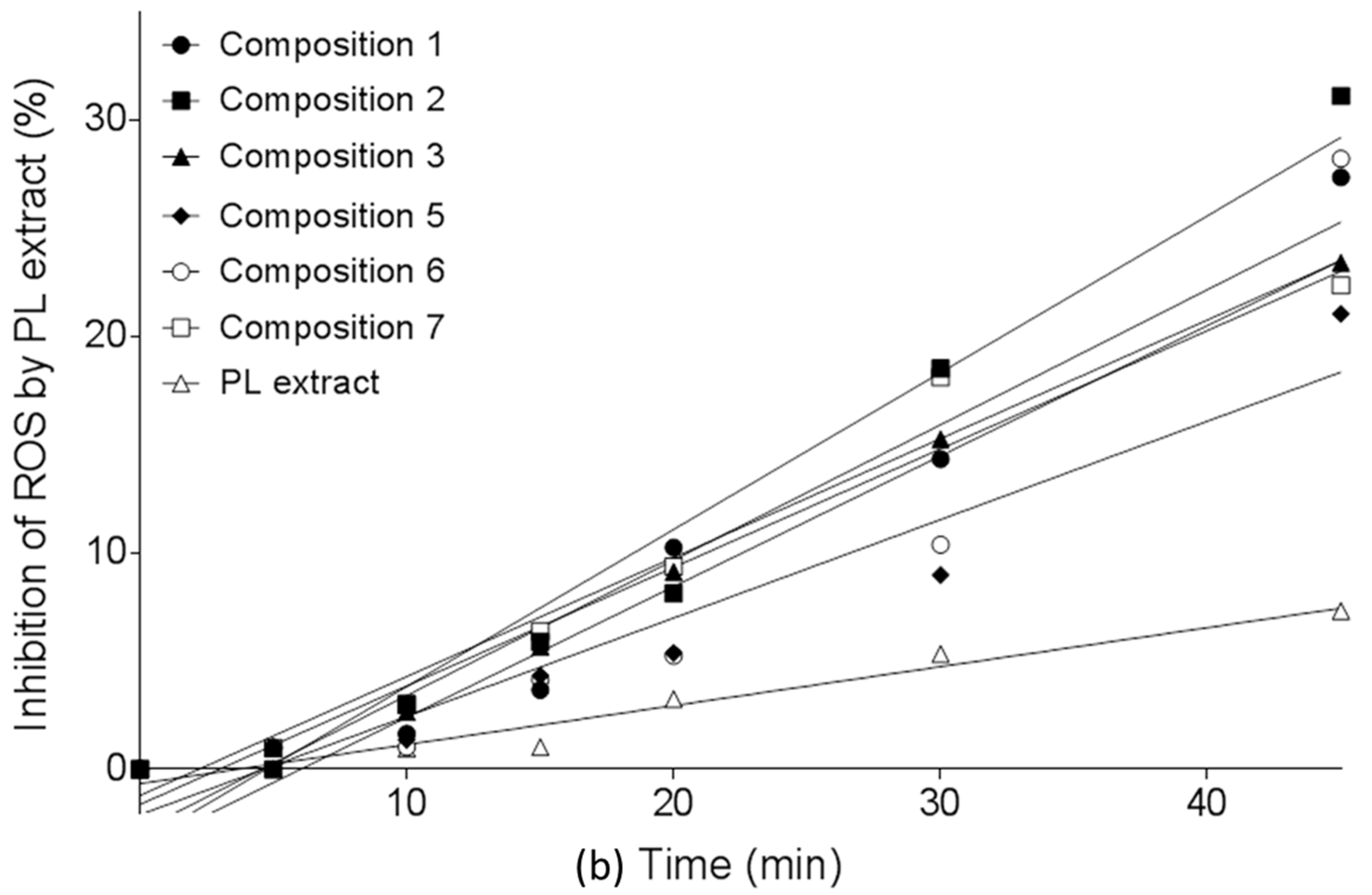
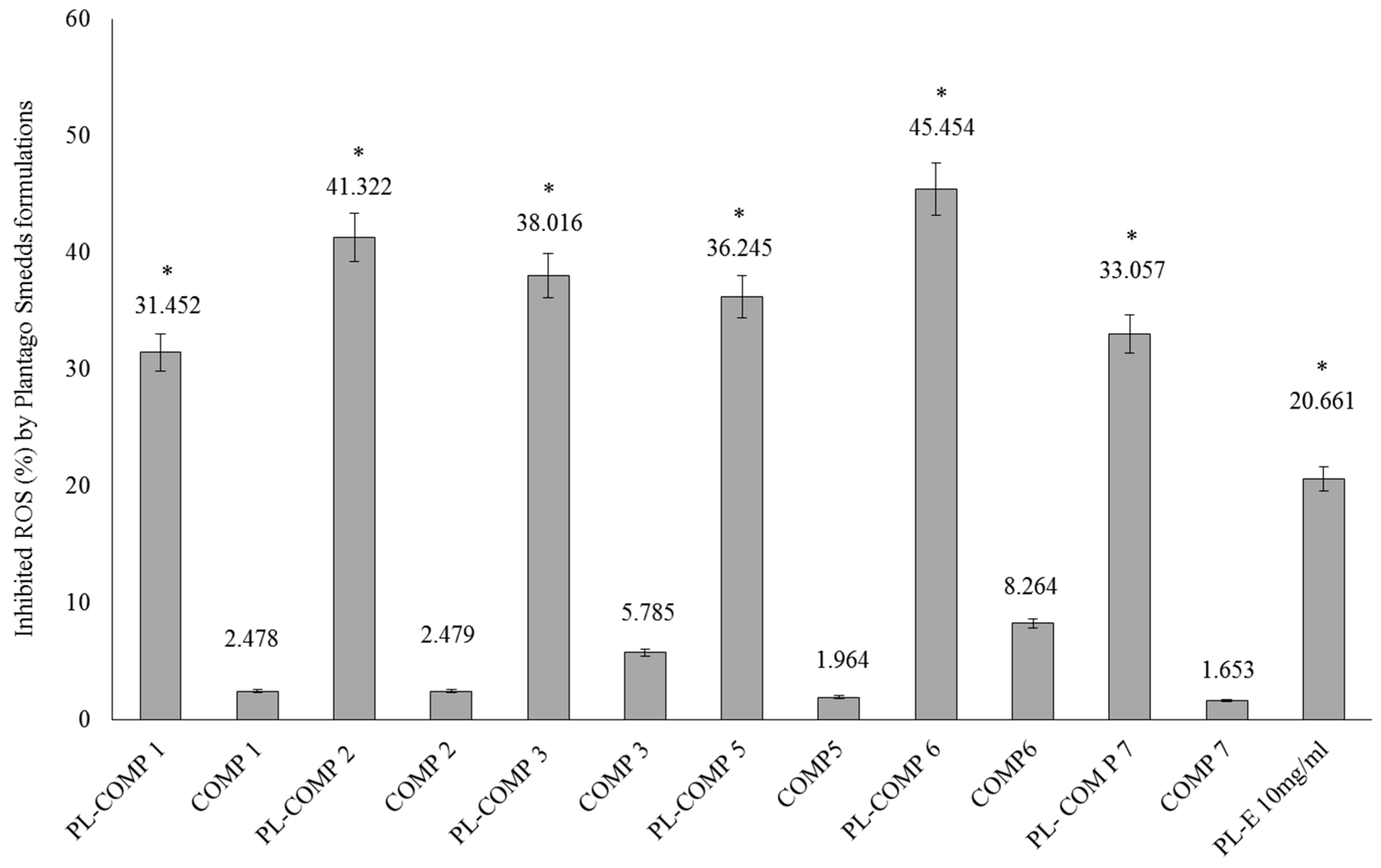
2.6. DPPH Radical Scavenging Activity of SNEDDS-PL Samples
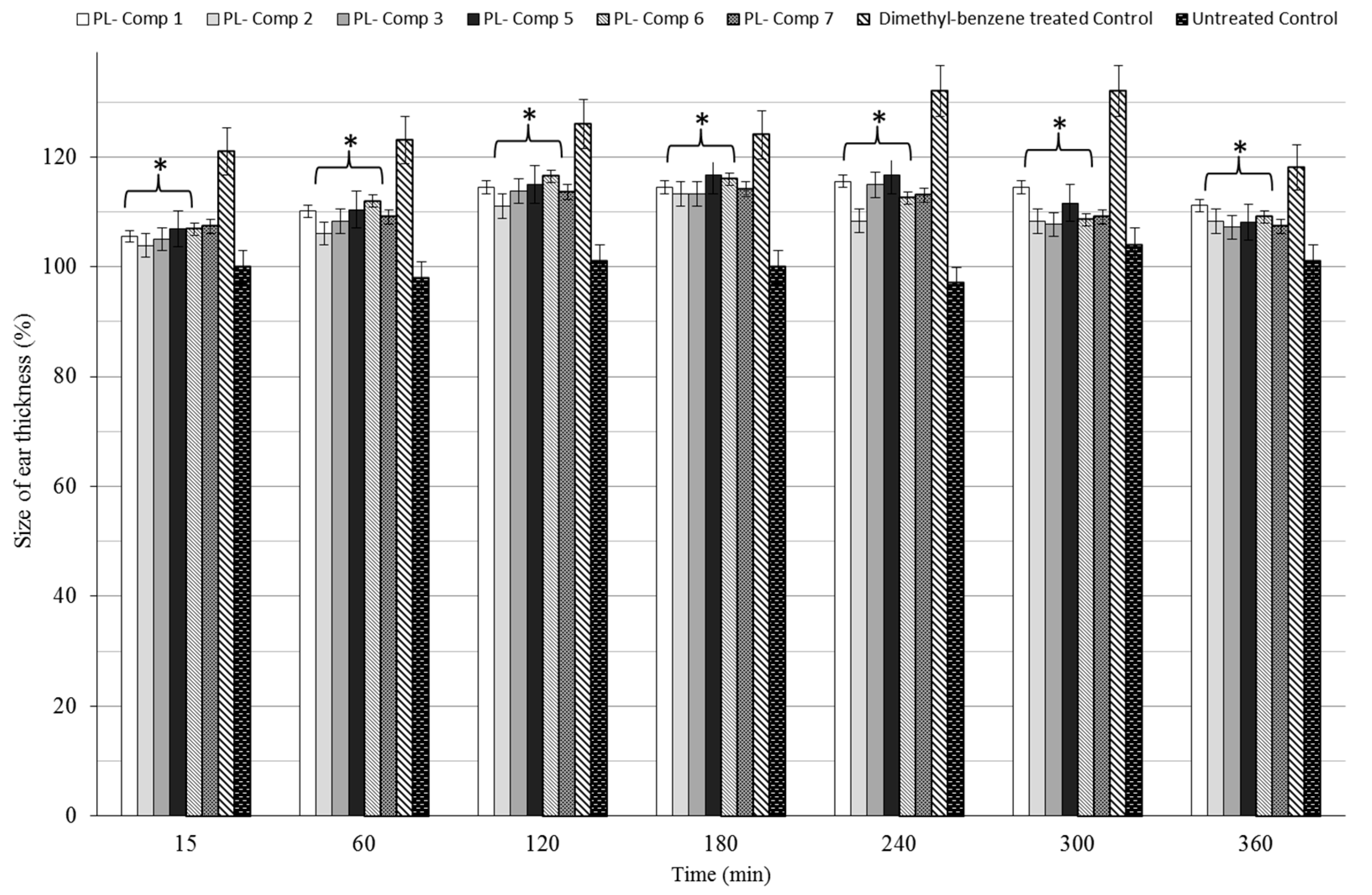
2.7. Dimethyl-Benzene-Induced Ear Edema
3. Discussion
4. Materials and Methods
4.1. Preparation and Characterization of Dry Plantago lanceolata Leaf Methanolic Extract
4.2. Preparation and Characterization of Dry Plantago lanceolata Leaf Methanolic Extract
4.3. Formulation and Evaluation of Self-Nano-Emulsifying Drug Delivery Systems
4.4. Determination of Droplet Size of PL-SNEDDS
4.5. Cell Culturing
4.6. In Vitro Cell Viability Assay
4.7. DPPH Radical Scavenging Activity of SNEDDS-PL Samples
4.8. In Vitro Dissolution Test
4.9. Animals and Experimental Groups
4.10. Preparation of Blood Plasma
4.11. Assay of Plasmatic Markers
4.12. Dimethyl-Benzene-Induced Inflammation Model
4.13. Measurement of Ear Oedema
4.14. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Goncalves, S.; Romano, A. The medicinal potential of plants form the genus Plantago (Plantaginaceae). Ind. Crops Prod. 2016, 82, 213–226. [Google Scholar] [CrossRef]
- Chiou, W.F.; Lin, L.C.; Chen, C.F. Acteoside protects endothelial cells against free radical-induced oxidative stress. J. Pharm. Pharmacol. 2004, 56, 743. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.Y.; Woo, E.R.; Kang, K.W. Inhibition of lipopolysaccharide-inducible nitric oxide synthase expression by acteoside through blocking of AP-1 activation. J. Ethnopharmacol. 2005, 97, 561. [Google Scholar] [CrossRef] [PubMed]
- Xiong, L.; Mao, S.; Lu, B.; Yang, J.; Zhou, F.; Hu, Y.; Jiang, Y.; Shen, C.; Zhao, Y. Osmathus fragrans Flower Extract and Acteoside Protect Agaianst d-Galactose-Induced Aging in an ICR Mouse Model. J. Med. Food 2016, 19, 54–61. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Yan, Y.; Liu, H.; Wang, J.; Hu, J. Protective effects of acteoside against X-ray induced damage in human skin fibroblast. Mol. Med. Rep. 2015, 12, 2301–2306. [Google Scholar] [CrossRef] [PubMed]
- Kovac, I.; Durkac, J.; Holly, M.; Jakubcova, K.; Perzelova, V.; Mucaji, P.; Svajdlenka, E.; Sabol, F.; Legath, J.; Belák, J.; et al. Plantago lanceolata L. water extract induces transition of fibroblasts into myofibroblasts and increases tensile strength of healing skin wounds. J. Pharm. Pharmacol. 2014, 67, 117–125. [Google Scholar] [CrossRef] [PubMed]
- Gonda, S.; Nguyen, N.M.; Batta, G.; Gyémánt, G.; Máthé, C.; Vasas, G. Determination of phenylethanoid glycosides and iridoid glycosides from therapeutically used Plantago species by CE-MEKC. Electrophoresis 2013, 34, 2577–2584. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Hu, X.P.; Zeng, Y.; Li, Y.; Wu, H.; Qiu, R.; Ma, W.; Li, T.; Li, C.; He, Z. Advanced research on acteoside for chemistry and bioactivities. J. Asian Nat. Prod. Res. 2011, 13, 449–464. [Google Scholar] [CrossRef] [PubMed]
- Isacchi, B.; Bergonzi, M.C.; Iacopi, R.; Ghelardini, C.; Galeotti, N.; Bilia, A.R. Liposomal Formulation to increase Stability and Prolong Antineuropathic Activity of Verbascoside. Planta Med. 2016, 83, 412–419. [Google Scholar] [CrossRef] [PubMed]
- Vertuani, S.; Beghell, E.; Scalambra, E.; Malisardi, G.; Copetti, S.; Dal Toso, R.; Baldisserotto, A.; Manfredini, S. Activity and Stability Studies of Verbascoside, a Novel Antioxidant, in Dermo-Cosmetic and Pharmaceutical Topical Formulations. Molecules 2011, 15, 7068–7080. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zhang, I.; Zhang, M.; Pang, Y.; Li, Z.; Zhao, A.; Feng, J. Self-emulsifying drug delivery system and the applications in herbal drug. Drug Deliv. 2013, 22, 475–486. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.; Zhao, Q.; Wang, Y.; Guo, T.; An, Y.; Shi, G. Design and evaluation of self-emulsifying drugdelivery systems of Rhizoma corydalis decumbentis extracts. Drug Dev. Ind. Pharm. 2012, 38, 1200–1206. [Google Scholar] [CrossRef] [PubMed]
- Eid, A.M.; El-Enshasy, H.A.; Aziz, R.; Elmarzug, N.A. The preparation and evaluation of self-nanoemulsifying system containing Swietenia oil and an examination of its anti-inflammatory effects. Int. J. Nanomed. 2014, 9, 4685–4695. [Google Scholar]
- European Pharmacopoiea 9th Edition. Available online: https://www.edqm.eu/en/european-pharmacopoeia-9th-edition (accessed on 29 June 2017).
- Ujhelyi, Z.; Fenyvesi, F.; Váradi, J.; Fehér, P.; Kiss, T.; Veszelka, S.; Deli, M.A.; Vecsernyés, M.; Bácskay, I. Evaluation of cytotoxicity of surfactants used in self-micro emulsifying drug delivery systems and their effects on paracellular transport in Caco-2 cell monolayer. Eur. J. Pharm. Sci. 2012, 47, 564–573. [Google Scholar] [CrossRef] [PubMed]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT—Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Grigore, A.; Colceru-Mihul, S.; Litescu, S.; Pantelli, M.; Rasit, I. Correlation between polyphenol content and anti-inflmammatory activity of Verbascum phlomoides (Mullein). Pharm. Biol. 2013, 51, 925–929. [Google Scholar] [CrossRef] [PubMed]
- Sinico, C.; Caddeo, C.; Valenti, D.; Fadda, A.M.; Bilia, A.R.; Vincieri, F.F. Liposomes as carriers for verbascoside: Stability and skin permeation. J. Liposome Res. 2008, 18, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Choudhrey, Q.N.; Kim, M.J.; Kim, T.G.; Pan, J.H.; Kim, J.H.; Park, S.J.; Lee, J.H.; Kim, Y.J. Saponin-Based Nanoemulsification Improves the Antioxidant Properties of Vitamin A and E in AML-12 cells. Int. J. Mol. Sci. 2016, 17, 1406. [Google Scholar] [CrossRef] [PubMed]
- Mello, J.C.; Gonzales, M.V.D.; Moraes, V.W.R.; Prieto, T.; Nascimento, O.R.; Rodrigues, T. Protective Effect of Plantago major Extract against t-BOOH-Induced Mitochondrial Oxidative Damage and Cytotoxicity. Molecules 2015, 20, 17747–177759. [Google Scholar] [CrossRef] [PubMed]
- Ujhelyi, J.; Ujhelyi, Z.; Szalai, A.; László, J.F.; Cayasso, M.; Vecsernyés, M.; Pórszász, R. Analgesic and anti-inflammatory effectiveness of sitagliptin and vitagliptin in mice. Regul. Pept. 2014. [Google Scholar] [CrossRef]
- Fakhrudin, N.; Astuti, E.D.; Sulistyawati, R.; Santosa, D.; Susandarini, R.; Nurrochmad, A.; Wahyuono, S. n-Hexane Insoluble Fraction of Plantago lanceolate Exerts Anti-Inflammatory Activity in Mice by Inhibiting Cyclooxygenase-2 and Reducing Chemokines Levels. Sci. Pharm. 2017, 85, 12. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Shaoling, Y.; Wang, Z.; Chen, S.; Xin, S.; Xie, J.; Zhao, C. Self-nanoemulsifying drug delivery system of persimmon leaf extract: Optimization and bioavailability studies. Int. J. Pharm. 2011, 420, 161–171. [Google Scholar] [CrossRef] [PubMed]
- Parikh, A.; Kathawala, K.; Tan, C.C.; Garg, S.; Zhou, X.F. Lipid-based nanosystem of edaravone: Development, optimization, characterization and in vitro/in vivo evaluation. Drug Deliv. 2017, 24, 962–978. [Google Scholar] [CrossRef] [PubMed]
- Pouton, C.W. Lipid formulations for oral administration of drugs: Non-emulsifying, self-emulsifying and ‘self-microemulsifying’ drug delivery systems. Eur. J. Pharm. Sci. 2000, 11 (Suppl. 2), 93–98. [Google Scholar] [CrossRef]
- Naguib, S.S.; Hathout, R.M.; Mansour, S. Optimizing novel penetration enhancing hybridized vesicles for augmenting the in vivo effect of an anti-glaucoma drug. Drug Deliv. 2017, 24, 99–108. [Google Scholar] [CrossRef] [PubMed]
- Powell, K.C.; Damitz, R.; Chauhan, A. Relating emulsion stability to interfacial properties for pharmaceutical emulsions stabilized by Pluronic F68 surfactant. Int. J. Pharm. 2017, 521, 8–18. [Google Scholar] [CrossRef] [PubMed]
- Ujhelyi, Z.; Róka, E.; Fenyvesi, F.; Fehér, P.; Váradi, J.; Réti-Nagy, K.; Vecsernyés, M.; Bácskay, I. Assessment of the hemolytic activity and cytotoxicity of different PEG-based solubilizing agents. Pharmazie 2013, 68, 383–384. [Google Scholar] [PubMed]
- Sandri, G.; Bonferoni, M.C.; Rossi, S.; Ferri, F.; Gibin, S.; Zambito, Y.; Di Colo, G.; Caramella, C. Nanoparticles Based on N-trimethylchitosan: Evaluation of absorption properties using in vitro (Caco-2 cells) and ex vivo (excised rat jejunum) models. Eur. J. Pharm. Biopharm. 2007, 65, 68–77. [Google Scholar] [CrossRef] [PubMed]
- Bigansoli, E.; Cavenaghi, L.A.; Rossi, R.; Brunati, M.C.; Nolli, M.L. Use of a Caco-2 cell culture model for the characterization of intestinal absorption of antibiotics. Farmaco 1999, 54, 594–599. [Google Scholar] [CrossRef]
- Fotakis, G.; Timbrell, J.A. In vitro cytotoxicity assays. Comparison of LDH, neutral red, MTT and protein assay in hepatoma cell lines following exposure to cadmium chloride. Toxicol. Lett. 2006, 160, 171–177. [Google Scholar] [CrossRef] [PubMed]
- Xing, J.Z.; Zhu, L.; Gabos, S.; Xie, L. Microelectronic cell sensor assay for detection of cytotoxicity and prediction of acute toxicity. Toxicol. Vitro 2006, 20, 995–1004. [Google Scholar] [CrossRef] [PubMed]
- Palamakula, A.; Khan, M.A. Evaluation of cytotoxicity of oils used in coenzyme Q10 Self-emulsifying Drug Delivery Systems (SEDDS). Int. J. Pharm. 2004, 273, 63–73. [Google Scholar] [CrossRef] [PubMed]
- Gursoy, N.; Garrigue, J.S.; Razafindratsita, A.; Lambert, G.; Benita, S. Excipient effects on in vitro cytotoxicity of a novel paclitaxel self-emulsifying drug delivery system. J. Pharm. Sci. 2003, 92, 2411–2418. [Google Scholar] [CrossRef] [PubMed]
- Mensor, L.L.; Menezes, F.S.; Leitao, G.G.; Reis, A.S.; dos Santos, T.C.; Coube, C.S.; Leitão, S.G. Screening of Brazilian plant extracts for antioxidant activity by the use of DPPH free radical method. Phytother. Res. 2001, 15, 127–130. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Plantago lanceolate extract is available from the authors. |
| Catalpol (CA) | Aucubin (AU) | Acteoside/Verbescoside (ACTE) | |
|---|---|---|---|
| Chemical structures of bioactive components |  |  |  |
| Content in MeOH extract | 1.21 ± 0.02% | 2.34 ± 0.01% | 5.99 ± 0.012% |
| Number of Compositions | Isopropyl-Myristate | Transcutol HP | Kolliphor RH 40 | Labrasol |
|---|---|---|---|---|
| 1. | 33% | 33% | 33% | - |
| 2. | 25% | 50% | 25% | - |
| 3. | 15% | 60% | 15% | - |
| 4. | 10% | 80% | 10% | - |
| 5. | 33% | 33% | - | 33% |
| 6. | 25% | 50% | - | 25% |
| 7. | 15% | 60% | - | 15% |
| 8. | 10% | 80% | - | 10% |
| Comp. 1 | Comp. 2 | Comp. 3 | Comp. 4 | Comp. 5 | Comp. 6 | Comp. 7 | Comp. 8 | |
|---|---|---|---|---|---|---|---|---|
| B0 | 59.16 | 55.65 | 47.21 | 39.53 | 57.13 | 57.72 | 44.76 | 36.85 |
| B1 | 0.1483 | 0.1551 | 0.1984 | 0.2363 | 0.15 | 0.1353 | 0.187 | 0.2282 |
| B2 | −2.793 × 10−4 | −2.73 × 10−4 | −3.577 × 10−4 | −4.222 × 10−4 | −2.848 × 10−4 | −2.477 × 10−4 | −3.303 × 10−4 | −4.08 × 10−4 |
| B3 | 1.738 × 10−7 | 1.631 × 10−7 | 2.092 × 10−7 | 2.414 × 10−7 | 1.783 × 10−7 | 1.541 × 10−7 | 1.924 × 10−7 | 2.366 × 10−7 |
| B0 SE | 1.42 | 1.283 | 1.766 | 1.771 | 1.43 | 1.283 | 1.752 | 1.627 |
| B1 SE | 1.785 × 10−2 | 1.613 × 10−2 | 2.22 × 10−2 | 2.226 × 10−2 | 1.798 × 10−2 | 1.613 × 10−2 | 2.202 × 10−2 | 2.045 × 10−2 |
| B2 SE | 4.45 × 10−5 | 4.022 × 10−5 | 5.535 × 10−5 | 5.551 × 10−5 | 4.483 × 10−5 | 4.022 × 10−5 | 5.49 × 10−5 | 5.1 × 10−5 |
| B3 SE | 2.916 × 10−8 | 2.635 × 10−8 | 3.627 × 10−8 | 3.637 × 10−8 | 2.937 × 10−8 | 2.635 × 10−8 | 3.597 × 10−8 | 3.341 × 10−8 |
| R2 | 0.9296 | 0.9501 | 0.9252 | 0.9399 | 0.9301 | 0.94 | 0.925 | 0.9502 |
| Control | Comp. 1 | Comp. 2 | Comp. 3 | Comp. 4 | Comp. 5 | Comp. 6 | Comp. 7 | Comp. 8 | |
|---|---|---|---|---|---|---|---|---|---|
| AST (IU/L) | 362.28 ± 12.3 | 183.6 ± 34 * | 225.78 ± 13.4 * | 285.90 ± 12.3 * | ND | 232.56 ± 10.6 * | 214.6 ± 12.3 * | 298.78 ± 33.2 * | ND |
| ALT (U/L) | 92.34 ± 23.4 | 113.23 ± 42.1 | 98.70 ± 29.7 | 145.62 ± 38.6 * | ND | 93.4 ± 21.4 | 119.21 ± 13.2 | 138.23 ± 12.6 * | ND |
| Comp. 1 | Comp. 2 | Comp. 3 | Comp. 5 | Comp. 6 | Comp. 7 | PL Extract | |
|---|---|---|---|---|---|---|---|
| Slope | 0.625 | 0.725 | 0.548 | 0.454 | 0.604 | 0.549 | 0.18 |
| (±SE) | (±0.061) | (±0.061) | (±0.027) | (±0.056) | (±0.098) | (±0.043) | (±0.017) |
| Y-intercept | −2.849 | −3.436 | −1.632 | −2.108 | −3.635 | −1.213 | −0.662 |
| (±SE) | (±1.408) | (±1.415) | (±0.627) | (±1.292) | (±2.235) | (±0.988) | (±0.382) |
| X-intercept | 4.556 | 4.737 | 2.979 | 4.638 | 6.023 | 2.207 | 3.677 |
| R2 | 0.954 | 0.9651 | 0.9877 | 0.9286 | 0.8844 | 0.9702 | 0.9588 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kalantari, A.; Kósa, D.; Nemes, D.; Ujhelyi, Z.; Fehér, P.; Vecsernyés, M.; Váradi, J.; Fenyvesi, F.; Kuki, Á.; Gonda, S.; et al. Self-Nanoemulsifying Drug Delivery Systems Containing Plantago lanceolata—An Assessment of Their Antioxidant and Antiinflammatory Effects. Molecules 2017, 22, 1773. https://doi.org/10.3390/molecules22101773
Kalantari A, Kósa D, Nemes D, Ujhelyi Z, Fehér P, Vecsernyés M, Váradi J, Fenyvesi F, Kuki Á, Gonda S, et al. Self-Nanoemulsifying Drug Delivery Systems Containing Plantago lanceolata—An Assessment of Their Antioxidant and Antiinflammatory Effects. Molecules. 2017; 22(10):1773. https://doi.org/10.3390/molecules22101773
Chicago/Turabian StyleKalantari, Azin, Dóra Kósa, Dániel Nemes, Zoltán Ujhelyi, Pálma Fehér, Miklós Vecsernyés, Judit Váradi, Ferenc Fenyvesi, Ákos Kuki, Sándor Gonda, and et al. 2017. "Self-Nanoemulsifying Drug Delivery Systems Containing Plantago lanceolata—An Assessment of Their Antioxidant and Antiinflammatory Effects" Molecules 22, no. 10: 1773. https://doi.org/10.3390/molecules22101773
APA StyleKalantari, A., Kósa, D., Nemes, D., Ujhelyi, Z., Fehér, P., Vecsernyés, M., Váradi, J., Fenyvesi, F., Kuki, Á., Gonda, S., Vasas, G., Gesztelyi, R., Salimi, A., & Bácskay, I. (2017). Self-Nanoemulsifying Drug Delivery Systems Containing Plantago lanceolata—An Assessment of Their Antioxidant and Antiinflammatory Effects. Molecules, 22(10), 1773. https://doi.org/10.3390/molecules22101773