Long Term Osmotic Mini Pump Treatment with Alpha-MSH Improves Myocardial Function in Zucker Diabetic Fatty Rats
Abstract
:1. Introduction
2. Results
2.1. Weight Gain, Serum Parameters, Blood Pressure and LV Mass/Whole Body Mass Ratio
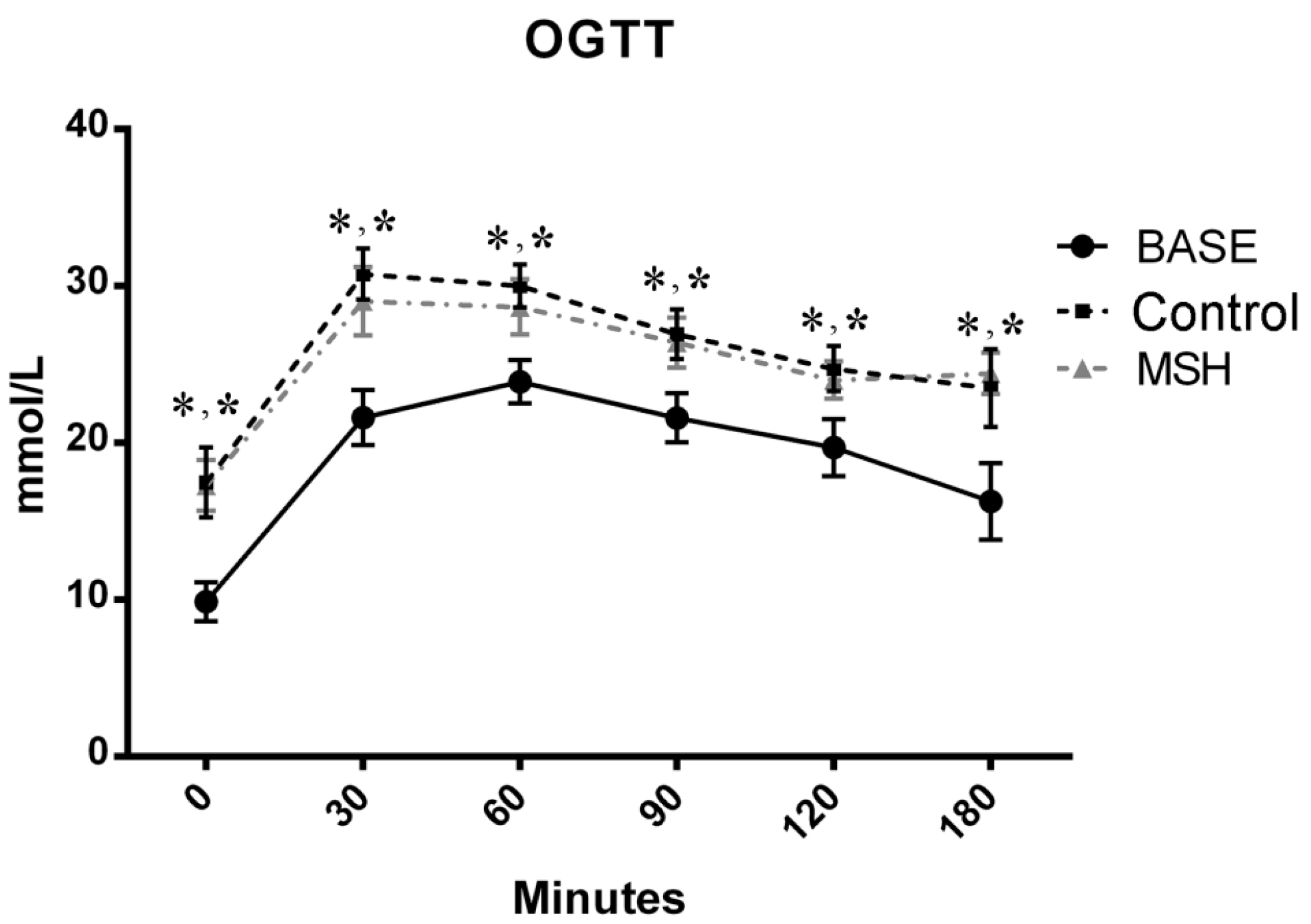
2.2. Results of OGT Tests
2.3. Echocardiography
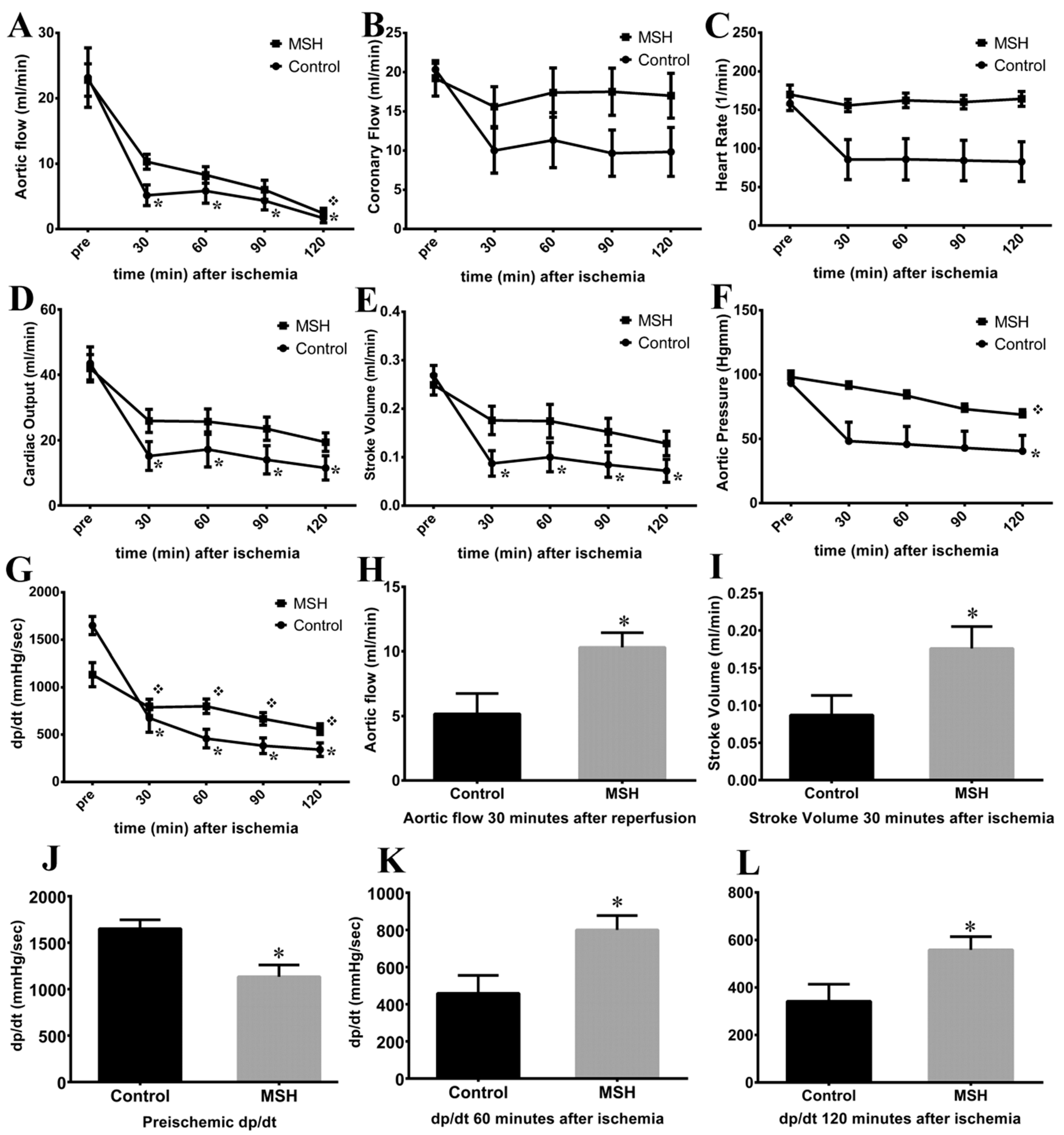
2.4. Isolated Working Heart Results
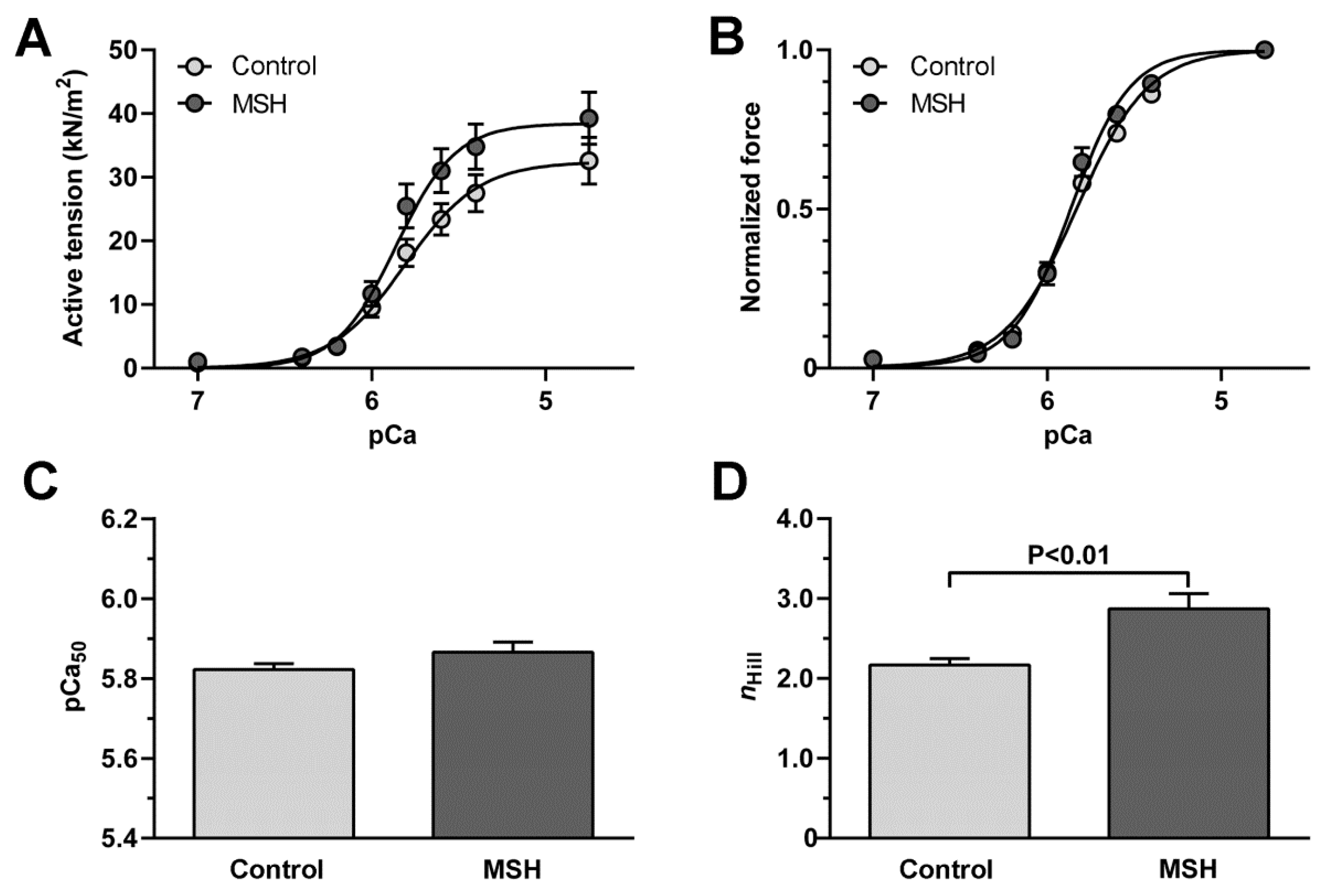
2.5. Enhancement of Cardiomyocyte Contractile Performance after Alpha-MSH Treatment
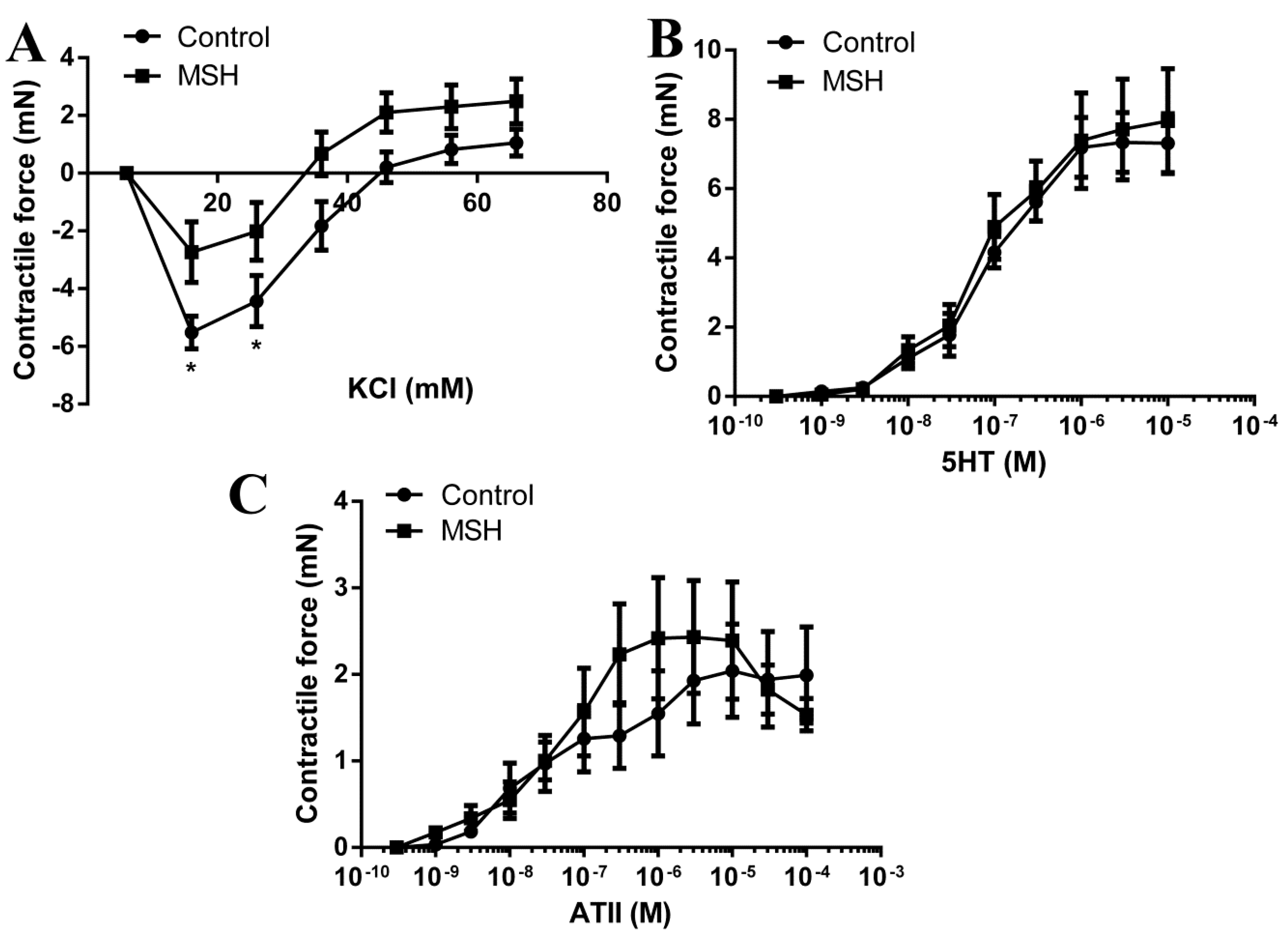
2.6. Vascular Status Brain Arteries
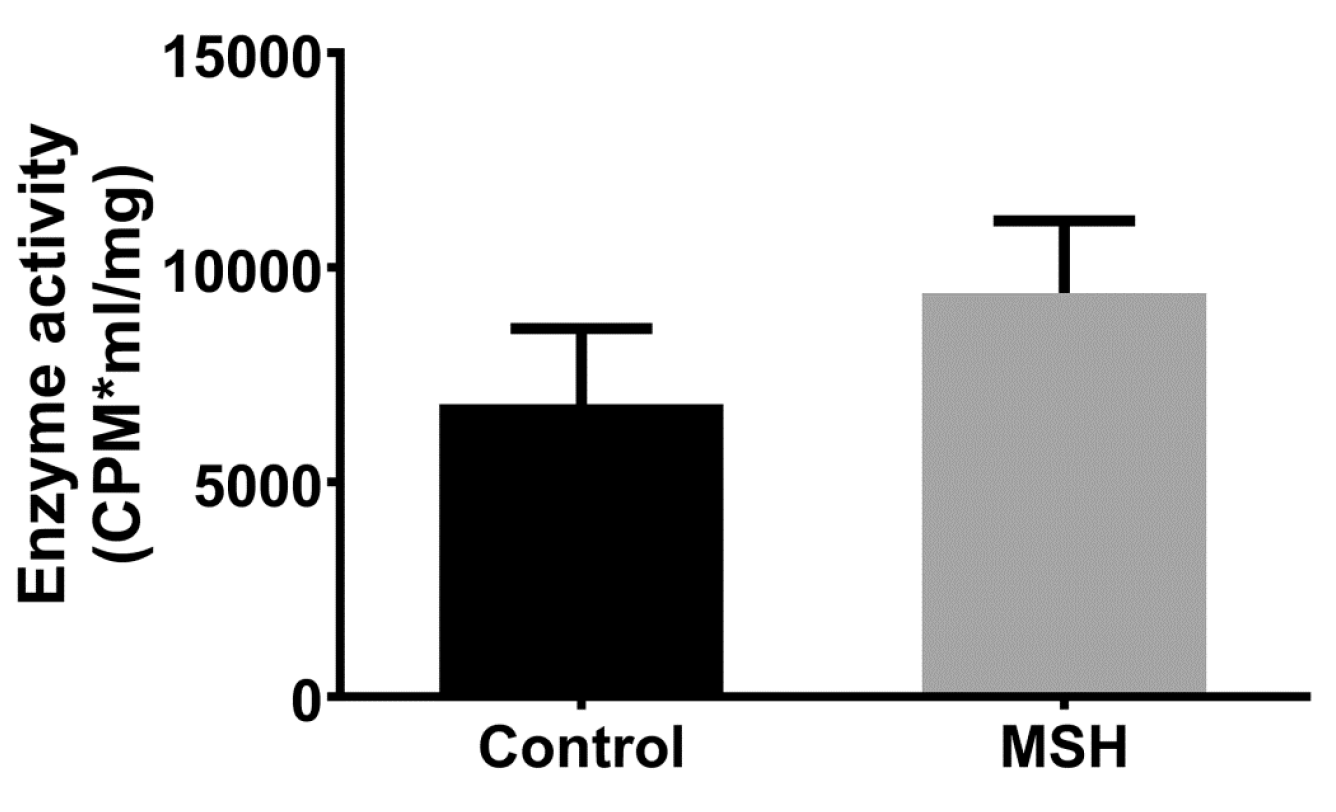
2.7. NADPH Oxidase Activity
3. Discussion
4. Materials and Methods
4.1. Animal Model and Chemicals
4.2. Study Design
4.3. Osmotic Pump Implant Surgery
4.4. Oral Glucose Tolerance Test (OGTT)
4.5. Blood Pressure Measurement of Conscious Rats
4.6. Analysis of Serum Parameters
4.7. Echocardiographic Studies
4.8. Isolated Working Heart Preparation
4.9. Force Measurements of Isolated Cardiomyocytes
4.10. Arterial Contractile Force Measurement
4.11. NADPH Oxidase Activity Measurement
4.12. Statistical Analyses
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Deylami, R.; Townson, J.; Mann, M.; Gregory, J.W. Systematic review of publicity interventions to increase awareness amongst healthcare professionals and the public to promote earlier diagnosis of type 1 diabetes in children and young people. Pediatr. Diabetes 2017. [Google Scholar] [CrossRef] [PubMed]
- Nadella, S.; Indyk, J.A.; Kamboj, M.K. Management of diabetes mellitus in children and adolescents: engaging in physical activity. Transl. Pediatr. 2017, 6, 215–224. [Google Scholar] [CrossRef] [PubMed]
- Adameova, A.; Kuzelova, M.; Andelova, E.; Faberova, V.; Pancza, D.; Svec, P.; Ziegelhoffer, A.; Ravingerova, T. Hypercholesterolemia abrogates an increased resistance of diabetic rat hearts to ischemia-reperfusion injury. Mol. Cell Biochem. 2007, 295, 129–136. [Google Scholar] [CrossRef] [PubMed]
- Kautzky-Willer, A.; Harreiter, J. Sex and gender differences in therapy of type 2 diabetes. Diabetes Res. Clin. Pract. 2017, 131, 230–241. [Google Scholar] [CrossRef] [PubMed]
- Baxter, M. Treatment of type 2 diabetes: A structured management plan. Adv. Ther. 2008, 25, 106–114. [Google Scholar] [CrossRef] [PubMed]
- Rana, B.; Bukhsh, A.; Khan, T.M.; Sarwar, A.; Omer, M.O.; Jamshed, S.Q. Evaluation of Therapeutic Effectiveness of Prescribed Medications in Patients with Type 2 Diabetes Mellitus: Findings from a Tertiary Care Hospital, Lahore, Pakistan. J. Pharm. Bioallied Sci. 2017, 9, 121–125. [Google Scholar] [PubMed]
- Naito, R.; Miyauchi, K. Coronary Artery Disease and Type 2 Diabetes Mellitus. Int. Heart J. 2017, 58, 475–480. [Google Scholar] [CrossRef] [PubMed]
- Gant, C.M.; Binnenmars, S.H.; Berg, E.V.D.; Bakker, S.J.L.; Navis, G.; Laverman, G.D. Integrated Assessment of Pharmacological and Nutritional Cardiovascular Risk Management: Blood Pressure Control in the DIAbetes and LifEstyle Cohort Twente (DIALECT). Nutrients 2017, 9. [Google Scholar] [CrossRef] [PubMed]
- Woo, V.C.; Berard, L.D.; Bajaj, H.S.; Ekoe, J.M.; Senior, P.A. Considerations for Initiating a Sodium-Glucose Co-Transporter 2 Inhibitor in Individuals with Type 2 Diabetes Using Insulin. Can. J. Diabetes 2017. [Google Scholar] [CrossRef] [PubMed]
- Maarman, G.J.; Mendham, A.E.; Lamont, K.; George, C. Review of a causal role of fructose-containing sugars in myocardial susceptibility to ischemia/reperfusion injury. Nutr. Res. 2017, 42, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Huisamen, B.; Dietrich, D.; Bezuidenhout, N.; Lopes, J.; Flepisi, B.; Blackhurst, D.; Lochner, A. Early cardiovascular changes occurring in diet-induced, obese insulin-resistant rats. Mol. Cell. Biochem. 2012, 368, 37–45. [Google Scholar] [CrossRef] [PubMed]
- Katakam, P.V.; Jordan, J.E.; Snipes, J.A.; Tulbert, C.D.; Miller, A.W.; Busija, D.W. Myocardial preconditioning against ischemia-reperfusion injury is abolished in Zucker obese rats with insulin resistance. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2007, 292, R920–R926. [Google Scholar] [CrossRef] [PubMed]
- Lear, T.; Liu, L.; O’Donnell, M.; McConn, B.R.; Denbow, D.M.; Cline, M.A.; Gilbert, E.R. Alpha-melanocyte stimulating hormone-induced anorexia in Japanese quail (Coturnix japonica) likely involves the ventromedial hypothalamus and paraventricular nucleus of the hypothalamus. Gen. Comp. Endocrinol. 2017, 252, 97–102. [Google Scholar] [CrossRef] [PubMed]
- Wellhauser, L.; Chalmers, J.A.; Belsham, D.D. Nitric Oxide Exerts Basal and Insulin-Dependent Anorexigenic Actions in POMC Hypothalamic Neurons. Mol. Endocrinol. 2016, 30, 402–416. [Google Scholar] [CrossRef] [PubMed]
- Lisak, R.P.; Benjamins, J.A. Melanocortins, Melanocortin Receptors and Multiple Sclerosis. Brain Sci. 2017, 7. [Google Scholar] [CrossRef]
- Getting, S.J.; Di Filippo, C.; Christian, H.C.; Lam, C.W.; Rossi, F.; D’Amico, M.; Perretti, M. MC-3 receptor and the inflammatory mechanisms activated in acute myocardial infarct. J. Leukoc. Biol. 2004, 76, 845–853. [Google Scholar] [CrossRef] [PubMed]
- Guarini, S.; Schioth, H.B.; Mioni, C.; Cainazzo, M.; Ferrazza, G.; Giuliani, D.; Wikberg, J.E.; Bertolini, A.; Bazzani, C. MC(3) receptors are involved in the protective effect of melanocortins in myocardial ischemia/reperfusion-induced arrhythmias. Naunyn Schmiedebergs Arch. Pharmacol. 2002, 366, 177–182. [Google Scholar] [CrossRef] [PubMed]
- Al-Barazanji, K.A.; Miller, J.E.; Rice, S.Q.; Arch, J.R.; Chambers, J.K. C-terminal fragments of ACTH stimulate feeding in fasted rats. Horm. Metab. Res. 2001, 33, 480–485. [Google Scholar] [CrossRef] [PubMed]
- Cerda-Reverter, J.M.; Schioth, H.B.; Peter, R.E. The central melanocortin system regulates food intake in goldfish. Regul. Pept. 2003, 115, 101–113. [Google Scholar] [CrossRef]
- Saneyasu, T.; Honda, K.; Kamisoyama, H.; Nakayama, Y.; Ikegami, K.; Hasegawa, S. Alpha-melanocyte stimulating hormone plays an important role in the regulation of food intake by the central melanocortin system in chicks. Peptides 2011, 32, 996–1000. [Google Scholar] [CrossRef] [PubMed]
- Wardlaw, S.L. Hypothalamic proopiomelanocortin processing and the regulation of energy balance. Eur. J. Pharmacol. 2011, 660, 213–219. [Google Scholar] [CrossRef] [PubMed]
- Zimanyi, I.A.; Pelleymounter, M.A. The role of melanocortin peptides and receptors in regulation of energy balance. Curr. Pharm. Des. 2003, 9, 627–641. [Google Scholar] [CrossRef] [PubMed]
- Chagnon, Y.C.; Chen, W.J.; Perusse, L.; Chagnon, M.; Nadeau, A.; Wilkison, W.O.; Bouchard, C. Linkage and association studies between the melanocortin receptors 4 and 5 genes and obesity-related phenotypes in the Quebec Family Study. Mol. Med. 1997, 3, 663–673. [Google Scholar] [PubMed]
- Jun, D.J.; Na, K.Y.; Kim, W.; Kwak, D.; Kwon, E.J.; Yoon, J.H.; Yea, K.; Lee, H.; Kim, J.; Suh, P.G.; et al. Melanocortins induce interleukin 6 gene expression and secretion through melanocortin receptors 2 and 5 in 3T3-L1 adipocytes. J. Mol. Endocrinol. 2010, 44, 225–236. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, A.R.; Almeida, H.; Gouveia, A.M. Alpha-MSH signalling via melanocortin 5 receptor promotes lipolysis and impairs re-esterification in adipocytes. Biochim. Biophys. Acta 2013, 1831, 1267–1275. [Google Scholar] [CrossRef] [PubMed]
- Cho, K.J.; Shim, J.H.; Cho, M.C.; Choe, Y.K.; Hong, J.T.; Moon, D.C.; Kim, J.W.; Yoon, D.Y. Signaling pathways implicated in alpha-melanocyte stimulating hormone-induced lipolysis in 3T3-L1 adipocytes. J. Cell. Biochem. 2005, 96, 869–878. [Google Scholar] [CrossRef] [PubMed]
- Minutoli, L.; Bitto, A.; Squadrito, F.; Irrera, N.; Rinaldi, M.; Nicotina, P.A.; Arena, S.; Magno, C.; Marini, H.; Spaccapelo, L.; et al. Melanocortin 4 receptor activation protects against testicular ischemia-reperfusion injury by triggering the cholinergic antiinflammatory pathway. Endocrinology 2011, 152, 3852–3861. [Google Scholar] [CrossRef] [PubMed]
- Ottani, A.; Giuliani, D.; Galantucci, M.; Spaccapelo, L.; Novellino, E.; Grieco, P.; Jochem, J.; Guarini, S. Melanocortins counteract inflammatory and apoptotic responses to prolonged myocardial ischemia/reperfusion through a vagus nerve-mediated mechanism. Eur. J. Pharmacol. 2010, 637, 124–130. [Google Scholar] [CrossRef] [PubMed]
- Rinne, P.; Silvola, J.M.; Hellberg, S.; Stahle, M.; Liljenback, H.; Salomaki, H.; Koskinen, E.; Nuutinen, S.; Saukko, P.; Knuuti, J.; et al. Pharmacological activation of the melanocortin system limits plaque inflammation and ameliorates vascular dysfunction in atherosclerotic mice. Arterioscler. Thromb. Vasc. Biol. 2014, 34, 1346–1354. [Google Scholar] [CrossRef] [PubMed]
- Varga, B.; Gesztelyi, R.; Bombicz, M.; Haines, D.; Szabo, A.M.; Kemeny-Beke, A.; Antal, M.; Vecsernyes, M.; Juhasz, B.; Tosaki, A. Protective effect of alpha-melanocyte-stimulating hormone (alpha-MSH) on the recovery of ischemia/reperfusion (I/R)-induced retinal damage in a rat model. J. Mol. Neurosci. 2013, 50, 558–570. [Google Scholar] [CrossRef] [PubMed]
- Ottani, A.; Neri, L.; Canalini, F.; Calevro, A.; Rossi, R.; Cappelli, G.; Ballestri, M.; Giuliani, D.; Guarini, S. Protective effects of the melanocortin analog NDP-alpha-MSH in rats undergoing cardiac arrest. Eur. J. Pharmacol. 2014, 745, 108–116. [Google Scholar] [CrossRef] [PubMed]
- Catania, A.; Lonati, C.; Sordi, A.; Leonardi, P.; Carlin, A.; Gatti, S. The peptide NDP-MSH induces phenotype changes in the heart that resemble ischemic preconditioning. Peptides 2010, 31, 116–122. [Google Scholar] [CrossRef] [PubMed]
- Bazzani, C.; Guarini, S.; Botticelli, A.R.; Zaffe, D.; Tomasi, A.; Bini, A.; Cainazzo, M.M.; Ferrazza, G.; Mioni, C.; Bertolini, A. Protective effect of melanocortin peptides in rat myocardial ischemia. J. Pharmacol. Exp. Ther. 2001, 297, 1082–1087. [Google Scholar] [PubMed]
- Vecsernyes, M.; Juhasz, B.; Der, P.; Kocsan, R.; Feher, P.; Bacskay, I.; Kovacs, P.; Tosaki, A. The administration of alpha-melanocyte-stimulating hormone protects the ischemic/reperfused myocardium. Eur. J. Pharmacol. 2003, 470, 177–183. [Google Scholar] [CrossRef]
- Bombicz, M.; Priksz, D.; Varga, B.; Kurucz, A.; Kertesz, A.; Takacs, A.; Posa, A.; Kiss, R.; Szilvassy, Z.; Juhasz, B. A Novel Therapeutic Approach in the Treatment of Pulmonary Arterial Hypertension: Allium ursinum Liophylisate Alleviates Symptoms Comparably to Sildenafil. Int. J. Mol. Sci. 2017, 18. [Google Scholar] [CrossRef] [PubMed]
- Van den Brom, C.E.; Huisman, M.C.; Vlasblom, R.; Boontje, N.M.; Duijst, S.; Lubberink, M.; Molthoff, C.F.; Lammertsma, A.A.; van der Velden, J.; Boer, C.; et al. Altered myocardial substrate metabolism is associated with myocardial dysfunction in early diabetic cardiomyopathy in rats: studies using positron emission tomography. Cardiovasc. Diabetol. 2009, 8, 39. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morton, G.J.; Cummings, D.E.; Baskin, D.G.; Barsh, G.S.; Schwartz, M.W. Central nervous system control of food intake and body weight. Nature 2006, 443, 289–295. [Google Scholar] [CrossRef] [PubMed]
- Shapiro, L.M.; McKenna, W.J. Left ventricular hypertrophy. Relation of structure to diastolic function in hypertension. Br. Heart J. 1984, 51, 637–642. [Google Scholar] [CrossRef] [PubMed]
- Akaishi, M.; Schneider, R.M.; Mercier, R.J.; Naccarella, F.F.; Agarwal, J.B.; Helfant, R.H.; Weintraub, W.S. Relation between left ventricular global and regional function and extent of myocardial ischemia in the canine heart. J. Am. Coll. Cardiol. 1985, 6, 104–112. [Google Scholar] [CrossRef]
- McDaniel, F.K.; Molden, B.M.; Mohammad, S.; Baldini, G.; McPike, L.; Narducci, P.; Granell, S.; Baldini, G. Constitutive cholesterol-dependent endocytosis of melanocortin-4 receptor (MC4R) is essential to maintain receptor responsiveness to alpha-melanocyte-stimulating hormone (alpha-MSH). J. Biol. Chem. 2012, 287, 21873–21890. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Malek, Z.A. Melanocortin receptors: their functions and regulation by physiological agonists and antagonists. Cell. Mol. Life Sci. 2001, 58, 434–441. [Google Scholar] [CrossRef] [PubMed]
- Huszar, D.; Lynch, C.A.; Fairchild-Huntress, V.; Dunmore, J.H.; Fang, Q.; Berkemeier, L.R.; Gu, W.; Kesterson, R.A.; Boston, B.A.; Cone, R.D.; et al. Targeted disruption of the melanocortin-4 receptor results in obesity in mice. Cell 1997, 88, 131–141. [Google Scholar] [CrossRef]
- Mountjoy, K.G.; Wong, J. Obesity, diabetes and functions for proopiomelanocortin-derived peptides. Mol. Cell. Endocrinol. 1997, 128, 171–177. [Google Scholar] [CrossRef]
- Moller, C.L.; Pedersen, S.B.; Richelsen, B.; Conde-Frieboes, K.W.; Raun, K.; Grove, K.L.; Wulff, B.S. Melanocortin agonists stimulate lipolysis in human adipose tissue explants but not in adipocytes. BMC Res. Notes 2015, 8, 559. [Google Scholar] [CrossRef] [PubMed]
- Pierroz, D.D.; Ziotopoulou, M.; Ungsunan, L.; Moschos, S.; Flier, J.S.; Mantzoros, C.S. Effects of acute and chronic administration of the melanocortin agonist MTII in mice with diet-induced obesity. Diabetes 2002, 51, 1337–1345. [Google Scholar] [CrossRef] [PubMed]
- Vecsernyes, M.; Szokol, M.; Bombicz, M.; Priksz, D.; Gesztelyi, R.; Fulop, G.A.; Varga, B.; Juhasz, B.; Haines, D.; Tosaki, A. Alpha-Melanocyte-stimulating Hormone Induces Vasodilation and Exerts Cardioprotection Through the Heme-Oxygenase Pathway in Rat Hearts. J. Cardiovasc. Pharmacol. 2017, 69, 286–297. [Google Scholar] [CrossRef] [PubMed]
- Alam, M.; Hoglund, C.; Thorstrand, C. Longitudinal systolic shortening of the left ventricle: An echocardiographic study in subjects with and without preserved global function. Clin. Physiol. 1992, 12, 443–452. [Google Scholar] [CrossRef] [PubMed]
- Jones, C.J.; Raposo, L.; Gibson, D.G. Functional importance of the long axis dynamics of the human left ventricle. Br. Heart J. 1990, 63, 215–220. [Google Scholar] [CrossRef] [PubMed]
- Hu, K.; Liu, D.; Herrmann, S.; Niemann, M.; Gaudron, P.D.; Voelker, W.; Ertl, G.; Bijnens, B.; Weidemann, F. Clinical implication of mitral annular plane systolic excursion for patients with cardiovascular disease. Eur. Heart J. Cardiovasc. Imaging 2013, 14, 205–212. [Google Scholar] [CrossRef] [PubMed]
- Alam, M.; Hoglund, C.; Thorstrand, C.; Philip, A. Atrioventricular plane displacement in severe congestive heart failure following dilated cardiomyopathy or myocardial infarction. J. Intern. Med. 1990, 228, 569–575. [Google Scholar] [CrossRef] [PubMed]
- Willenheimer, R.; Cline, C.; Erhardt, L.; Israelsson, B. Left ventricular atrioventricular plane displacement: An echocardiographic technique for rapid assessment of prognosis in heart failure. Heart 1997, 78, 230–236. [Google Scholar] [CrossRef] [PubMed]
- Emilsson, K.; Wandt, B. The relation between ejection fraction and mitral annulus motion before and after direct-current electrical cardioversion. Clin. Physiol. 2000, 20, 218–224. [Google Scholar] [CrossRef] [PubMed]
- Emilsson, K.; Wandt, B. The relation between mitral annulus motion and ejection fraction changes with age and heart size. Clin. Physiol. 2000, 20, 38–43. [Google Scholar] [CrossRef] [PubMed]
- Rydberg, E.; Arlbrandt, M.; Gudmundsson, P.; Erhardt, L.; Willenheimer, R. Left atrioventricular plane displacement predicts cardiac mortality in patients with chronic atrial fibrillation. Int. J. Cardiol. 2003, 91, 1–7. [Google Scholar] [CrossRef]
- Goroshi, M.; Chand, D. Myocardial Performance Index (Tei Index): A simple tool to identify cardiac dysfunction in patients with diabetes mellitus. Indian Heart J. 2016, 68, 83–87. [Google Scholar] [CrossRef] [PubMed]
- Gong, H.; Wang, W.; Kwon, T.H.; Jonassen, T.; Li, C.; Ring, T.; Froki, A.J.; Nielsen, S. EPO and alpha-MSH prevent ischemia/reperfusion-induced down-regulation of AQPs and sodium transporters in rat kidney. Kidney Int. 2004, 66, 683–695. [Google Scholar] [CrossRef] [PubMed]
- Ottani, A.; Giuliani, D.; Neri, L.; Calevro, A.; Canalini, F.; Vandini, E.; Cainazzo, M.M.; Ruberto, I.A.; Barbieri, A.; Rossi, R.; et al. NDP-alpha-MSH attenuates heart and liver responses to myocardial reperfusion via the vagus nerve and JAK/ERK/STAT signaling. Eur. J. Pharmacol. 2015, 769, 22–32. [Google Scholar] [CrossRef] [PubMed]
- Strelkov, R.B.; Belykh, A.G.; Karash Iu, M.; Kir’ianov, I.; Matiushin, A.I. Enhancement of the body’s capacity to resist various extreme factors by using normobaric hypoxic stimulation. Vestn. Akad. Med. Nauk SSSR 1988, 5, 77–80. [Google Scholar]
- Tsatmali, M.; Ancans, J.; Thody, A.J. Melanocyte function and its control by melanocortin peptides. J. Histochem. Cytochem. 2002, 50, 125–133. [Google Scholar] [CrossRef] [PubMed]
- Getting, S.J.; Di Filippo, C.; D’Amico, M.; Perretti, M. The melanocortin peptide HP228 displays protective effects in acute models of inflammation and organ damage. Eur. J. Pharmacol. 2006, 532, 138–144. [Google Scholar] [CrossRef] [PubMed]
- Penny, R.J.; Tilders, F.J.; Thody, A.J. The effect of hypothalamic lesions on immuno-reactive alpha-melanocyte stimulating hormone secretion in the rat. J. Physiol. 1979, 292, 59–67. [Google Scholar] [CrossRef] [PubMed]
- Gregersen, S.; Bystrup, S.; Overgaard, A.; Jeppesen, P.B.; Sonderstgaard Thorup, A.C.; Jensen, E.; Hermansen, K. Effects of whey proteins on glucose metabolism in normal Wistar rats and Zucker diabetic fatty (ZDF) rats. Rev. Diabet. Stud. 2013, 10, 252–269. [Google Scholar] [CrossRef] [PubMed]
- Bayorh, M.A.; Rollins-Hairston, A.; Adiyiah, J.; Lyn, D.; Eatman, D. Eplerenone suppresses aldosterone/ salt-induced expression of NOX-4. J. Renin Angiotensin Aldosterone Syst. 2011, 12, 195–201. [Google Scholar] [CrossRef] [PubMed]
- Bombicz, M.; Priksz, D.; Varga, B.; Gesztelyi, R.; Kertesz, A.; Lengyel, P.; Balogh, P.; Csupor, D.; Hohmann, J.; Bhattoa, H.P.; et al. Anti-Atherogenic Properties of Allium ursinum Liophylisate: Impact on Lipoprotein Homeostasis and Cardiac Biomarkers in Hypercholesterolemic Rabbits. Int. J. Mol. Sci. 2016, 17. [Google Scholar] [CrossRef] [PubMed]
- Juhasz, B.; Kertesz, A.; Balla, J.; Balla, G.; Szabo, Z.; Bombicz, M.; Priksz, D.; Gesztelyi, R.; Varga, B.; Haines, D.D.; et al. Cardioprotective effects of sour cherry seed extract (SCSE) on the hypercholesterolemic rabbit heart. Curr. Pharm. Des. 2013, 19, 6896–6905. [Google Scholar] [CrossRef] [PubMed]
- Kovacs, A.; Fulop, G.A.; Kovacs, A.; Csipo, T.; Bodi, B.; Priksz, D.; Juhasz, B.; Beke, L.; Hendrik, Z.; Mehes, G.; et al. Renin overexpression leads to increased titin-based stiffness contributing to diastolic dysfunction in hypertensive mRen2 rats. Am. J. Physiol. Heart Circ. Physiol. 2016, 310, H1671–H1682. [Google Scholar] [CrossRef] [PubMed]
- Moro, C.; Grauzam, S.; Ormezzano, O.; Toufektsian, M.C.; Tanguy, S.; Calabrese, P.; Coll, J.L.; Bak, I.; Juhasz, B.; Tosaki, A.; et al. Inhibition of cardiac leptin expression after infarction reduces subsequent dysfunction. J. Cell. Mol. Med. 2011, 15, 1688–1694. [Google Scholar] [CrossRef] [PubMed]
- Bak, I.; Lekli, I.; Juhasz, B.; Nagy, N.; Varga, E.; Varadi, J.; Gesztelyi, R.; Szabo, G.; Szendrei, L.; Bacskay, I.; et al. Cardioprotective mechanisms of Prunus cerasus (sour cherry) seed extract against ischemia-reperfusion-induced damage in isolated rat hearts. Am. J. Physiol. Heart Circ. Physiol. 2006, 291, H1329–H1336. [Google Scholar] [CrossRef] [PubMed]
- Juhasz, B.; Der, P.; Szodoray, P.; Gesztelyi, R.; Lekli, I.; Bak, I.; Antal, M.; Maulik, N.; Tosaki, A.; Vecsernyes, M. Adrenocorticotrope hormone fragment (4–10) attenuates the ischemia/reperfusion-induced cardiac injury in isolated rat hearts. Antioxid. Redox. Signal. 2007, 9, 1851–1861. [Google Scholar] [CrossRef] [PubMed]
- Juhasz, B.; Thirunavukkarasu, M.; Pant, R.; Zhan, L.; Penumathsa, S.V.; Secor, E.R., Jr.; Srivastava, S.; Raychaudhuri, U.; Menon, V.P.; Otani, H.; et al. Bromelain induces cardioprotection against ischemia-reperfusion injury through Akt/FOXO pathway in rat myocardium. Am. J. Physiol. Heart Circ. Physiol. 2008, 294, H1365–H1370. [Google Scholar] [CrossRef] [PubMed]
- Thirunavukkarasu, M.; Penumathsa, S.V.; Koneru, S.; Juhasz, B.; Zhan, L.; Otani, H.; Bagchi, D.; Das, D.K.; Maulik, N. Resveratrol alleviates cardiac dysfunction in streptozotocin-induced diabetes: Role of nitric oxide, thioredoxin, and heme oxygenase. Free Radic. Biol. Med. 2007, 43, 720–729. [Google Scholar] [CrossRef] [PubMed]
- Kertesz, A.; Bombicz, M.; Priksz, D.; Balla, J.; Balla, G.; Gesztelyi, R.; Varga, B.; Haines, D.D.; Tosaki, A.; Juhasz, B. Adverse impact of diet-induced hypercholesterolemia on cardiovascular tissue homeostasis in a rabbit model: Time-dependent changes in cardiac parameters. Int. J. Mol. Sci. 2013, 14, 19086–19108. [Google Scholar] [CrossRef] [PubMed]
- Papp, Z.; Szabo, A.; Barends, J.P.; Stienen, G.J. The mechanism of the force enhancement by MgADP under simulated ischaemic conditions in rat cardiac myocytes. J. Physiol. 2002, 543, 177–189. [Google Scholar] [CrossRef] [PubMed]
- Koshikawa, S.; Nishikimi, T.; Inaba, C.; Akimoto, K.; Matsuoka, H. Fasudil, a Rho-kinase inhibitor, reverses L-NAME exacerbated severe nephrosclerosis in spontaneously hypertensive rats. J Hypertens. 2008, 26, 1837–1848. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, E.; Kataoka, K.; Yamashita, T.; Tokutomi, Y.; Dong, YF.; Matsuba, S.; Ogawa, H.; Kim-Mitsuyama, S. Role of xanthine oxidoreductase in the reversal of diastolic heart failure by candesartan in the salt-sensitive hypertensive rat. Hypertension 2007, 50, 657–662. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are not available from the authors. |
| Parameter | Control | MSH |
|---|---|---|
| Endpoint weight (g) | 358.7 ± 7.154 | 391.8 ± 17.00 |
| Weight gain (%) | 20.23 ± 2.444 | 17.65 ± 2.225 |
| Baseline LV mass/Baseline bodyweight (%) | 0.337 ± 0.011 | 0.321 ± 0.017 |
| Endpoint LV mass/Endpoint bodyweight (%) | 0.320 ± 0.011 | 0.2770 ± 0.010 * |
| Total cholesterol (mmol/L) | 3.445 ± 0.210 | 3.295 ± 0.074 |
| HDL (mmol/L) | 2.082 ± 0.194 | 1.768 ± 0.092 |
| Triglyceride (mmol/L) | 3.487 ± 0.318 | 3.068 ± 0.344 |
| Systolic BP (mmHg) | 134.00 ± 4.073 | 142.2 ± 1.900 |
| Diastolic BP (mmHg) | 96.25 ± 3.484 | 105.5 ± 4.400 |
| Parameter | BASE | Control | MSH |
|---|---|---|---|
| LA/Ao ratio | 1.133 ± 0.039 | 1.104 ± 0.043 | 0.945 ± 0.029 * |
| LV Ejection Fraction (%) | 73.17 ± 1.973 | 66.50 ± 0.067 ❖ | 72.00 ± 0.774 * |
| LV Fractional Shortening (%) | 37.50 ± 1.500 | 32.33 ± 0.421 ❖ | 36.83 ± 0.703 * |
| IVSd (mm) | 1.845 ± 0.164 | 1.550 ± 0.044 | 1.613 ± 0.099 |
| LVIDd (mm) | 7.038 ± 0.048 | 7.747 ± 0.328 | 7.688 ± 0.248 |
| IVSs (mm) | 2.603 ± 0.075 | 2.143 ± 0.093 ❖ | 2.097 ± 0.103 |
| LVIDs (mm) | 4.385 ± 0.103 | 5.232 ± 0.226 | 4.858 ± 0.117 |
| Stroke volume (mL) | 0.428 ± 0.037 | 0.406 ± 0.046 | 0.581 ± 0.030 * |
| Cardiac Output (mL/min) | 99.89 ± 8.236 | 77.55 ± 7.763 | 112.30 ± 6.110 * |
| HR (bpm) | 235.8 ± 8.462 | 192.7 ± 4.185 ❖ | 193.3 ± 6.259 ❖ |
| LVOT maxPG (mmHg) | 3.173 ± 0.217 | 2.698 ± 0.254 | 3.765 ± 0.284 * |
| LVOT meanPG (mmHg) | 1.178 ± 0.138 | 1.095 ± 0.088 | 1.592 ± 0.106 * |
| LVOT Vmax (m/s) | 0.887 ± 0.029 | 0.818 ± 0.038 | 0.965 ± 0.036 * |
| LVOT Vmean (m/s) | 0.447 ± 0.032 | 0.441 ± 0.024 | 0.553 ± 0.019 * |
| Lateral e’ (mm/s) | 39.50 ± 1.803 | 26.83 ± 1.939 ❖ | 28.33 ± 0.614 |
| MV E velocity (m/s) | 0.887 ± 0.025 | 0.743 ± 0.014 ❖ | 0.710 ± 0.027 |
| MV A velocity (m/s) | 0.477 ± 0.027 | 0.438 ± 0.022 | 0.403 ± 0.018 |
| MV E/A ratio | 1.885 ± 0.109 | 1.802 ± 0.048 | 1.770 ± 0.101 |
| MV Deceleration Time (ms) | 55.67 ± 3.333 | 66.67 ± 3.201 | 85.50 ± 5.258 * |
| E/e’ ratio | 22.59 ± 0.832 | 28.47 ± 2.220 ❖ | 25.11 ± 1.070 |
| MAPSE (mm) | 2.167 ± 0.061 | 1.602 ± 0.045 ❖ | 2.268 ± 0.010 * |
| Ejection Time (ms) | 100.7 ± 2.459 | 83.17 ± 2.926 ❖ | 93.17 ± 3.877 |
| IVCT (ms) | 18.50 ± 1.708 | 23.50 ± 1.727 | 17.83 ± 0.703 * |
| IVRT (ms) | 25.00 ± 1.390 | 58.00 ± 1.826 ❖ | 43.00 ± 1.125 * |
| MPI (Tei-index) | 0.305 ± 0.012 | 0.491 ± 0.014 ❖ | 0.392 ± 0.013 * |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szokol, M.; Priksz, D.; Bombicz, M.; Varga, B.; Kovacs, A.; Fulop, G.A.; Csipo, T.; Posa, A.; Toth, A.; Papp, Z.; et al. Long Term Osmotic Mini Pump Treatment with Alpha-MSH Improves Myocardial Function in Zucker Diabetic Fatty Rats. Molecules 2017, 22, 1702. https://doi.org/10.3390/molecules22101702
Szokol M, Priksz D, Bombicz M, Varga B, Kovacs A, Fulop GA, Csipo T, Posa A, Toth A, Papp Z, et al. Long Term Osmotic Mini Pump Treatment with Alpha-MSH Improves Myocardial Function in Zucker Diabetic Fatty Rats. Molecules. 2017; 22(10):1702. https://doi.org/10.3390/molecules22101702
Chicago/Turabian StyleSzokol, Miklos, Daniel Priksz, Mariann Bombicz, Balazs Varga, Arpad Kovacs, Gabor Aron Fulop, Tamas Csipo, Aniko Posa, Attila Toth, Zoltan Papp, and et al. 2017. "Long Term Osmotic Mini Pump Treatment with Alpha-MSH Improves Myocardial Function in Zucker Diabetic Fatty Rats" Molecules 22, no. 10: 1702. https://doi.org/10.3390/molecules22101702




