PrLPAAT4, a Putative Lysophosphatidic Acid Acyltransferase from Paeonia rockii, Plays an Important Role in Seed Fatty Acid Biosynthesis
Abstract
:1. Introduction
2. Results
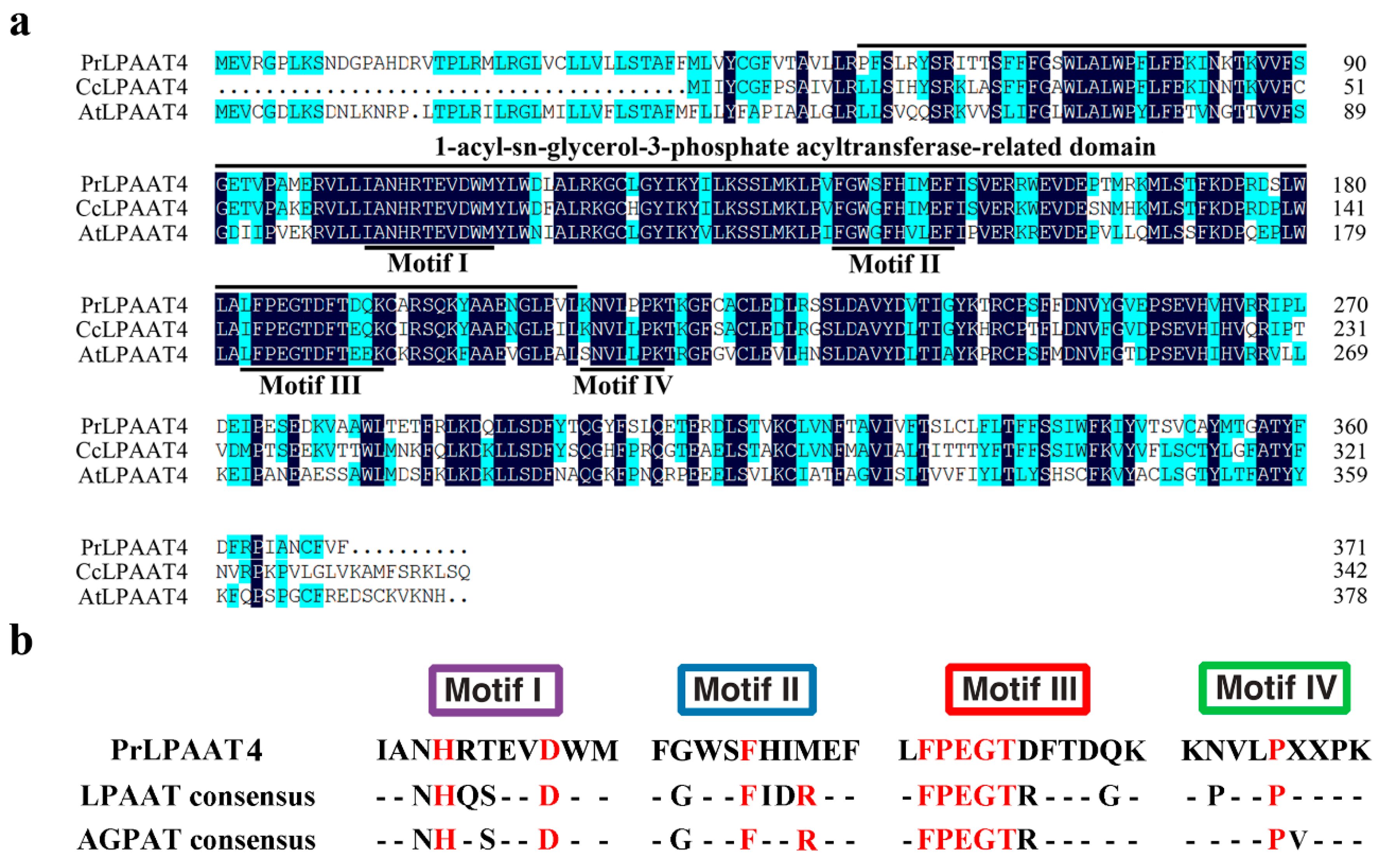
2.1. Isolation and Characterization of PrLPAAT4
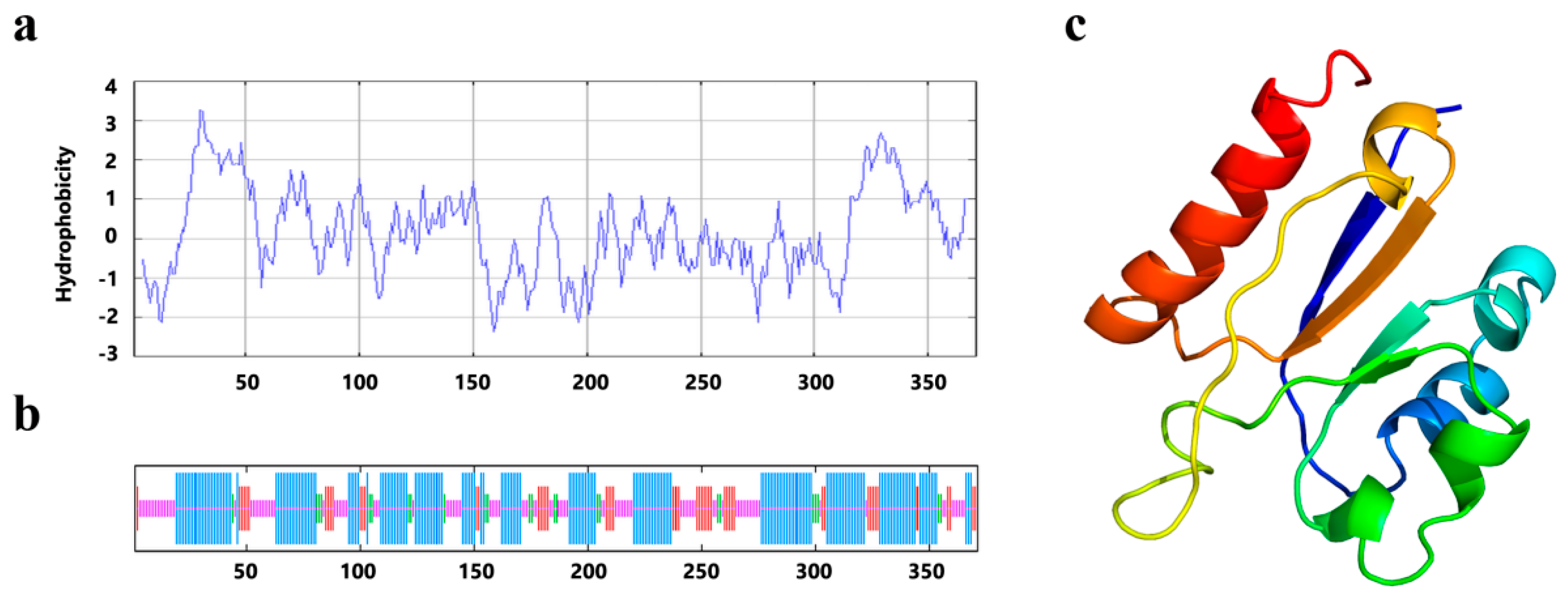
2.2. The Predicted Structure of PrLPAAT4
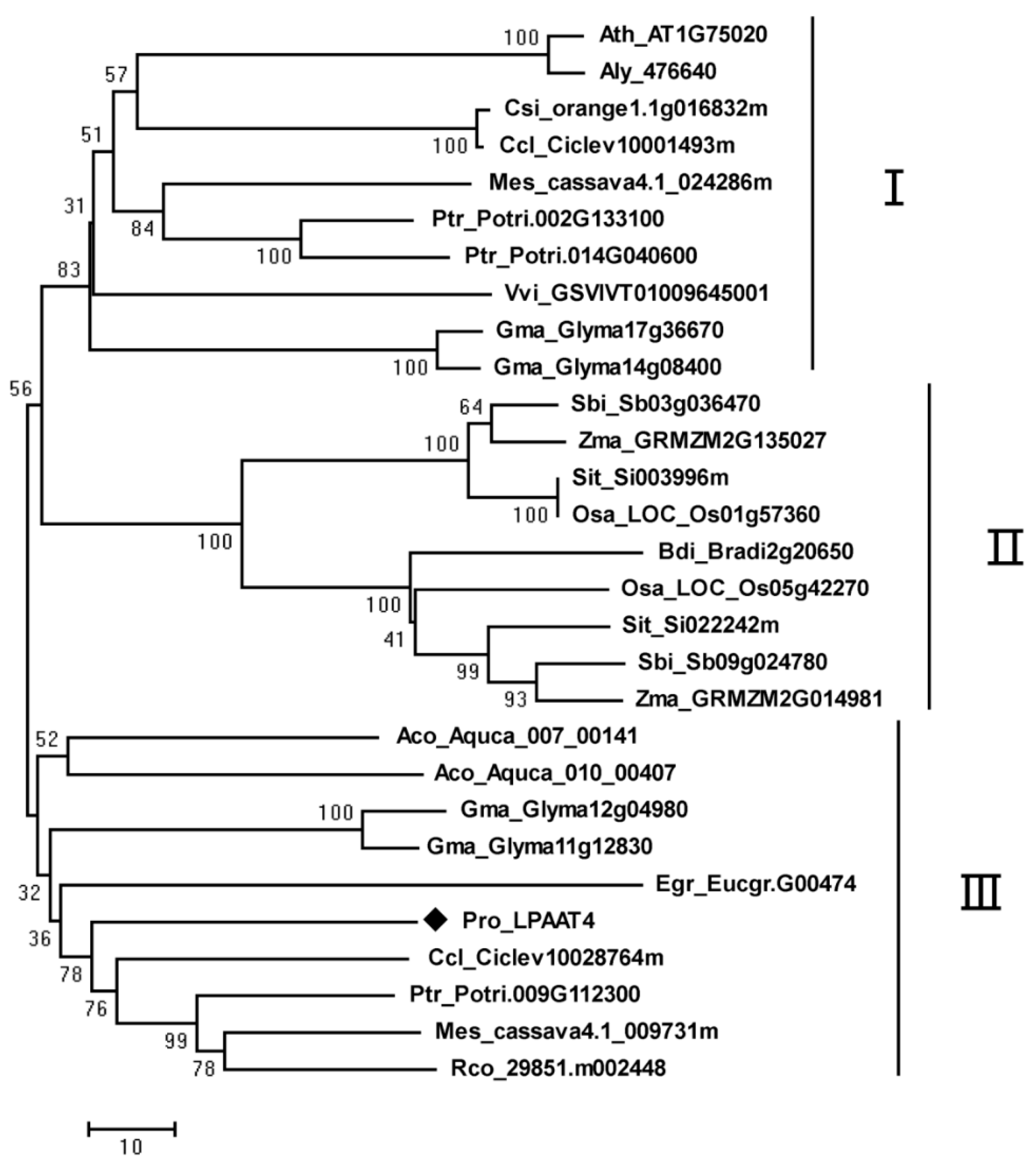
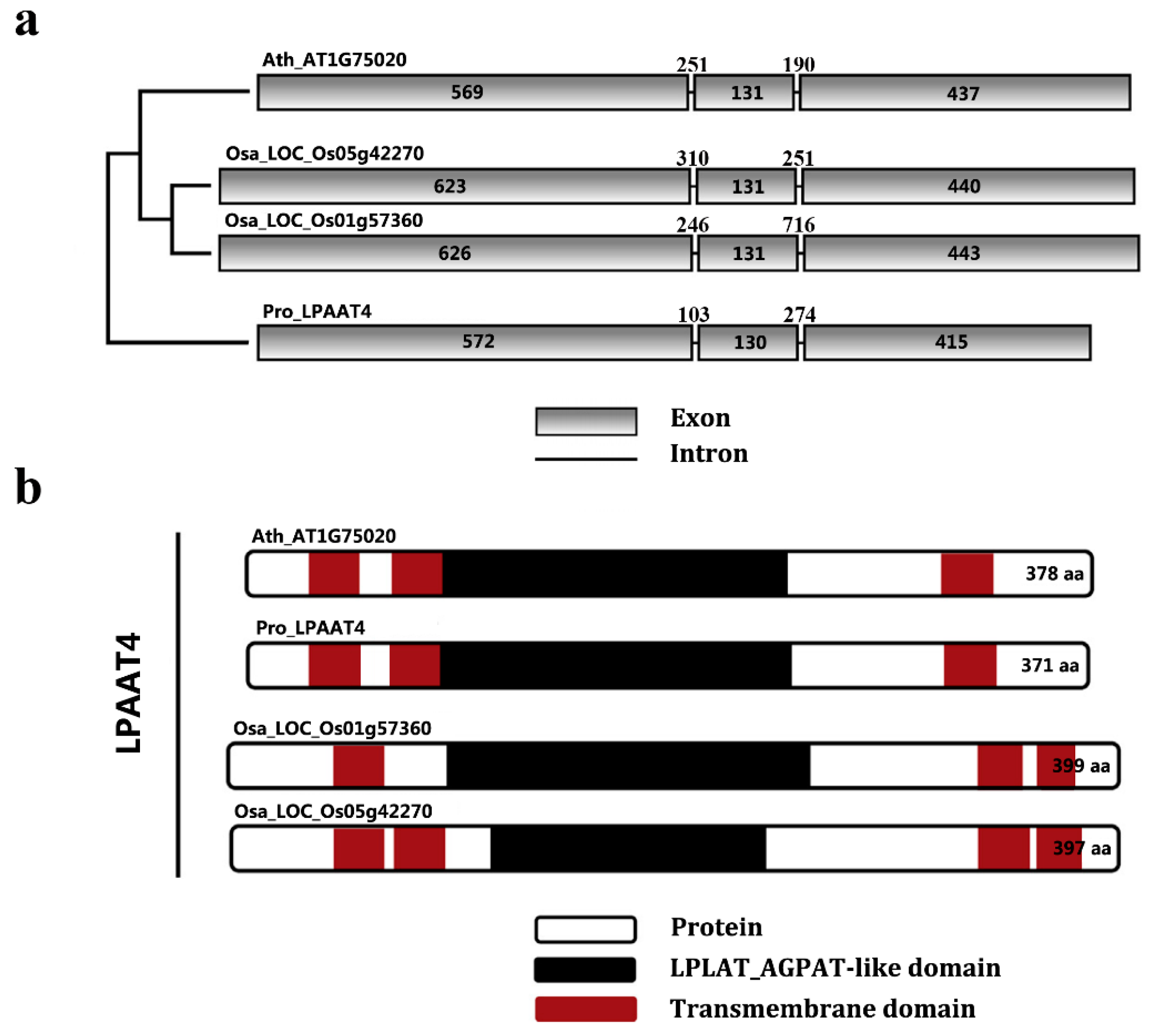
2.3. Phylogenetic Analysis of PrLPAAT4
2.4. Comparative Analysis of PrLPAAT4 and Its Deduced Protein Structure
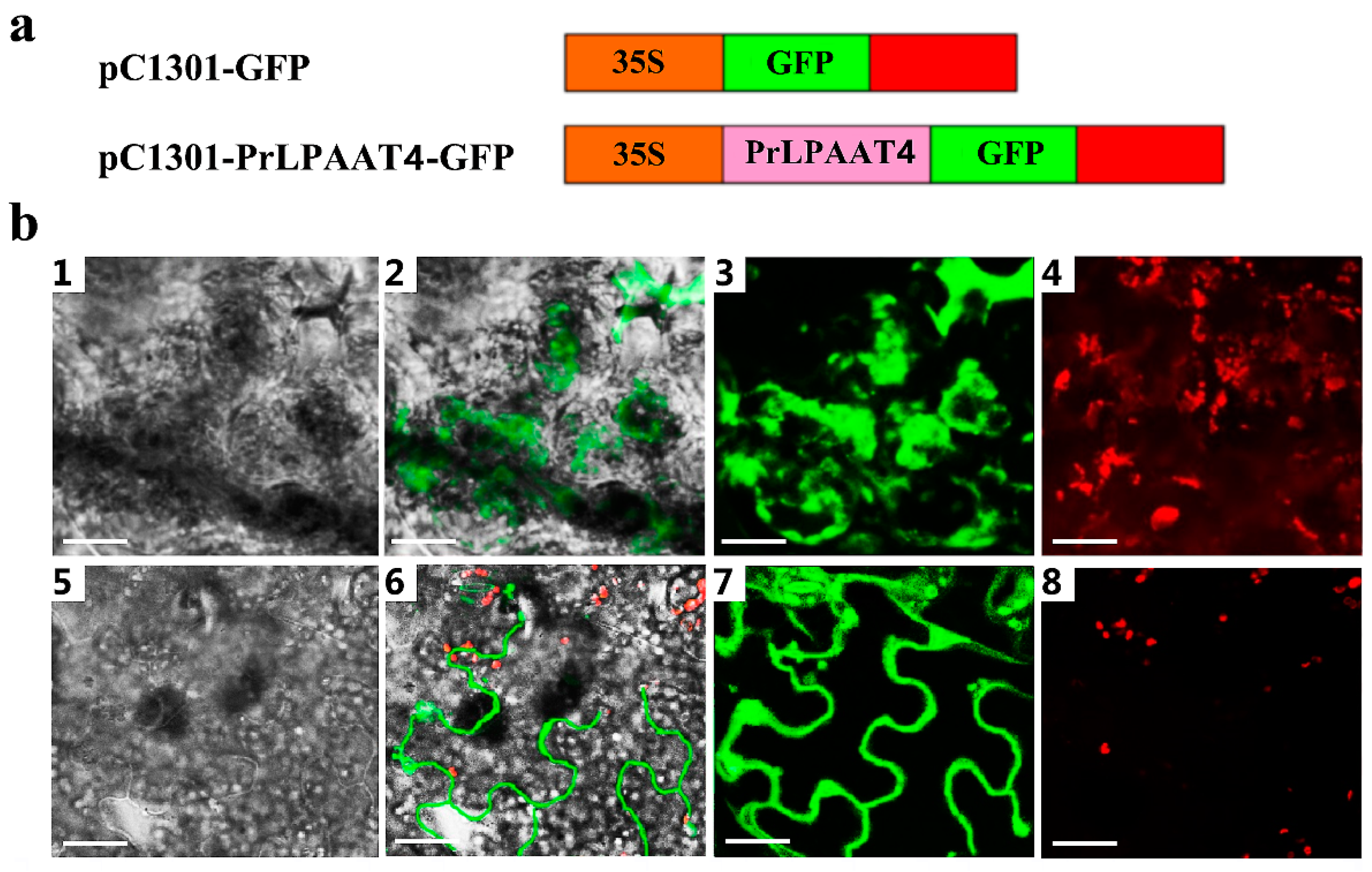
2.5. PrLPAAT4 Is Localized to the Plasma Membrane
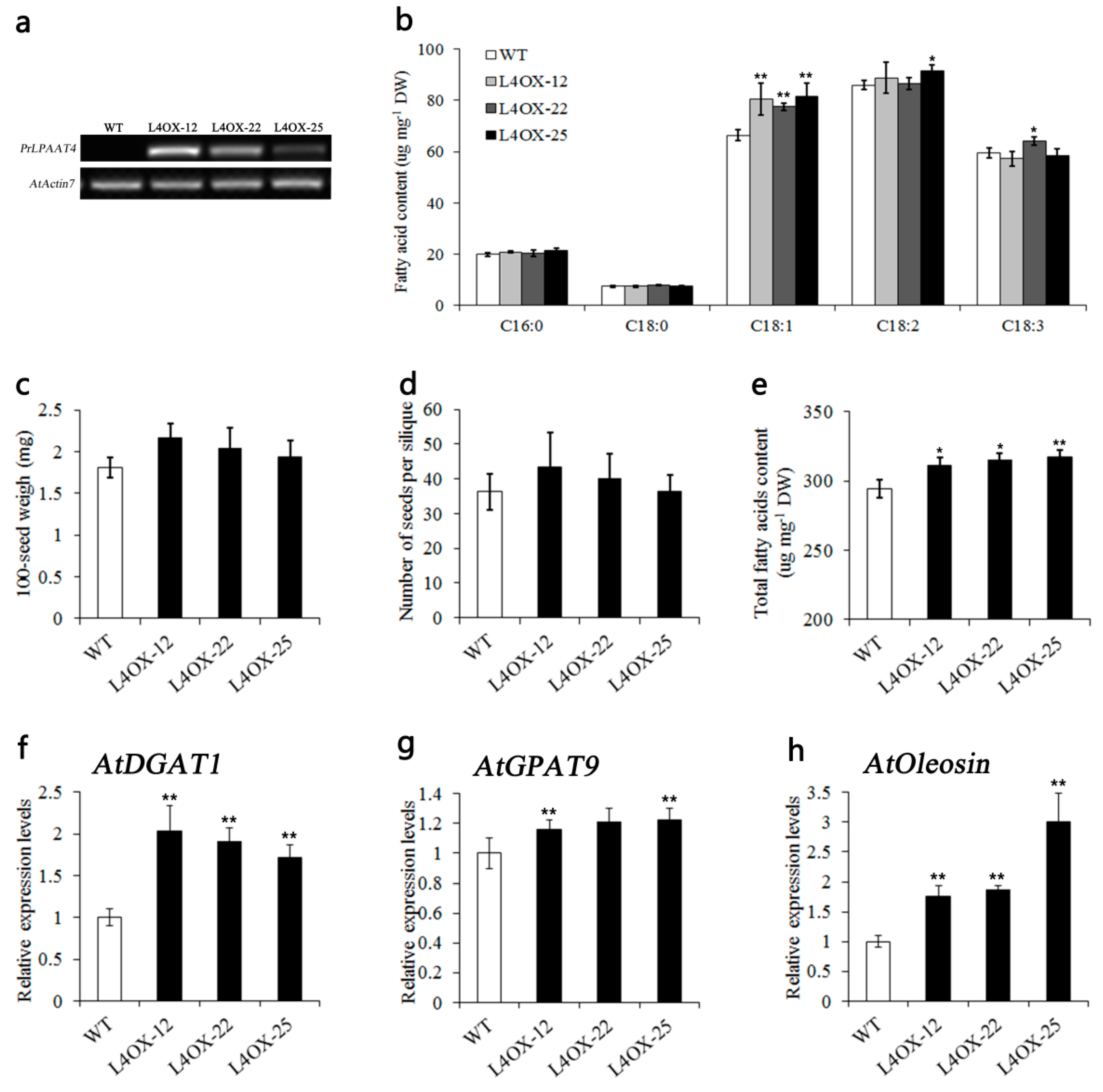
2.6. Overexpression of PrLPAAT4 in Arabidopsis Increases FA Content and Expression of FA and Lipid Biosynthetic Genes
3. Discussion
4. Materials and Methods
4.1. Plant Materials
4.2. Genomic DNA and Total RNA Isolation
4.3. Full-Length Cloning of PrLPAAT4
4.4. Sequence and Phylogeny Analysis
4.5. Subcellular Localization
4.6. Overexpression Construct and Stable Transformation
4.7. FA Measurement
4.8. Quantitative Real-Time PCR
4.9. Statistical Analysis
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Bewley, J.D.; Black, M. Seeds: Physiology of Development and Germination; Springer: New York, NY, USA, 1986. [Google Scholar]
- Ohlrogge, J.; Browse, J. Lipid biosynthesis. Plant Cell 1995, 7, 957–970. [Google Scholar] [CrossRef] [PubMed]
- Bates, P.D.; Stymne, S.; Ohlrogge, J. Biochemical pathways in seed oil synthesis. Curr. Opin. Plant Biol. 2013, 16, 358–364. [Google Scholar] [CrossRef] [PubMed]
- Körbes, A.P.; Kulcheski, F.R.; Margis, R.; Margis-Pinheiro, M.; Turchetto-Zolet, A.C. Molecular evolution of the lysophosphatidic acid acyltransferase (LPAAT) gene family. Mol. Phylogenet. Evol. 2016, 96, 55–69. [Google Scholar] [CrossRef] [PubMed]
- Ohlrogge, J.B.; Browse, J.; Somerville, C.R. The genetics of plant lipids. Biochim. Biophys. Acta 1991, 1082, 1–26. [Google Scholar] [CrossRef]
- Banaś, W.; Sanchez, G.A.; Banaś, A.; Stymne, S. Activities of acyl-CoA:diacylglycerol acyltransferase (DGAT) and phospholipid:diacylglycerol acyltransferase (PDAT) in microsomal preparations of developing sunflower and safflower seeds. Planta 2013, 237, 1627–1636. [Google Scholar] [CrossRef] [PubMed]
- Dahlqvist, A.; Stahl, U.; Lenman, M.; Banas, A.; Lee, M.; Sandager, L.; Ronne, H.; Stymne, S. Phospholipid:diacylglycerol acyltransferase: An enzyme that catalyzes the acyl-CoA-independent formation of triacylglycerol in yeast and plants. Proc. Natl. Acad. Sci. USA 2000, 97, 6487–6492. [Google Scholar] [CrossRef] [PubMed]
- Baud, S.; Lepiniec, L. Physiological and developmental regulation of seed oil production. Prog. Lipid Res. 2010, 49, 235–249. [Google Scholar] [CrossRef] [PubMed]
- Misra, N.; Panda, P.K.; Parida, B.K. Genome-wide identification and evolutionary analysis of algal LPAT genes involved in TAG biosynthesis using bioinformatic approaches. Mol. Biol. Rep. 2014, 41, 8319–8332. [Google Scholar] [CrossRef] [PubMed]
- Brown, A.P.; Coleman, J.; Tommey, A.M.; Watson, M.D.; Slabas, A.R. Isolation and characterisation of a maize cDNA that complements a 1-acyl sn-glycerol-3-phosphate acyltransferase mutant of Escherichia coli and encodes a protein which has similarities to other acyltransferases. Plant Mol. Biol. 1994, 26, 211–223. [Google Scholar] [CrossRef] [PubMed]
- Knutzon, D.S.; Lardizabal, K.D.; Nelsen, J.S.; Bleibaum, J.L.; Davies, H.M.; Metz, J.G. Cloning of a coconut endosperm cDNA encoding a 1-acyl-sn-glycerol-3-phosphate acyltransferase that accepts medium-chain-length substrates. Plant Physiol. 1995, 109, 999–1006. [Google Scholar] [CrossRef] [PubMed]
- Bourgis, F.; Kader, J.C.; Barret, P.; Renard, M.; Robinson, D.; Robinson, C.; Delseny, M.; Roscoe, T.J. A plastidial lysophosphatidic acid acyltransferase from oilseed rape. Plant Physiol. 1999, 120, 913–922. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.U.; Li, Y.; Huang, A.H.C. Ubiquitous and endoplasmic reticulum-located lysophosphatidyl acyltransferase, LPAT2, is essential for female but not male gametophyte development in Arabidopsis. Plant Cell 2005, 17, 1073–1089. [Google Scholar] [CrossRef] [PubMed]
- Taylor, D.C.; Francis, T.; Lozinsky, S.; Hoffman, T.; Giblin, M.; Marillia, E.F. Cloning and characterization of a constitutive lysophosphatidic acid acyltransferase 2 (LPAT2) gene from Tropaeolum majus L. Open Plant Sci. J. 2010, 4, 7–17. [Google Scholar] [CrossRef]
- Chen, S.L.; Huang, J.Q.; Lei, Y.; Zhang, Y.T.; Ren, X.P.; Chen, Y.N.; Jiang, H.F.; Yan, L.Y.; Li, Y.R.; Liao, B.S. Identification and characterization of a gene encoding a putative lysophosphatidyl acyltransferase from Arachis hypogaea. J. Biosci. 2012, 37, 1029–1039. [Google Scholar] [CrossRef] [PubMed]
- Arroyocaro, J.M.; Chileh, T.; Kazachkov, M.; Zou, J.; Alonso, D.L.; Garcíamaroto, F. The multigene family of lysophosphatidate acyltransferase (LPAT)-related enzymes in Ricinus communis: Cloning and molecular characterization of two LPAT genes that are expressed in castor seeds. Plant Sci. 2013, 199–200, 29–40. [Google Scholar] [CrossRef] [PubMed]
- Maisonneuve, S.; Bessoule, J.J.; Lessire, R.; Delseny, M.; Roscoe, T.J. Expression of rapeseed microsomal lysophosphatidic acid acyltransferase isozymes enhances seed oil content in Arabidopsis. Plant Physiol. 2010, 152, 670–684. [Google Scholar] [CrossRef] [PubMed]
- Rao, S.S.; Hildebrand, D. Changes in oil content of transgenic soybeans expressing the yeast SLC1 gene. Lipids 2009, 44, 945–951. [Google Scholar] [CrossRef] [PubMed]
- Zou, J.; Katavic, V.; Giblin, E.M.; Barton, D.L.; Mackenzie, S.L.; Keller, W.A.; Hu, X.; Taylor, D.C. Modification of seed oil content and acyl composition in the brassicaceae by expression of a yeast sn-2 acyltransferase gene. Plant Cell 1997, 9, 909–923. [Google Scholar] [CrossRef] [PubMed]
- Yu, B.; Wakao, S.; Fan, J.; Benning, C. Loss of plastidic Lysophosphatidic acid acyltransferase causes embryo-lethality in Arabidopsis. Plant Cell Physiol. 2004, 45, 503–510. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.U.; Huang, A.H.C. Plastid lysophosphatidyl acyltransferase is essential for embryo development in Arabidopsis. Plant Physiol. 2004, 134, 1206–1216. [Google Scholar] [CrossRef] [PubMed]
- Chapman, K.D.; Ohlrogge, J.B. Compartmentation of triacylglycerol accumulation in plants. J. Biol. Chem. 2011, 287, 2288–2294. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Du, H.; Wang, L.; Shu, Q.; Zheng, Y.; Xu, Y.; Zhang, J.; Zhang, J.; Yang, R.; Ge, Y. Flavonoid composition and antioxidant activity of tree peony (Paeonia section Moutan) yellow flowers. J. Agric. Food Chem. 2009, 57, 8496–8503. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Chen, L.; Xu, Y.; Wang, L.; Wang, L. Identification of floral fragrances in tree peony cultivars by gas chromatography–mass spectrometry. Sci. Hortic. 2012, 142, 158–165. [Google Scholar] [CrossRef]
- Yuan, J.; Cheng, F.Y.; Zhou, S.L. The phylogeographic structure and conservation genetics of the endangered tree peony, Paeonia rockii (Paeoniaceae), inferred from chloroplast gene sequences. Conserv. Genet. 2011, 12, 1539–1549. [Google Scholar] [CrossRef]
- Qi, J.C.; Zhou, H.M.; Ma, J.Q.; Li, P. Analysis of the chemical constituents in peony seed oil by GC-MS. J. Cereals Oils 2005, 11, 22–23. [Google Scholar]
- Shi, G.; Guo, X.F.; Jin, B.L.; Huang, H.X.; Wang, W.; Zhang, S.X.; Wang, F.L. Optimization of supercritical CO2 extraction and analysis of antioxidation activity of peony seed oil. J. Chin. Cereals Oils Assoc. 2013, 28, 47–50. [Google Scholar]
- Wu, J.; Cai, C.; Cheng, F.; Cui, H.; Zhou, H. Characterisation and development of EST-SSR markers in tree peony using transcriptome sequences. Mol. Breed. 2014, 34, 1853–1866. [Google Scholar] [CrossRef]
- Zhang, Q.Y.; Niu, L.X.; Yu, R.; Zhang, X.X.; Bai, Z.Z.; Duan, K.; Gao, Q.H.; Zhang, Y.L. Cloning, characterization, and expression analysis of a gene encoding a putative lysophosphatidic acid acyltransferase from seeds of Paeonia rockii. Appl. Biochem. Biotechnol. 2016, 182, 721–741. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; You, F.M.; Lazo, G.R.; Luo, M.C.; Thilmony, R.; Gordon, S.; Kianian, S.F.; Gu, Y.Q. PIECE: A database for plant gene structure comparison and evolution. Nucleic Acids Res. 2013, 41, D1159–D1166. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, A.; Hayashi, Y.; Matsumoto, N.; Nemotosasaki, Y.; Oka, S.; Tanikawa, T.; Sugiura, T. Glycerophosphate/Acylglycerophosphate acyltransferases. Biology 2014, 3, 801–830. [Google Scholar] [CrossRef] [PubMed]
- Lewin, T.M.; Wang, P.; Coleman, R.A. Analysis of amino acid motifs diagnostic for the sn-glycerol-3-phosphate acyltransferase reaction. Biochemistry 1999, 38, 5764–5771. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, A.K.; Ramakrishnan, G.; Chandramohan, C.; Rajasekharan, R. CGI-58, the causative gene for Chanarin-Dorfman syndrome, mediates acylation of lysophosphatidic acid. J. Biol. Chem. 2008, 283, 24525–24533. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, A.; Nakanishi, H.; Suzuki, H.; Kamata, R.; Tanaka, K.; Waku, K.; Sugiura, T. Topology of acyltransferase motifs and substrate specificity and accessibility in 1-acyl-sn-glycero-3-phosphate acyltransferase 1. Biochim. Biophys. Acta 2007, 1771, 1202–1215. [Google Scholar] [CrossRef] [PubMed]
- Blundy, K.S.; Blundy, M.A.; Carter, D.; Wilson, F.; Park, W.D.; Burrell, M.M. The expression of class I patatin gene fusions in transgenic potato varies with both gene and cultivar. Plant Mol. Biol. 1991, 16, 153–160. [Google Scholar] [CrossRef] [PubMed]
- Dean, C.; Jones, J.; Favreau, M.; Dunsmuir, P.; Bedbrook, J. Influence of flanking sequences on variability in expression levels of an introduced gene in transgenic tobacco plants. Nucleic Acids Res. 1988, 16, 9267–9283. [Google Scholar] [CrossRef] [PubMed]
- Mlynárová, L.; Nap, J.P. Reduced position effect in mature transgenic plants conferred by the chicken lysozyme matrix-associated region. Plant Cell 1994, 6, 417–426. [Google Scholar] [CrossRef] [PubMed]
- Peach, C.; Velten, J. Transgene expression variability (position effect) of CAT and GUS reporter genes driven by linked divergent T-DNA promoters. Plant Mol. Biol. 1991, 17, 49–60. [Google Scholar] [CrossRef] [PubMed]
- Bernerth, R.; Frentzen, M. Utilization of erucoyl-CoA by acyltransferases from developing seeds of Brassica napus (L.) involved in triacylglycerol biosynthesis. Plant Sci. 1990, 67, 21–28. [Google Scholar] [CrossRef]
- Cao, Y.Z.; Huang, A.H.C. Lysophosphatidate acyltransferase in the microsomes from maturing seeds of Meadowfoam (Limnanthes alba). Plant Physiol. 1990, 94, 1199–1206. [Google Scholar] [CrossRef] [PubMed]
- Frentzen, M. Acyltransferases from basic science to modified seed oils. Eur. J. Lipid Sci. Technol. 1998, 100, 161–166. [Google Scholar]
- Laurent, P.; Huang, A.H. Organ- and development-specific acyl coenzyme a lysophosphatidate acyltransferases in palm and meadowfoam. Plant Physiol. 1992, 99, 1711–1715. [Google Scholar] [CrossRef] [PubMed]
- Ichihara, K.I.; Asahi, T.; Fujii, S. 1-Acyl-sn-glycerol-3-phosphate acyltransferase in maturing safflower seeds and its contribution to the non-random fatty acid distribution of triacylglycerol. Eur. J. Biochem. 1987, 167, 339–347. [Google Scholar] [CrossRef] [PubMed]
- Griffiths, G.; Stobart, A.K.; Stymne, S. The acylation of sn-glycerol 3-phosphate and the metabolism of phosphatidate in microsomal preparations from the developing cotyledons of safflower (Carthamus tinctorius L.) seed. Biochem. J. 1985, 230, 379–388. [Google Scholar] [CrossRef] [PubMed]
- Oo, K.C.; Huang, A.H.C. Lysophosphatidate acyltransferase activities in the microsomes from palm endosperm, maize scutellum, and rapeseed cotyledon of maturing seeds. Plant Physiol. 1989, 91, 1288–1295. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.L.; Lei, Y.; Xu, X.; Huang, J.Q.; Jiang, H.F.; Wang, J.; Cheng, Z.S.; Zhang, J.A.; Song, Y.H.; Liao, B.S.; et al. The peanut (Arachis hypogaea L.) gene AhLPAT2 increases the lipid content of transgenic Arabidopsis seeds. PLoS ONE 2015, 10, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Allen, G.C.; Floresvergara, M.A.; Krasynanski, S.; Kumar, S.; Thompson, W.F. A modified protocol for rapid DNA isolation from plant tissues using cetyltrimethylammonium bromide. Nat. Protoc. 2006, 1, 2320–2325. [Google Scholar] [CrossRef] [PubMed]
- Tamura, K.; Peterson, D.; Peterson, N.; Stecher, G.; Nei, M.; Kumar, S. MEGA5: Molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol. Biol. Evol. 2011, 28, 2731–2739. [Google Scholar] [CrossRef] [PubMed]
- Kelley, L.A.; Sternberg, M.J. Protein structure prediction on the Web: A case study using the Phyre server. Nat. Protoc. 2009, 4, 363–371. [Google Scholar] [CrossRef] [PubMed]
- Rambaldi, D.; Ciccarelli, F.D. FancyGene: Dynamic visualization of gene structures and protein domain architectures on genomic loci. Bioinformatics 2009, 25, 2281–2282. [Google Scholar] [CrossRef] [PubMed]
- Clough, S.J.; Bent, A.F. Floral dip: A simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J. 1998, 16, 735–743. [Google Scholar] [CrossRef] [PubMed]
- Li, S.S.; Yuan, R.Y.; Chen, L.G.; Wang, L.S.; Hao, X.H.; Wang, L.J.; Zheng, X.C.; Du, H. Systematic qualitative and quantitative assessment of fatty acids in the seeds of 60 tree peony (Paeonia section Moutan DC.) cultivars by GC-MS. Food Chem. 2015, 173, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−△△CT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are available from the authors. |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Q.; Yu, R.; Sun, D.; Bai, Z.; Li, H.; Xue, L.; Zhang, Y.; Niu, L. PrLPAAT4, a Putative Lysophosphatidic Acid Acyltransferase from Paeonia rockii, Plays an Important Role in Seed Fatty Acid Biosynthesis. Molecules 2017, 22, 1694. https://doi.org/10.3390/molecules22101694
Zhang Q, Yu R, Sun D, Bai Z, Li H, Xue L, Zhang Y, Niu L. PrLPAAT4, a Putative Lysophosphatidic Acid Acyltransferase from Paeonia rockii, Plays an Important Role in Seed Fatty Acid Biosynthesis. Molecules. 2017; 22(10):1694. https://doi.org/10.3390/molecules22101694
Chicago/Turabian StyleZhang, Qingyu, Rui Yu, Daoyang Sun, Zhangzhen Bai, Hong Li, Liang Xue, Yanlong Zhang, and Lixin Niu. 2017. "PrLPAAT4, a Putative Lysophosphatidic Acid Acyltransferase from Paeonia rockii, Plays an Important Role in Seed Fatty Acid Biosynthesis" Molecules 22, no. 10: 1694. https://doi.org/10.3390/molecules22101694




